Abstract
The effect of administration of the concentration of five strains of Debaryomyces hansenii on protecting the Mexican lime from Penicillium italicum was measured. Imazalil fungicide was applied as a control treatment for comparison. The most effective treatments for reducing the severity of P. italicum on fruit at room temperature were intermediate (1 × 106 cells mL–1) and high (1 × 108 cells mL–1) doses of antagonistic yeasts. Cold-stored fruits were protected better by the highest dose of yeast. The protection of antagonistic yeasts in both temperatures was similar to imazalil.
Se evaluó el efecto de la cantidad de inóculo de cinco cepas de Debaryomyces hansenii para proteger frutos de limón mexicano contra el hongo fitopatógeno Penicillium italicum comparando su efecto protector con el fungicida imazalil. El tratamiento más efectivo para reducir la severidad de P. italicum en las frutas almacenadas a temperatura ambiente fue la cantidad intermedia (1 × 106 células mL–1) y alta (1 × 108 células mL–1) de inóculo de las levaduras antagónicas. Las frutas almacenadas bajo frío fueron protegidas con la dosis más alta de levadura. La protección ejercida por las levaduras antagónicas en ambas temperaturas fue similar a la cuantificada con el fungicida imazalil.
Introduction
Mexico is by far the biggest exporter of fresh limes, with more than 90,000 acres producing 1 million 500,000 tons of Mexican lime (Citrus aurantiifolia Christm. Swingle). Of the harvested fruit, approximately 45% is for fresh consumption and the remaining 55% is used in industry. The main producing states are Colima, Michoacán, Guerrero and Oaxaca. The characteristic aroma, color and acid content of the juice make Mexican lime an important commodity in national and international markets.
Production technology activities involve grafting, fertilizing and insecticide treatment during cultivation and application of fungicides during post-harvest handling and storage of fruit. Harvesting, packaging and transport can damage the fruit and affect storage quality and shelf life. Surface wounds allow infection, mainly from fungi (Prusky & Lichter, Citation2008). Penicillium italicum Wehmer (blue mold) is one of the most common post-harvest pathogens of citrus, responsible for 40–50% decay of the harvest (Ochoa, Hernández-Montiel, Latisnere-Barragán, León de La Luz, & Larralde-Corona, Citation2007). Chemical control of this fungus is based on applications of imazalil (1-[2-(2,4-dichlorophenyl)-2-(2-propeniloxi) ethyl)]-1H-imidazole) and thiabendazole (2-(4-thiazolyl)-1H-benzimidazole) (Hao et al., Citation2010; Medina et al., Citation2001). Use of this type of synthetic fungicides may lead to resistant strains (Kanetis, Förster, Jones, Borkovich, & Adaskaveg, Citation2008) as well as environmental pollution and public health problems. The Mexican lime sold in local markets usually has no fungicide treatment or wax to protect against pathogens. At the national and international level, limes are treated with these agents and stored at low temperatures (5–15°C) with high relative humidity (>90%) to maintain fruit quality prior to and during transport (Smilanick, Sorenson, Mansour, Aieyabei, & Plaza, Citation2003). Unfortunately, storing fruits under these conditions is conducive to growth of fungi (Smilanick & Mansour, Citation2008).
The search for alternatives to control plant pathogens has become a priority, since the market trend is to demand high quality nutritional foods with little or no chemical residues (Fan, Tian, Li, Xu, & Wang, Citation2000; Karabulut & Baykal, Citation2003). In recent years, biological control has been considered a viable option for fighting fungal pathogens of fruit, especially by using the antagonistic activity of bacteria and yeast (Abraham, Laing, & Bower, Citation2010; Liu et al., Citation2010). Application of bacteria, such as Pseudomonas putida and Pseudomona fluorescens, has declined as resistance to antibiotics has increased (Janisiewicz & Buyer, Citation2010). Also, application of these bacteria appears to enhance the growth of yeast (Droby, Wisniewski, Macarisin, & Wilson, Citation2009; Mari & Guizzardi, Citation1998; Zong, Liu, Li, Qin, & Tian, Citation2010), which possesses several advantages over other biological antagonists. This includes the following: (a) they can colonize and survive for extended periods on the surface of the fruit and (b) they limit the growth of fungi through different modes of action (Lahlali, Massart, Serrhini, & Jijakli, Citation2008), including competition for space (Leibinger, Breuker, Hahn, & Mendgen, Citation1997) and nutrients (Droby & Chalutz, Citation1994), killer toxins (Dubash, Gupta, Prakash, & Bairy, Citation2010), hydrolytic enzymes (Friel, Gomez Pessoa, Vandenbol, & Jijakli, Citation2007) and mycoparasitism, and induction of resistance in the fruit (Macarisin, Droby, Bauchan, & Wisniewski, Citation2010).
