Abstract
The purpose of this study was to assess the conjugated linoleic acid (CLA) content of some commercial beef cuts and dairy products. Levels of CLA ranged between 3.75 and 20.45 mg/g fat, corresponding to 2% milk and cooked ground beef. On the basis of serving size, CLA content ranged between 12.87 and 93.62 mg/portion, corresponding to cream cheese (portion of 15 g) and cooked ground beef (portion of 100 g). There was no direct relationship between CLA content and the amount of fat provided per serving size in dairy foods. This study showed the presence of significant amounts of natural CLA in beef and dairy fats in the foods analyzed.
El propósito de este estudio fue evaluar el contenido de ácidos grasos y CLA encontrados en algunos cortes de carne de res y lácteos comerciales selectos. Los niveles de CLA fluctuaron entre 3,75 y 20,45 mg/g de grasa correspondientes a leche con 2% de grasa y carne de res molida cocida, respectivamente. En base a sus respectivas porciones alimenticias, el contenido de CLA en los productos estudiados varió entre 12,87 a 93,62 mg/porción, que correspondieron a queso crema (porción de 15 g) y carne de res molida cocida (porción de 100 g). Este estudio mostró la presencia de cantidades importantes de CLA en las carnes de res y lácteos analizados.
Palabras clave:
Introduction
Conjugated linoleic acid (CLA) refers to a group of positional and geometrical isomers of linoleic acid (LA). Interest in CLA has arisen due to reports of its many beneficial health effects. Both animal and in vitromodels have shown that CLA is able to inhibit tumorigenesis in skin, colon, prostate, and breast tissues (Ou, Ip, Lisafeld, & Ip, Citation2007). Antiatherogenic properties have also been associated with dietary CLA in hamster models (Ribot, Portillo, Picó, Macarulla, & Palou, Citation2007). Additionally, CLA was reported to modify energy metabolism by decreasing body fat and increasing lean tissue mass (Park & Pariza, Citation2007). The major isomer detected in animal tissues is the Δ9c,11t–18:2 isomer, and this chemical species has been reported to be further esterified in the phospholipid fraction (Chin, Liu, Storkson, Ha, & Pariza, Citation1992). Therefore, detection of CLA in this fraction suggests a functional role. CLA has been measured in various human tissues, and levels of CLA can be modulated by dietary intervention. CLA is produced naturally in ruminant animals such as beef, sheep, and goats and is a component of most western diets. The ruminal bacterium Butyrivibrio fibrisolvensis responsible for the synthesis of the Δ9c,11t–18:2 isomer as an intermediate in the biohydrogenation of LA to vaccenic acid (Kepler, Tucker, & Tove, Citation1971). Consequently, this single isomer ofCLA is found in the highest proportion in dairy and beefproducts (Ma, Wierzbicki, Field, & Clandinin, Citation1999). Other authors have reported the presence of some of the positional and geometrical isomers of LA: Δ9,11–18:2 and Δ10,12–18:2 (Lin, Boylston, Chang, Luedecke, & Shultz,Citation1995; Ritzenthaler et al., Citation2001). Variation in CLA content in milk has been associated with several factors such as stage of lactation, parity (Kelly, Kolver, Bauman, Van Amburgh, & Muller, Citation1998), and breed (Secchiari et al., Citation2001). However, diet is the most important factor influencing milkCLA concentrations (Collomb et al., Citation2008; Collomb, Butikofer, Sieber, Jeangros, & Bosset, Citation2002). Nudda, McGuire, Battacone, and Pulina (Citation2005) published seasonal variations in fatty acid (FA) composition particularly CLA and transvaccenic acid in milk fat of pasture-grazed ewes. The authors noted that these seasonal changes were probably related to the changes in pasture quality. Other factors reported that affect the lipid content and composition of bovine milk include breed (Ellis et al., Citation2006; Ferlay, Martin, Pradel, Coulon, & Chilliard, Citation2006), geographical locations (Collomb et al., Citation2002), access to fresh grazing (Ellis et al., Citation2006), and feeding system (Chilliard, Ferlay, & Doreau, Citation2001; Collomb et al., Citation2008; Mushi, Thomassen, Kifaro, & Eik, Citation2010; Romano et al., Citation2010). French et al. (Citation2000) reported a linear increase in CLA-beef fat content with increased percentage of grass in the diet. While these studies indicate the likelihood that beef from pasture-fed or pasture-finished cattle will contain higher concentrations of CLA than feedlot cattle, small sample sizes and the limited selection of muscles for analysis prevent a firm conclusion. Although some factors before mentioned are important, appropriate supplements and correct feed rations can increase the proportion of monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) and decrease the saturated fatty acid (SFA) level at the same time (Stockdale et al., Citation2003). CLA are widely found in various foods, including dairy products, meat and meat products, certain plant oils, and some seafood products (Chin et al., Citation1992). Animal sources and, in general, foods from ruminants contain more CLA than foods from non-ruminants (Bu, Wang, Dhiman, & Liu, Citation2007; Lin et al., Citation1995). CLA intake in Germany was estimated to be of 360mg/day for women and 440 mg/day for men (Fritsche & Steinhart, Citation1998); this level of intake is considerably less than the 3 g/day minimum value extrapolated from animal studies that would result in beneficial effects (Ou et al., Citation2007). However, the exact intake levels in humans that confer these beneficial effects are still undetermined. Nevertheless, increasing CLA consumption or assessment of its consumption requires documentation of CLA composition and content in the food supply. Current compositional information on the CLA content in foods is limited and has been conducted in several countries (Fritsche & Steinhart, Citation1998; Glew et al., Citation2006; Lin et al., Citation1995; Ma et al., Citation1999; Prandini, Sigolo, Tansini, Brogna, & Piva, Citation2007; Ritzenthaler et al., Citation2001; Talpur, Bangher, & Khuhawar, Citation2006), but to the best of our knowledge, not in Mexico. Therefore, the aim of the present study was to provide data on the CLA content and the fatty acid composition of some common foods consumed in the country.