Among antagonistic yeast, Debaryomyces hansenii is known for its effectiveness in controlling post-harvest fungal pathogens in the fruits of lemon (Citrus limon), grapefruit (Citrus paradisi) and sweet orange (Citrus sinensis) (Chalutz & Wilson, Citation1990; Droby et al. Citation1999; Taqarort et al. Citation2008; Wilson & Chalutz, Citation1989). In Mexican lime, a concentration of 106 cells mL–1 of strains of D. hansenii decreased the incidence rate of decay to less than 50% P. italicum (Hernández-Montiel et al., Citation2010). This protection by yeast can be enhanced if the concentration of the antagonist inoculum is high. For example, Droby et al. (Citation1997) reported a decrease in disease caused by Penicillium digitatum on grapefruit by increasing the inoculum of the yeast Pichia guilliermondii. Bar-Shimon et al. (Citation2004) observed the same effect of increased concentration of inoculum to inhibit the yeast fungus Candida oleophila. Zhang, Zheng, and Xi (Citation2005) reported a reduction in disease caused by P. italicum with higher levels of Cryptococcus laurentii on sweet oranges. Zheng, Zhang, and Sun (Citation2005) found lower incidence of P. digitatum in oranges with increasing doses of Rhodotorula glutinis and Liu et al. (Citation2010) found that higher levels of C. laurentii and Rhodosporidium paludigenum inoculum reduced infection by Geotrichum citri-aurantii on mandarin (C. reticulata).
In Mexican lime, no record of dose assessment of yeasts to control post-harvest fungal pathogens has been carried out. The objective of this work was evaluate and compare the effect of different concentrations of several strains of D. hansenii and fungicide imazalil on the incidence and severity of P. italicum in Mexican limes stored under different conditions of temperature and humidity.
Materials and methods
Fruits
Limes were harvested in the municipality of Llera in the State of Tamaulipas, washed with water, disinfected with 1% sodium hypochlorite for 2 min, and air-dried for 20 min (Yao, Tian, & Wang, Citation2004).
Pathogen
P. italicum was isolated from decayed citrus fruit (Hernández-Montiel & Ochoa, Citation2007). The fungus was grown in Petri dishes with potato-dextrose agar (PDA, Difco Laboratories, Detroit, MI, USA) and incubated for seven days at 25°C. Spores were suspended in sterile, distilled water over 0.05% (v/v) Tween 80 (P4780, Sigma-Aldrich, St. Louis, MO, USA), and their concentration was adjusted to 104 spores mL–1.
Antagonists
Five strains of D. hansenii (DhhBCS03, DhhBCS05, DhhBCS06, LL01 and LL02) from the collection of yeasts in the plant pathology laboratory of the Centro de Investigaciones Biológicas del Noroeste and the control strain P. guilliermondii (NRRL-Y-50426) from the industrial biotechnology laboratory of the Centro de Biotecnología Genómica-Instituto Politecnico Nacional were used. Yeasts were grown in liquid yeast extract-peptone-dextrose (LPD) for 24 h at 25°C and were harvested by centrifugation, suspending the cells in sterile, distilled water to adjust its concentration to 104, 106 and 108 cells mL–1 using a hemocytometer (Sigma)).
Effect of the fungicide imazalil (IMZ) (1-[2-(2,4-dichlorophenyl)-2-(2-propeniloxi) ethyl]-1H-imidazole) on P. italicum
The IMZ CL50 (lethal concentration) that inhibited the diameter of mycelial growth in P. italicum by 50% was determined on PDA medium (Kinay, Mansour, Gabler, Margosan, & Smilanick, Citation2007) and it was tested with IMZ at doses of 50, 100, 150, 200, 250, 300, 350 and 400 ppm. Petri dishes with PDA without fungicide were included as controls. Each dose was applied to six Petri dishes and was incubated at 25 ± 2°C with continuous fluorescent light for five days. The experiment was repeated twice. The percentage of inhibition of mycelial growth of P. italicum was estimated using the formula by Abbott (Citation1925). Probit analysis was performed on the data to determine the CL50 using a logistic regression curve with computer software (Statistica 8.0, Statsoft, Tulsa, OK).
Biocontrol assessment
Mexican lime fruit was immersed once for 5 min in each the yeast suspension and dried at room temperature for 20 min. Each lime was damaged by cutting a 2 mm wide by 1 mm deep incision with a sterile scalpel. One of the tested fungal suspensions (20 ml) was applied to the wound (Long, Zheng, & Deng, Citation2005; Hernandez-Montiel et al. 2010). The control treatment consisted of wounded fruit sprayed with the IMZ fungicide solution at a concentration of 250 ppm and inoculated with the same dose of the pathogen. Twelve fruits were used per treatment and the experiment was repeated twice.
Storage
One batch of 12 fruits were stored for 21 days under ambient conditions (25°C, 80% RH); a second batch of 12 fruits were stored in a climate chamber (13°C, 90% RH) (KBF 720, Binder, Tuttlingen, Germany). After incubation period, the size lesion and percentage of disease incidence was determined (Taqarort et al. Citation2008).
Micrographs from scanning electron microscope
To observe the arrangement of the hyphae, conidia of fungal and yeast cells were sampled from wounds inoculated with both organisms, fixed by immersion in 2.5% glutaraldehyde in phosphate buffer at pH 7 for 24 h. The preparation methodology of Usall, Teixido, Torres, Ochoa, and Viñas (Citation2001) was used and the specimens were observed under a scanning electron microscope (Hitachi S-3000N, San Jose, CA, USA).
Statistical analysis
We used one-way ANOVA with significance level set at P < 0.05. To test statistically significant differences, we used post-hoc analysis and the Fisher's test of least significant difference for comparing treatment group means after the ANOVA null hypothesis of equal means has been rejected using the ANOVA F-test. All statistical analyses were done using computer software (Statistica 8.0, Statsoft, Tulsa, OK).
Results
Lethal concentration (CL50)
The fungus P. italicum was sensitive to IMZ fungicide at doses starting at 200 ppm. At 350 ppm IMZ, fungal inhibition was 100%. The median lethal concentration (CL50) of IMZ was 233 ppm.
Control of P. italicum with antagonistic yeasts in limes stored at 25°C and 80% RH
Incidence of the disease
Different levels of protection were found with the different strains of D. hansenii (). Strains DhhBCS03, DhhBCS05, DhhBCS06, LL01, and LL02 at the three application concentrations (104, 106 and 108 cells mL–1) reduced the incidence of disease compared with the fruits used as a negative control (). Fruit inoculated with yeast at concentrations of 104 cells mL–1 did exhibit fruit decay; however, those treated with the concentration 106 cells L–1 with strains DhhBCS06, LL01, and LL02 and at the concentration of 108 cells mL–1 did not exhibit decay. All fruits treated with IMZ were free of decay.
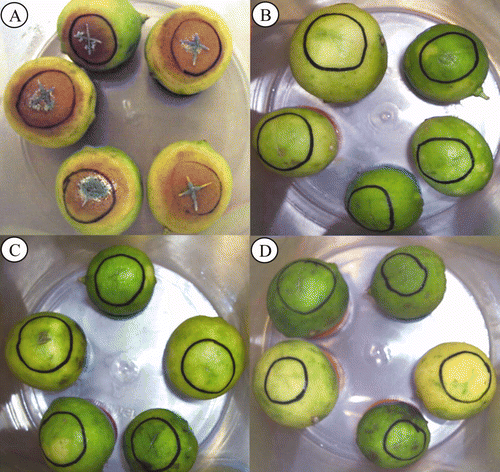
Figure 1. Fruit rot caused by Penicillium italicum (Pi) on Mexican lime and inoculated with different strains of Debaryomyces hansenii. (A) Inoculated only with Pi. (B) Pi and DhhBCS05. (C) Pi and DhhBCS06. (D) Pi and LL02. The fungal pathogen was adjusted to 104 spores mL–1 and the yeasts were adjusted to 108 cells mL–1. Fruit were stored for three weeks at 25°C and 80% RH.
Figura 1. Pudrición ocasionada por Penicillium italicum (Pi) sobre limón mexicano inoculado con diferentes cepas de Debaryomyces hansenii. (A) frutos inoculados con el fitopatógeno. (B) Pi más DhhBCS05. (C) Pi más DhhBCS06. (D) Pi más LL02. La concentración del hongo fue ajustada a 104 esporas mL–1 y de las levaduras a 108 células mL–1. Los frutos fueron almacenados durante tres semanas a 25°C y 80% de HR.