Materials and methods
A mixture of commercial CLA as free fatty acids was obtained from Nu-Chek Prep (Elysian, MN, USA, 99% purity) and Matreya (Ontario, Canada) and was used as a quantitative standard. Concentrated CLA (86% pure) was also prepared from corn oil as described elsewhere (Martínez, Vinay, Brieva, Hill, & Garcia, Citation2005). All solvents were reagent grade or better from Baker (Mexico City). Methanolic HCl (3 M) and margaric acid (C17:0) used as internal standard were obtained from Supelco (Mexico City). The foods investigated for this study were selected based on typical southwestern Mexico consumption. A total of eight different dairy products: 2% milk, whole milk, sweet cream, farmer's, panela (fresh), and Oaxaca cheeses, cream cheese, and yoghurt; and four beef products: ground beef, roast beef, sirloin roast, and rib roast were randomly purchased from different local shops or supermarkets in the city of Tuxtepec, Oaxaca, México. The beef was produced locally.
Sampling and sample preparation
Food samples were representative of several commercial brands. Meat samples were: rib roast, sirloin tip roast, roast beef, and ground beef, and dairy products were yogurt, 2% milk, whole milk, farmer's, panela, and Oaxaca chesses, cream cheese, and sweet cream. For all the types of meat and dairy products selected, we analyzed seven samples of each product in triplicate, thus considering n = 21. Meat samples were analyzed for CLA as both raw and cooked preparations (broiled) using 20–50 g samples and a cooking treatment of 0.5–1 h and homogenized. The cooking treatment was assessed by making sure that an internal temperature of 80 ± 2°C was reached at the coldest point of the samples. Forwhole milk and 2% milk, 50 mL samples were used according to the methods proposed by Ma et al. (Citation1999) and Shantha, Crum, and Decker (Citation1994) with slight modifications in terms of sample quantities.
Lipid extraction
Lipid material was extracted in triplicate following the method for the isolation and purification of total lipids from animal tissues; fat content was determined from dry solids (Folch, Lees, & Stanley, Citation1957). All lipid extracts were reconstituted in chloroform (Ma et al., Citation1999).
Saponification and methylation of lipid extracts for gas chromatography (GC)analysis
In a glass screw-cap tube, 5 mg of extracted fat sample and 25 μg of margaric acid (internal standard) were saponified with 2 mL of 0.5 M NaOH in methanol for 1 h at 110°C in a heating block, and then cooled. n-Hexane (2 mL) and 0.2 M HCl–methanol (1 mL) were then added to each sample. The sample vial was then flushed with N2and held overnight in a heating block at 80°C; then, 200 μL of distilled water was added and the mixture was extracted with 2 mL of hexane. Samples were vortexed briefly and then centrifuged at 224 × gfor 10 min. The upper n-hexane phase was collected and the lower phase re-extracted with n-hexane (2 mL). Both supernatants were mixed and dried with anhydrous sodium sulfate. Samples were vortexed briefly and then centrifuged at224 × gfor 10 min again. The upper n-hexane phase was collected and 1 μL from each of the paired samples were injected to the gas chromatograph.
Chromatographic analysis
CLA content was determined using a HP 6890 gas chromatograph fitted with a Supelcowax–10 capillary column (60 m × 0.32 mm i.d., 0.25 μm film thickness) with a 40:1 split injector and flame ionization detector (FID) temperatures set at 250°C. Nitrogen was employed as the carrier gas. The temperature program used was from 60 to 200°C at 20°C/min and held for 45 min (Martínez et al., Citation2005). The results were expressed as absolute values (mg CLA/g fat). Samples were injected in triplicate from each lipid extraction. CLA isomers were identified by comparison of their retention times with commercial and prepared CLA standards. CLA content was expressed in mg/g fat or mg/g sample. Content was alsoexpressed per serving using values from the tables of nutritional values for diet calculations of Mexico (Quintín, Citation2001).