Size of lesions
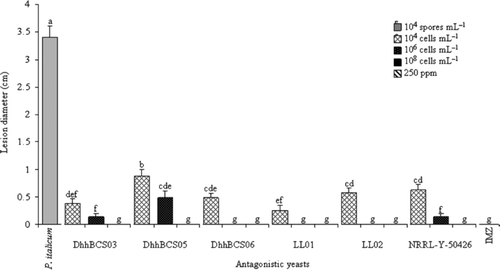
Damage from P. italicum was minimized or not detected in fruits co-inoculated with strains D. hansenii (). Diameters of lesions (visible infection area) were not quantified because there were no lesions on fruits treated with the intermediate and high concentrations of DhhBCS06, LL01, or LL02, as well as fruits treated with IMZ or the high concentration of P. guilliermondii NRRL-Y-50426. Fruits with the largest lesions were only inoculated with the pathogen; the average diameter of the lesion was 3.4 cm.
Figure 2. Incidence of Penicillium italicum in Mexican lime fruit inoculated with three concentrations of antagonistic yeasts. Strains of Debaryomyces hansenii used were: DhhBCS03, DhhBCS05, DhhBCS06, LL01, LL02, and P. guilliermondii NRRL-Y-50426. The fungicide imazalil (IMZ) was applied at 250 ppm. Fruit were stored at 25°C and 80% RH for 21 days. Columns with the same letter are not significantly different (LSD, P < 0.05).
Figura 2. Incidencia de la pudrición ocasionada por Penicillium italicum en limón mexicano inoculado con tres dosis de levaduras antagónicas. Las cepas utilizadas de Debaryomyces hansenii fueron DhhBCS03, DhhBCS05, DhhBCS06, LL01, LL02, y de P. guilliermondii NRRL-Y-50426. El fungicida imazalil (IMZ) fue aplicado a 250 ppm. Los frutos fueron almacenados a 25°C y 80% de HR durante 21 días. Columnas con la misma letra no son significativamente diferentes (LSD, P < 0,05).
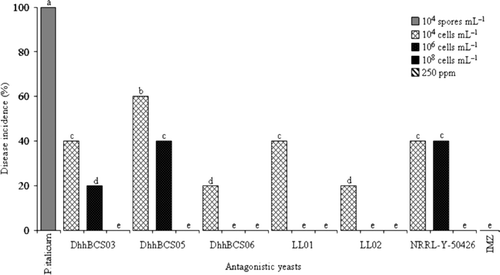
Figure 3. Lesions caused by Penicillium italicum in Mexican lime fruit inoculated with three concentrations of antagonistic yeasts. Strains of D. hansenii used were: DhhBCS03, DhhBCS05, DhhBCS06, LL01, LL02, and P. guilliermondii NRRL-Y-50426. The fungicide imazalil (IMZ) was applied at 250 ppm. Fruit were stored at 25°C and 80% RH for 21 days. Columns with the same letter are not significantly different (LSD, P < 0.05).
Figura 3. Tamaño de lesión ocasionado por Penicillium italicum en limón mexicano inoculado con tres dosis de levaduras antagónicas. Las cepas utilizadas de Debarymoyces hansenii fueron DhhBCS03, DhhBCS05, DhhBCS06, LL01, LL02, y de P. guilliermondii NRRL-Y-50426. El fungicida imazalil (IMZ) fue aplicado a 250 ppm. Los frutos fueron almacenados a 25°C y 80% de HR durante 21 días. Columnas con la misma letra no son significativamente diferentes (LSD, P < 0,05).
Control of P. italicum with antagonistic yeasts in limes stored at 13°C and 90% RH
Incidence of the disease
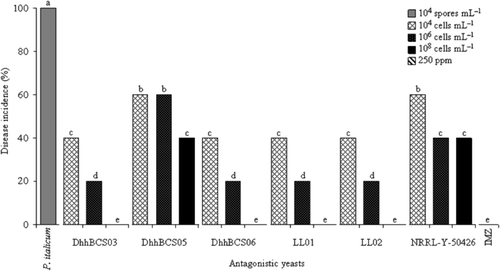
The disease was not detected in fruits inoculated with strains of DhhBCS03, DhhBCS06, LL01, LL02 and IMZ at a concentration of 108 cells mL–1 (). In the controls, incidence of decay from P. italicum was 100%.
Figure 4. Incidence of Penicillium italicum in Mexican lime fruit inoculated with three concentrations of antagonistic yeasts. Strains of Debaryomyces hansenii used were: DhhBCS03, DhhBCS05, DhhBCS06, LL01, LL02, and P. guilliermondii NRRL-Y-50426. The fungicide imazalil (IMZ) was applied at 250 ppm. Fruit were stored at 13°C and 90% RH for 21 days. Columns with the same letter are not significantly different (LSD, P < 0.05).
Figura 4. Incidencia de la pudrición ocasionada por Penicillium italicum en limón mexicano inoculado con tres dosis de levaduras antagonistas. Las cepas utilizadas de Debaryomyces hansenii fueron: DhhBCS03, DhhBCS05, DhhBCS06, LL01, LL02, y P. guilliermondii NRRL-Y-50426. El fungicida imazalil (IMZ) fue aplicado a 250 ppm. Los frutos fueron almacenados a 13°C y 90% de HR durante 21 días. Columnas con la misma letra no son significativamente diferentes (LSD, P < 0,05).
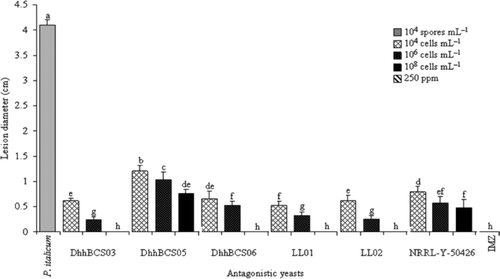
Size of lesions
Fruits treated with 108 cells mL–1 of DhhBCS03, DhhBCS06, LL01, LL02, or IMZ had no visible decay (). The strain DhhBCS05 failed to control P. italicum in fruits inoculated at three concentrations. The average size of injury to fruits treated only with the plant pathogen was 4.1 cm.
Figure 5. Lesions caused by Penicillium italicum in Mexican lime fruit inoculated with three concentrations of antagonistic yeasts. Strains of D. hansenii used were: DhhBCS03, DhhBCS05, DhhBCS06, LL01, LL02, and P. guilliermondii NRRL-Y-50426. The fungicide imazalil (IMZ) was applied at 250 ppm. Fruit were stored at 13°C and 90% RH for 21 days. Columns with the same letter are not significantly different (LSD, P < 0.05).
Figura 5. Tamaño de lesión ocasionado por Penicillium italicum en limón mexicano inoculado con tres dosis de levaduras antagónicas. Las cepas utilizadas de Debarymoyces hansenii fueron DhhBCS03, DhhBCS05, DhhBCS06, LL01, LL02, y de P. guilliermondii NRRL-Y-50426. El fungicida imazalil (IMZ) fue aplicado a 250 ppm. Los frutos fueron almacenados a 13°C y 90% de HR durante 21 días. Columnas con la misma letra no son significativamente diferentes (LSD, P < 0,05).
Microphotographs of antagonistic yeast and P. italicum
In fruits where the disease had not progressed, there were many yeast cells and a few germinated spores of P. italicum, and generally poor development of its mycelium (A and B). Infected fruits had few yeast cells (C); in these cases, spore germination and penetration of mycelium of P. italicum into the fruits were extensive (D).
Figure 6. Microphotographs of the interaction between strains of Debaryomyces hansenii and Penicillium italicum in wounds of Mexican lime. (A) Fruit wound inoculated with the strain LL2 at a concentration of 108 cells mL–1 after 21 days in storage. (B) Parasitism of LL02 yeast and mycelium of P. italicum (Pi). (C) Interaction between strain DhhBCS05 and Penicillium italicum (Pi) in inoculated wounds of Mexican lime at a concentration of 108 cells mL–1 and 104 spores mL–1, respectively. (D) Spore E and mycelium M of Penicillium italicum penetrating wounds on the surface of the lime. Samples were taken from fruit stored for 21 days at 13°C and 90% RH.