Statistical analysis
The mean values and standard deviations of the samples were calculated. Tukey's pairwise comparison test was applied to determine the significance between different treatments (p < 0.05) using the statistical software Minitab for Windows version 13.20 (Minitab, Inc., State College, PA, USA).
Results and discussion
CLA content in commercial dairy foods
In this study, only the peak corresponding to the cis9, trans11 isomer of CLA was detected in all the samples analyzed and it appeared as a single peak among all of the remaining fatty acid peaks. depicts a chromatogram corresponding to the quantitative standard and another chromatogram of a meat fat sample. shows fat content and CLA concentrations of various milk and dairy products based on lipid content and sample weight. Fat content differed in some products but such values were consistent with the type of product, except 2% milk. These products showed the lowest values and there was no statistical difference between them (p > 0.05). Sweet cream and cream cheeses were the products with the highest fat content. When compared on fat basis, a significant difference (p < 0.05) in CLA content was observed among different groups of dairy products, except in yoghurt, whole milk, panela, and cream cheeses. Content of CLA ranged between 3.75 and 7.01 mg/g fat, with farmer's cheese (7.01 mg/g fat) and sweet cream (6.61 mg/g fat) having the highest values (p < 0.05). These values were closely related with those reported by Eynard andLopez (2003), who found that CLA concentrations inArgentinean dairy products typically range from 2.9 to 8.92 mg/g fat, of which the 9-cis, 11-transisomer makes up to 73–93% of the total. CLA contents were different when expressed as mg/g sample, and the values ranged from 0.11 (for 2% milk) to 1.31 mg/g (for sweet cream). There was no significant difference (p > 0.05) in foods according to the groups analyzed except in cream products. The differences in CLA contents found in this work could be due to some factors that are known to affect the CLA concentration in milk fat mentioned by Pariza, Park, and Cook (Citation2001), Collomb et al. (Citation2008) and others. Additionally, the diet of the animals involved could have played an important role in the CLA content of milk, as previous investigations have proposed (AbuGhazaleh, Schingoethe, Hippen, & Kalscheur, Citation2004; Chilliard et al., Citation2001; Kelly et al., Citation1998). A high CLA content in cream could be attributed to the concentration of fat in this product. However, such an increase was not important compared to the large natural variations of CLA in dairy products reported by others (Chin et al., Citation1992; Coakley et al., Citation2007; Shantha et al., Citation1994). Among other compositional factors recognized to contribute to elevated CLA contents are high protein quality acting as hydrogen donor, and originally high contents of CLA and CLA precursors in the raw milk (Ha, Grimm, & Pariza, Citation1989). Comparisons between products were made assuming the existence of a number of unknown and uncontrolled variables because of the significant variability in the CLA content of raw milk and consequently for dairy products. For example, samples in this work have been obtained from different animals, feed regimes, or breeds; when compared to previously published values, the concentration of CLA in samples of dairy products used in this study was similar to values reported by Chin et al. (Citation1992) for whole milk, with a mean value of 5.5 mg CLA/g fat and yoghurt mean of 4.8 mg CLA/g fat, and Ma et al. (Citation1999) values for whole milk and 2% milk varied between 3.4 and 5.0 mg CLA/g fat, respectively, and yoghurt 4.4 mg CLA/g fat. Variability in the protein, lipid, moisture contents and the titratable acidity of these dairy products as a result of differences in processing conditions may be associated to observed differences in their CLA content. Due to the lipid nature of CLA, fat content had the most significant influence on the CLA content of dairy products when compared based on sample weight. Moreover, the positive relationship between protein, CLA content, and isomerase activity of starter cultures could contribute to the formation of CLA in cheeses and fermented dairy products in comparison to milk values according to previous studies reported by Ha et al. (Citation1989). Significant differences (p < 0.05) were observed when concentrations of CLA were expressed per serving size and this ranged from 12.87 (cream cheese) to 47.88 mg/serving (yoghurt) except Oaxaca cheese and whole milk. There is no direct relationship between CLA content and the amount of fat provided per usual serving size in dairy foods and this is due to differences inherent to variations in quantity of each portion according to nutritionist recommendations. Yoghurt and farmer's cheese had the highest concentrations when CLA concentration was expressed on serving size basis.
Figure 1. GC chromatogram of (A) the separated isomers cis9, trans11 and trans10, cis12 C18:2 of the quantitative standard used and (B) a sample of beef fat. The sample contains a single peak corresponding to the cis9, trans11 18:2 isomer.