Figura 6. Micrografías de la interacción entre cepas de Debaryomyces hansenii y Penicillium italicum en heridas de limón mexicano. (A) Herida del fruto inoculado con la cepa LL02 a una concentración de 108 células mL–1 después de 21 días de almacenamiento. (B) Parasitismo de la levadura LL02 en el micelio de P. italicum (Pi). (C) Interacción entre la cepa DhhBCS05 y Penicillium italicum (Pi) dentro de la herida del limón mexicano inoculada a una concentración de 108 células mL–1 y 104 esporas mL–1 respectivamente. (D) Espora E y micelio M de Penicillium italicum penetrando en las heridas del limón mexicano. Las muestras fueron tomadas de frutos almacenados por 21 días a 13°C y 90% de HR.

Discussion
In vitro inhibition of growth of P. italicum occurs when IMZ is applied in doses greater than 200 ppm; however, there is a relationship between dose levels of IMZ and the zone of inhibition. Resistance of the fungus at in vitro doses between 50 and 150 ppm of IMZ is probably related to the low accumulation of the fungicide on the mycelium of P. italicum, a level that is not sufficient to inhibit biosynthesis of sterols in the fungal membrane and cause cell damage (Zhu, Xie, & Li, Citation2006). Resistance to low doses of IMZ may be related to the small concentration of P. italicum in the citrus-packing plants compared with other fungi, such as P. digitatum (Bus, Bongers, & Risse, Citation1991; Holmes & Eckert, Citation1995), which reduces the contact time with fungicides, hence preventing it from generating strains resistant to this chemical (Boubaker, Saadi, Boudyach, & Benaoumar, Citation2009). Other factors that may affect the resistance of this fungus to IMZ, include package design and various programs for the application of fungicides used in packing house (Holmes & Eckert, Citation1999). Early detection of P. italicum in the citrus packing industry is important because it can lead to losses > ;40% of the harvested fruits, especially in production areas in the tropics and subtropics (Kanetis, Förster, & Adaskaveg, Citation2008). Therefore, it is important to determine the sensitivity levels of the populations of P. italicum to fungicides to determine the degree of resistance. With this information, post-harvest management strategies can be planned to minimize infestations of strains in citrus packing houses that are resistant to fungicides. Additionally, the type of fungicide and dose of each product will depend on national and export regulations.
In our trials, we found that under ambient (25°C, 80% RH) and controlled (13°C, 90% RH) storage, some strains of D. hansenii protects all the limes inoculated with P. italicum. The efficiency of the yeast antagonist is similar to other reports. Chalutz and Wilson (Citation1990) could control P. italicum and P. digitatum (green mold) on lemon (Citrus limon L.) and grapefruit (C. paradisi M.). Droby et al. (Citation1999) could considerably reduce green mold decay on grapefruit fruits. Taqarort et al. (Citation2008) could reduce by 80% decay caused by P. digitatum in oranges (C. sinensis O.).
The differences in the level of protection against decay provided by D. hansenii and P. guilliermondii on Mexican lime could be explained by the ability of the yeasts to assimilate carbon sources from the wounds of the fruit. Torres et al. (Citation2006) report that variations in the adaptability of yeasts to particular fruit are an important factor in reducing colonization of fungal pathogens, while concentration of yeast inoculum perhaps influences competition for space and nutrients, thereby reducing energy sources for germination of spores and mycelium development and reducing infection. Diğrak and Özçelik (Citation2001), Miedes and Lorences (Citation2006), and Qin, Tian, Chan, and Li (Citation2007) remark that P. italicum requires carbon sources for spore germination and production of enzymes, such as β-1,3- and β-1,6-glucanase, which are essential in the process of infecting fruits. There are other mechanisms in yeast to limit growth of P. italicum. Hernández-Montiel et al. (Citation2010) found that some strains of D. hansenii produced hydrolytic enzymes, such as β-1,3-glucanase, chitinase, protease and killer toxins, which have already been reported as the main metabolites produced by yeasts (Ganiger, Bhat, Chettri, & Kuruvinashetti, Citation2008; Santos, Mauro, Bravo, & Marquina, Citation2009) for disintegration and collapse of hyphae of fungal pathogens (Oelofse, Dubery, & Berger, Citation2008).
D. hansenii protects fruit, similar to that conferred by the fungicide imazalil. Applications of this yeast can be an alternative to control populations of P. italicum that are resistant to IMZ or other chemicals (Cabañas, Abarca, Bragulata, & Cabañes, Citation2009). The protection provided by antagonistic yeasts on Mexican lime stored at room temperature and under refrigerated conditions demonstrated the ability of D. hansenii to grow and adapt in both environments. This is very important for selecting an antagonist effective under a variety of climatic conditions (Bouzerda, Boubaker, Boudyach, Akhayat, & Bin-Aoumar, Citation2003). Determining the effective concentration of yeast is important to implement commercial use in agriculture, since the cost of production is one of the main factors that determine the profitability of the use and application of antagonistic microorganisms in any agricultural system (Kinay & Yildiz, Citation2008). Our results indicate that application of yeast after harvest of Mexican lime can be an ecologically and economically viable option in the growing demand for agricultural products raised organically or in cases where only a few inorganic substances are used to control pests (Castoria, Caputo, de Curtis, & de Cicco, 2003).