Figura 1. Cromatograma (A) de los isómeros separados cis9, trans11 y trans10, cis12 C18:2 del estándar para cuantificación utilizado, y (B) muestra de grasa de res. La muestra contiene un único pico correspondiente al isómero cis9, trans11 18:2.
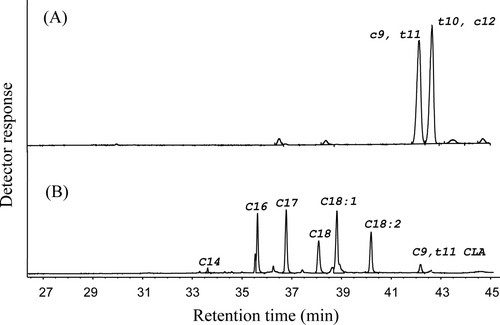
Table 1. Fat and CLA concentrations in milk and dairy products.
Tabla 1. Concentración de grasa y ácido linoleico conjugado en leche y productos lácteos.
CLA content in commercial beef products
shows no significant differences (p > 0.05) between raw and cooked meat of the same type with respect to fat content. Raw and cooked ground beef had the highest fat content and this was statistically different with the rest of the cuts. The meat with the highest significant (p > 0.05) CLA concentration was cooked and raw ground beef with 20.45 and 20.61 mg CLA/g fat, respectively. Among raw samples the CLA content ranged from 9.84 (rib roast) to 20.61 mg CLA/g fat (ground beef). Cooked samples ranged from 10.21 (rib roast) to 20.45 mg CLA/g fat for ground beef showing significant differences (p > 0.05) among cuts. These results suggest that cooking did not induce isomerization, according to other authors that have reported that neither the cooking procedure nor the extent of cooking had a significant influence on the amounts of CLA in beef (Fritsche & Steinhart, Citation1998; Shantha et al., Citation1994). The CLA content of raw meats in this study was higher than that measured by Shantha et al. (Citation1994), who reported that CLA content in raw beef cuts (rib eye, round, T-bone, and sirloin) ranged from 3.1 to 8.5 mg/g fat. Other authors studied the level and type of CLA isomers in commercial beef and dairy products from Canada (Ma et al., Citation1999). They found that raw beef ranged from 1.2 to 3.0 mg/g fat and cooked beef ranged from 1.2 to 3.2 mg CLA/g fat. Values found in the present study are higher than those observed in Canadian beef fat maybe because cows in Mexico are fed mainly by grass diets and this is in agreement with data reported by French et al. (Citation2000). In this study, the only CLA isomer detected was c9,t11 in all the samples analyzed (as we mentioned before), because more than 80% of total CLA is represented by this isomer (Fritsche & Steinhart, Citation1998). The t10,c12 isomer has been reported to be responsible for the portioning (of fat to muscle) effect (Park, Storkson, Albright, Liu, & Pariza, Citation1999). The c11,t13 isomer was reported to selectively concentrate in heart phospholipids of pigs (Kramer et al., Citation1998). The t7,c9 isomer, which is usually the second most predominant CLA in bovine and human milk (Yurawecz et al., Citation1998), has been found to represent as high as 40% of total CLA in lactating cows fed milk fat-depressing diets (Piperova et al., Citation2000). Even though other authors had found that in beef fat specifically the second most important isomer is 18:2 trans10, cis12 (Turk & Smith, Citation2009). Since individual CLA isomers have different biological activities, the determination of the CLA isomer profile in ruminant-derived fat is required. This determination could be achieved using more precise techniques like silver ion-high-performance liquid chromatography (HPLC) and GC with complementary identification by GC–mass spectrometry and GC–Fourier transform infrared analyses (Roach, Mossoba, Yurawecz, & Kramer, Citation2002). CLA content differed between cuts, maybe because CLA is located in the interstitial, non-visible fat evenly distributed along muscle fibers, as well as in subcutaneous depots (Eynard & Lopez, Citation2003), and these depots are located in different arrays in each cut. Maria, Colnago, Forato, and Bouchard (Citation2010) showed that the average CLA content in beef fat is about 25% higher in subcutaneous fat (SCF) than in intramuscular fat (IMF). They observed a good correlation (r = 0.85) between the CLA content in SCF and IMF samples, indicating that it is possible to measure the CLA content in SCF and then use these data to estimate the IMF content. This could be the reason by which a high content of SCF in ground beef could increase the CLA content on meat. CLA content in beef fat can also be influenced by the same factors mentioned for dairy products, such as breed (Salamon, Varga-Visi, Sára, Csapó-Kiss, & Csapó, Citation2006), feeding regime, feed allowance (Castañeda-Gutiérrez, Overton, Butler, & Bauman, Citation2005), and dietary oils from the feed (Ryhänen et al., Citation2005). There were differences in beef fat CLA content: cooked ground beef contained the highest CLA concentrations of all samples analyzed (p < 0.05).
Table 2. Fat concentration and CLA concentrations in beef fat.
Tabla 2. Concentración de grasa y ácido linoleico conjugado en grasa de carne de res.
Significant differences (p < 0.05) were observed when concentrations of CLA were expressed per serving size and this ranged from 22.98 (rib roast) to 93.36 mg/serving (ground beef). Raw and cooked ground beef had the highest concentrations when CLA concentration was expressed on serving size basis.
On the basis of a serving size considered for beef and dairy products, the levels of CLA ranged from 12.87 to 93.62 mg per portion (cream cheese and cooked ground beef, respectively). We estimated the CLA daily intake in Mexico in the range of 0.3–0.63 g. This estimation was done taking into account an average daily intake of men and women of ages between 18 and 35 years, and diets for normal and moderate physical activity (Quintín, Citation2001) were considered (theoretical estimation). A report estimated the daily intake of CLA in Germany as 0.36 g/day for women and 0.44 g/day for men (Fritsche & Steinhart, Citation1998). The average daily CLA intake in Australia was calculated as 0.5–1.5 g (Ryhänen et al., Citation2005). In general, estimates of daily intake of CLA in people from different countries ranged from 0.015 to 1 g (Eynard & Lopez, Citation2003; Lin et al., Citation1995). These amounts are smaller than the 3.5 g/day suggested that could provide anticarcinogenic protection in laboratory animals (Ou et al., Citation2007). Increasing dietary CLA consumption to these levels could be accomplished by altering the consumption of CLA-containing foods. However, this should be promoted with caution because a diet rich in CLA is frequently associated with diets rich in fat. Alternatively, food products recognized as a source of CLA can be substituted for similar foods containing lower amounts of CLA or none (e.g. butter vs. margarine). An alternative approach for increasing dietary CLA intake is to naturally enhance CLA concentration in foods. Ryhänen et al. (Citation2005) studied the effect of production of CLA-enriched milk and dairy products from cows fed grass silage supplemented with a cereal-based concentrate containing rapeseed oil. Their results showed that the inclusion of the rapeseed oil as concentrate supplements increased the mean concentrations of CLA in milk fat from 0.46 to 1.02 g/100 g of total fatty acids. Moreover, feeding safflower oil, linseed oil, fish oil, or full-fat extruded soybean meal increased the CLA content in lamb and beef fat (Dhiman et al., Citation2000). We found differences in the CLA contents between milk, dairy foods, and beef; the latter showed the highest CLA content expressed as mg/g fat and the highest CLA concentration of all samples analyzed. On the basis of a serving size, yoghurt and cooked ground beef samples had the highest CLA content.
Fatty acid profile
In addition to the analysis of CLA, the composition of some of the major fatty acids was also investigated and the results are depicted in and . Values are reported as mg/g sample of fatty acids analyzed. A fairly high variability of milk, dairy, and beef fat fatty acid composition among different samples was observed. We determined a lower proportion (0.61–1.15mg/g sample vs. 2.82–13.33 mg/g sample) of myristic acid (C14:0) in beef fat values compared to dairy products, respectively. A lower proportion of myristic acid in beef fat seems to be favorable for human health because of their negative role in atherosclerosis (Pfeuffer & Schrezenemeir, Citation2000). Mean values for stearic acid contents (C18:0) varied from 5.71 to 13.94 mg/g sample in dairy products (). MUFA oleic acid (C18:1) showed mean contents of 1.61–11.02 mg/g of total FA in milk fat, in comparison to beef fat (8.52–12.57 mg/g sample). Cream cheese, sweet cream, and farmer's cheese showed the higher percentage of CLA, but significant difference was observed among them. Significant difference (p < 0.05) was noted among the rest of the dairy products studied (). Moreover, cream cheese and 2% milk showed a higher proportion in PUFA content.
Table 3. Fatty acid profile in milk and dairy products (mg/g sample of the fatty acid analyzed).
Tabla 3. Perfil de ácidos grasos en leche y productos lácteos (mg/g de muestra de los ácidos grasos analizados).
Table 4. Fatty acid profile in beef fat (mg/g sample of the fatty acid analyzed).
Tabla 4. Perfil de ácidos grasos en grasa de carne de res (mg/g de muestra de los ácidos grasos analizados).