Conclusions
Yeasts of D. hansenii DhhBCS03, DhhBCS05, DhhBCS06, LL1 and LL2 protected Mexican lime (C. aurantiifolia) that were stored under ambient or cold conditions to the fungus P. italicum. Protection was similar to the application of the fungicide imazalil. Consequently, some D. hansenii strains are attractive alternatives to traditional post-harvest treatment of Mexican lime with agrochemicals to control plant pathogens.
Acknowledgments
We thank Daniel Téliz-Ortiz for comments and Miguel Cordoba for translation; Maria G. López Aburto and Isabel Rodríguez-Luna provided technical assistance during the trials. Ira Fogel of CIBNOR provided final editorial services. This research was funded by the Consejo Nacional de Ciencia y Tecnología (CONACYT grant 144710). L.G.H.M. was a recipient of a CONACYT doctoral fellowship.
References
- Abbott , W.S. 1925 . A method of computing the effectiveness of an insecticide . Journal of Economic Entomology , 18 : 265 – 267 .
- Abraham , A.O. , Laing , M.D. and Bower , J.P. 2010 . Isolation and in vivo screening of yeast and Bacillus antagonists for the control of Penicillium digitatum of citrus fruit . Biological Control , 53 : 32 – 38 .
- Bar-Shimon , M. , Yehuda , H. , Cohen , L. , Weiss , B. , Kobeshnikov , A. , Daus , A. and Droby , S. … . 2004 . Characterization of extracellular lytic enzymes produced by the yeast biocontrol agent . Candida oleophila. Current Genetics , 45 : 140 – 148 .
- Boubaker , H. , Saadi , B. , Boudyach , E.H. and Benaoumar , A.A. 2009 . Sensitivity of Penicillium digitatum and P. italicum to Imazalil and Thiabendazole in Morocco . Plant Pathology Journal , 8 : 152 – 158 .
- Bouzerda , L. , Boubaker , H. , Boudyach , E.H. , Akhayat , O. and Bin-Aoumar , A.A. 2003 . Selection of antagonistic yeasts to green mold disease of citrus in Morocco . Journal of Food Agriculture and Environment , 1 : 215 – 218 .
- Bus , V.G. , Bongers , A.J. and Risse , L.A. 1991 . Occurrence of Penicillium digitatum and P. italicum resistant to benomyl, thiabendazole, and imazalil on citrus fruit from different geographic origins . Plant Disease , 75 : 1098 – 1100 .
- Cabañas , R. , Abarca , M.L. , Bragulata , M.R. and Cabañes , F.J. 2009 . In vitro activity of imazalil against Penicillium expansum: Comparison of the CLSI M38-A broth microdilution method with traditional techniques . International Journal of Food Microbiology , 129 : 26 – 29 .
- Castoria , R. , Caputo , L. , de Curtis , F. and de Cicco , V. 2003 . Resistance to oxidative stress of postharvest biocontrol yeasts: A possible new mechanism of action . Phytopathology , 93 : 564 – 572 .
- Chalutz , E. and Wilson , C.L. 1990 . Postharvest biocontrol of green and blue mold and sour rot of citrus fruit by Debaryomyces hansenii . Plant Disease , 74 : 134 – 137 .
- Diğrak , M. and Özçelik , S. 2001 . Determination of some fungal metabolite as influenced by temperature, time, pH and sugars by bioassay method . Turkisk Journal of Biology , 25 : 197 – 203 .
- Droby , S. and Chalutz , E. 1994 . “ Mode of action of biocontrol agents for postharvest disease ” . In Biological control of postharvest disease of fruits and vegetables-Theory and practice , Edited by: Wilson , C.L. and Wisniewski , M.E. 63 – 75 . Boca Raton , FL : CRC Press .