Concerning to beef products, the higher percentage of CLA was showed for raw and cooked ground beef and no significant difference was observed between them, but a significant difference was noted with respect to the rest of the products analyzed. The mean content of PUFAs ranged between 0.34 and 1.69 mg/g in dairy products in comparison to 1.21 and 2.87 mg/g in beef fat. CLA content varied from 0.11 to 1.71 mg/g sample considering both dairy products and beef fat. Although these PUFAs are present in small concentrations in milk fat, they could exert many health promoting effects including anticarcinogenic, antimutagenic, hypocholesterolemic, and antiatherosclerotic properties (Jahries et al., Citation1999; Jensen, Citation2002). Differences found in fatty acid composition of milk and beef fat in this study could be in agreement with some factors reported by Collomb et al. (Citation2008) and Stockdale et al. (Citation2003). According to data presented in this work, consumers should avoid high intake of sweet cream and milk (due to the high SFA content) and prefer farmer's chesses and yoghurt with respect to their CLA content. The different types of cheeses analyzed were rich in MUFA and have similar values of CLA content according to the serving size recommended by nutritionists, in comparison to milk and yoghurt products. Finally, cooked ground beef had high values of MUFA and SFA but contained the highest CLA concentration (according to the serving size) of the total of the selected food products analyzed.
Acknowledgment
The authors acknowledge the financial support of the Dirección General de Educación Superior Tecnológica (DGEST) through grant 592.02-P.
References
- AbuGhazaleh , A.A. , Schingoethe , D.J. , Hippen , A.R. and Kalscheur , K.F. 2004 . Conjugated linoleic acid increases in milk when cows fed fish meal and extruded soybeans for an extended period of time . Journal of Dairy Science , 87 : 1758 – 1766 .
- Bu , D.P. , Wang , J.Q. , Dhiman , T.R. and Liu , S.J. 2007 . Effectiveness of oils rich in linoleic and linolenic acids to enhance conjugated linoleic acid in milk from dairy cows . Journal of Dairy Science , 90 : 998 – 1007 .
- Castañeda-Gutiérrez , E. , Overton , T.R. , Butler , W.R. and Bauman , D.E. 2005 . Dietary supplements of two doses of calcium salts of conjugated linoleic acid during the transition period and early lactation . Journal of Dairy Science , 88 : 1078 – 1089 .
- Chilliard , Y. , Ferlay , A. and Doreau , M. 2001 . Effect of different types of forages, animal fat or marine oils in cow's diet on milk fat secretion and composition, especially conjugated linoleic acid (CLA) and polyunsaturated fatty acids . Livestock Production Science , 70 : 31 – 48 .
- Chin , S.F. , Liu , W. , Storkson , J.M. , Ha , Y.L. and Pariza , M.W. 1992 . Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens . Journal of Food Composition and Analysis , 5 : 185 – 197 .
- Coakley , M. , Barret , E. , Murphy , J.J. , Ross , P. , Devery , R. and Stanton , C. 2007 . Cheese manufacture with milk with elevated conjugated linoleic acid levels caused by dietary manipulation . Journal of Dairy Science , 90 : 2919 – 2927 .
- Collomb , M. , Bisig , W. , Bütikofer , U. , Sieber , R. , Bregy , M. and Etter , L. 2008 . Fatty acid composition of mountain milk from Switzerland: Comparison of organic and integrated farming systems . International Dairy Journal , 18 : 976 – 982 .
- Collomb , M. , Butikofer , U. , Sieber , R. , Jeangros , B. and Bosset , J.O. 2002 . Composition of fatty acids in cow's milk fat produced in the lowlands, mountains and highlands of Switzerland using high-resolution gas chromatography . International Dairy Journal , 12 : 649 – 659 .
- Dhiman , T.R. , Satter , L.D. , Pariza , M.W. , Galli , M.P. , Albright , K. and Tolosa , M.X. 2000 . Conjugated linoleic acid (CLA) content of milk from cows offered diets rich in linoleic and linolenic acid . Journal of Dairy Science , 83 : 1016 – 1027 .
- Ellis , K.A. , Innocent , G. , Grove-White , D. , Cripps , P. , McLean , W.G. , Howard , C.V. and Mihm , M. 2006 . Comparing the fatty acid composition of organic and convectional milk . Journal of Dairy Science , 89 : 1938 – 1950 .
- Eynard , A.R. and Lopez , C.B. 2003 . Conjugated linoleic acid (CLA) versus saturated fats/cholesterol: Their proportion in fatty and lean meats may affect the risk of developing colon cancer . Lipids in Health and Disease , 2 : 6 – 10 .
- Ferlay , A. , Martin , B. , Pradel , Ph. , Coulon , J. and Chilliard , Y. 2006 . Influence of grass-based diets on milk fatty acid composition and milk lipolytic system in Tarentaise and Montbéliarde cow breeds . Journal of Dairy Science , 89 : 4026 – 4041 .
- Folch , J. , Lees , M. and Stanley , G.H.S. 1957 . A simple method for the isolation and purification of total lipids from animal tissues . Journal of Biological Chemistry , 226 : 497 – 509 .
- French , P. , Stanton , C. , Lawless , F. , O'Riordan , E.G. , Monahan , F.J. , Caffrey , P.J. and Maloney , A.P. 2000 . Fatty acid composition, including conjugated linoleic acid, of intramuscular fat from steers offered grazed grass, grass silage, or concentrate-based diets . Journal of Animal Science , 78 : 2849 – 2855 .
- Fritsche , J. and Steinhart , H. 1998 . Amounts of conjugated linoleic acid (CLA) in German foods and evaluation of daily intake . Zeitschrift für Lebensmittel-Untersuchung und -Forschung A , 206 : 77 – 82 .
- Glew , R.H. , Herbein , J.H. , Ma , I. , Obadofin , M. , Wark , W.A. and Vanderjagt , D.J. 2006 . The transfatty acid and conjugated linoleic acid content of Fulani butter oil in Nigeria . Journal of Food Composition and Analysis , 19 : 704 – 710 .
- Ha , Y.L. , Grimm , N.K. and Pariza , M.W. 1989 . Newly recognized anticarcinogenic fatty acids: Identification and quantification in natural and process cheeses . Journal of Agricultural and Food Chemistry , 37 : 75 – 81 .
- Jahries , G.J. , Fritsche , J. , Mockel , P. , Schone , F. , Moller , U. and Steinhart , H. 1999 . The potential anticarcinogenic conjugated linoleic acid, cis-9 trans-11 C18:2 in milk of different species: Cow, goat, ewe, sow, mare, woman . Nutrition Research , 19 : 1541 – 1549 .
- Jensen , R.G. 2002 . The composition of bovine milk lipids: January 1995 to December 2000 . Journal of Dairy Science , 85 : 295 – 350 .
- Kelly , M.L. , Kolver , E.S. , Bauman , D.E. , Van Amburgh , M.E. and Muller , L.D. 1998 . Effect of intake of pasture on concentrations of conjugated linoleic acid in milk of lactating cows . Journal of Dairy Science , 81 : 1630 – 1636 .
- Kepler , C.R. , Tucker , W.P. and Tove , S.B. 1971 . Biohydrogenation of unsaturated fatty acids. V. Stereospecificity of proton addition and mechanism of action of linoleic Δ12-cis, Δ11-trans-isomerase of Butyrivibrio fibrisolvens . Journal of Biological Chemistry , 246 : 2765 – 2771 .
- Kramer , J.K.G. , Sehat , N. , Dugan , M.E.R. , Mossoba , M.M. , Yurawecz , M.P. , Roach , J.A.G. and Ku , Y. 1998 . Distribution of conjugated linoleic acid (CLA) isomers in tissue lipid classes ofpigs fed a commercial CLA mixture determined by gas chromatography and silver ion-high-performance liquid chromatography . Lipids , 33 : 549 – 558 .
- Lin , H. , Boylston , T.D. , Chang , M.J. , Luedecke , L.O. and Shultz , T.D. 1995 . Survey of the conjugated linoleic acid contents of dairy products . Journal of Dairy Science , 78 : 2358 – 2365 .
- Ma , D.W. , Wierzbicki , L.A.A. , Field , C.J. and Clandinin , T.M. 1999 . Conjugated linoleic acid in Canadian dairy and beef products . Journal of Agricultural and Food Chemistry , 47 : 1956 – 1960 .
- Maria , R.M. , Colnago , L.A. , Forato , L.A. and Bouchard , D. 2010 . Fast and simple nuclear magnetic resonance method to measure conjugated linoleic acid in beef . Journal of Agricultural and Food Chemistry , 58 : 6562 – 6564 .
- Martínez , C.E. , Vinay , J.C. , Brieva , R. , Hill , C.G.Jr. and Garcia , H.S. 2005 . Preparation of mono- and diacylglycerols by enzymatic esterification of glycerol with conjugated linoleic acid in n-hexane . Applied Biochemistry and Biotechnology , 125 : 63 – 75 .
- Mushi , D.E. , Thomassen , M.S. , Kifaro , G.C. and Eik , L.O. 2010 . Fatty acid composition of minced meat, longissimus muscle and omental fat from Small East African goats finished on different levels of concentrate supplementation . Meat Science , 86 : 337 – 342 .
- Nudda , M. , McGuire , A. , Battacone , G. and Pulina , G. 2005 . Seasonal variations in conjugated linoleic acid and vaccenic acid in milk fat of sheep and its transfer to cheese and Riccota . Journal of Dairy Science , 88 : 1311 – 1319 .
- Ou , L. , Ip , C. , Lisafeld , B. and Ip , M.M. 2007 . Conjugated linoleic acid induces apoptosis of murine mammary tumor cells via Bcl-2 loss . Biochemical and Biophysical Research Communications , 356 : 1044 – 1049 .
- Pariza , M.W. , Park , Y. and Cook , M.E. 2001 . The biologically active isomers of conjugated linoleic acid . Progress in Lipid Research , 40 : 283 – 298 .
- Park , Y. and Pariza , M.W. 2007 . Mechanisms of body fat modulation by conjugated linoleic acid (CLA) . Food Research International , 40 : 311 – 323 .
- Park , Y. , Storkson , J.M. , Albright , K.J. , Liu , W. and Pariza , M.W. 1999 . Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice . Lipids , 34 : 235 – 241 .
- Pfeuffer , M. and Schrezenemeir , J. 2000 . Bioactive substances in milk with properties decreasing risk of cardiovascular disease . British Journal of Nutrition , 84 : 155 – 159 .
- Piperova , L.S. , Teter , B. , Bruckental , I. , Sampugna , J. , Mills , S.E. , Yurawecz , M.P. and Erdman , R.A. 2000 . Mammary lipogenic enzyme activity, transfatty acids and conjugated linoleic acids are altered in lactating dairy cows fed a milk fat-depressing diet . Journal of Nutrition Science , 130 : 2568 – 2574 .
- Prandini , A. , Sigolo , S. , Tansini , G. , Brogna , N. and Piva , G. 2007 . Different level of conjugated linoleic acid (CLA) in dairy products from Italy . Journal of Food Composition and Analysis , 20 : 472 – 479 .
- Quintín , J. 2001 . Tablas de valores nutritivos para cálculos dietéticos (90 pp.) , México : Edit. Méndez Cervantes .
- Ribot , J. , Portillo , M.P. , Picó , C. , Macarulla , M.T. and Palou , A. 2007 . Effects of trans-10 cis-12 conjugated linoleic acid on the expression of uncoupling proteins in hamsters fed and atherogenic diet . British Journal of Nutrition , 97 : 1074 – 1082 .
- Ritzenthaler , K.L. , McGuire , M.K. , Falen , R. , Shultz , T.D. , Dasgupta , N. and McGuire , M.A. 2001 . Estimation of conjugated linoleic acid intake by written dietary assessment methodologies underestimates actual intake evaluated by food duplicated methodology . Journal of Nutrition , 131 : 1548 – 1554 .
- Roach , J.A.G. , Mossoba , M.M. , Yurawecz , M.P. and Kramer , J.K.G. 2002 . Chromatographic separation and identification of conjugatedlinoleic acid isomers . Analytica Chimica Acta , 465 : 207 – 226 .
- Romano , R. , Masucci , F. , Giordano , A. , Musso , S.S. , Naviglio , D. and Santini , A. 2010 . Effect of tomato by-products in the diet of Comisana sheep on composition and conjugated linoleic acid content of milk fat . International Dairy Journal , 20 : 858 – 862 .
- Ryhänen , E.L. , Tallavaara , K. , Griinari , J.M. , Jaakkola , S. , Mantere-Alhonen , S. and Shingfield , K.J. 2005 . Production of conjugated linoleic acid enriched milk and dairy products from cows receiving grass silage supplemented with a cereal-based concentrate containing rapeseed oil . International Dairy Journal , 15 : 207 – 217 .
- Salamon , R. , Varga-Visi , É , Sára , P. , Csapó-Kiss , Zg. and Csapó , J. 2006 . The influence of the season on the fatty acid composition and conjugated linoleic acid of the milk . Krmiva , 48 : 193 – 200 .
- Secchiari , P. , Mele , M. , Serra , A. , Buccioni , A. , Antongiovanni , M. , Ferruzzi , G. and Andreotti , L. 2001 . Conjugated linoleic acid (CLA) content in milk of three dairy sheep breeds . Progress in Nutrition , 3 : 37 – 42 .
- Shantha , N.C. , Crum , A.D. and Decker , E.A. 1994 . Evaluation of conjugated linoleic acid concentrations in cooked beef . Journal of Agricultural and Food Chemistry , 42 : 1757 – 1760 .
- Stockdale , C.R. , Walker , G.P. , Wales , W.J. , Dalley , D.E. , Birkett , A. , Zhiping , S. and Doyle , P.T. 2003 . Influence of pasture and concentrates in the diet of grazing dairy cows on the fatty acid composition . Journal of Dairy Research , 70 : 267 – 276 .
- Talpur , F.N. , Bangher , N.I. and Khuhawar , M.Y. 2006 . Comparison of fatty acids and cholesterol content in the milk of Pakistani cow breeds . Journal of Food Composition and Analysis , 19 : 698 – 703 .
- Turk , S.N. and Smith , S.B. 2009 . Carcass fatty acid mapping . Meat Science , 81 : 658 – 663 .
- Yurawecz , M.P. , Roach , J.A.G. , Sehat , N. , Mossoba , M.M. , Kramer , J.K.G. , Fritsche , J. and Ku , Y. 1998 . A new conjugated linoleic acid (CLA) isomer, 7-trans, 9-cis-octadecadienoic acid in cow milk, cheese, beef and human milk and adipose tissue . Lipids , 33 : 803 – 809 .