- Droby , S. , Lischinski , S. , Cohen , L. , Weiss , B. , Daus , A. , Chand-Goyal , T. and Manulis , S. … . 1999 . Characterization of an epiphytic yeast population of grapefruit capable of suppression of green mold decay caused by Penicillum digitatum . Biological Control , 16 : 27 – 34 .
- Droby , S. , Wisniewski , M. , Macarisin , D. and Wilson , C. 2009 . Twenty years of postharvest biocontrol research: Is it time for a new paradigm? . Postharvest Biology and Technology , 52 : 137 – 145 .
- Droby , S. , Wisniewski , M.E. , Cohen , L. , Weiss , B. , Touitou , D. , Eilam , Y. and Chalutz , E. 1997 . Influence of CaCl2 on Penicillium digitatum, grapefruit peel tissue, and biocontrol activity of Pichia guilliermondii . Phytopathology , 87 : 310 – 315 .
- Dubash , T. , Gupta , S. , Prakash , P.Y. and Bairy , I. 2010 . Isolation of yeasts from various food products and detection of killer toxin activity in vitro . Journal of Scientific Research , 2 : 407 – 411 .
- Fan , Q. , Tian , S.P. , Li , Y. , Xu , Y. and Wang , Y. 2000 . Biological control of postharvest brown rot in peach and nectarine fruits by Bacillus subtilis (B-912) . Acta Botanica Sinica , 42 : 1137 – 1143 .
- Friel , D. , Gomez Pessoa , N.M. , Vandenbol , M. and Jijakli , M.H. 2007 . Separate and combined disruptions of two exo-β-1,3-glucanase genes decrease the efficiency of Pichia anomala (Strain K) biocontrol against Botrytis cinerea on apple . Molecular Plant Microbe Interactions , 20 : 371 – 379 .
- Ganiger , M.C. , Bhat , S. , Chettri , P. and Kuruvinashetti , M.S. 2008 . Cloning and Expression of Endoglucanase genes from Trichoderma species in Saccharomyces cerevisiae . Journal of Applied Sciences Research , 11 : 1546 – 1556 .
- Hao , W. , Zhong , G. , Hu , M. , Luo , J. , Weng , Q. and Rizwan-ul-Haq , M. 2010 . Control of citrus postharvest green and blue mold and sour rot by tea saponin combined with imazalil and prochloraz . Postharvest Biology and Technology , 56 : 39 – 43 .
- Hernández-Montiel , L.G. , Larralde-Corona , C.P. , Vero , S. , López-Aburto , M.G. , Ochoa , J.L. and Ascencio-Valle , F. 2010 . Characterization of yeast Debaryomyces hansenii for the biological control of blue mold decay of Mexican lemon . CyTA-Journal of Food , 8 : 49 – 56 .
- Hernández-Montiel , L.G. and Ochoa , J.L. 2007 . Fruit Rot Caused by Penicillium italicum on Lemon (Citrus aurantifolia) in Colima, Mexico . Plant Disease , 91 : 767
- Holmes , G.J. and Eckert , J.W. 1995 . Relative fitness of imazalil resistant and sensitive biotypes of Penicillium digitatum . Plant Disease , 79 : 1068 – 1073 .
- Holmes , G.J. and Eckert , J.W. 1999 . Sensitivity of Penicillium digitatum and P. italicum to postharvest citrus fungicides in California . Phytopathology , 89 : 716 – 721 .
- Janisiewicz , W.J. and Buyer , J.S. 2010 . Culturable bacterial microflora associated with nectarine fruit and their potential for control of brown rot . Canadian Journal of Microbiology , 56 : 480 – 486 .
- Kanetis , L. , Förster , H. and Adaskaveg , J.E. 2008 . Optimizing efficacy of new postharvest fungicides and evaluation of sanitizing agents for managing citrus green mold . Plant Disease , 92 : 261 – 269 .
- Kanetis , L. , Förster , H. , Jones , C.A. , Borkovich , K.A. and Adaskaveg , J.E. 2008 . Characterization of genetic and biochemical mechanisms of fludioxonil and pyrimethanil resistance in field isolates of Penicillium digitatum . Phytopathology , 98 : 205 – 214 .
- Karabulut , O.A. and Baykal , N. 2003 . Biological control of postharvest diseases of peaches and nectarines by yeast . Journal of Phytopathology , 151 : 130 – 134 .
- Kinay , P. , Mansour , M.F. , Gabler , F.M. , Margosan , D.A. and Smilanick , J.L. 2007 . Characterization of fungicide-resistant isolates of Penicillium digitatum collected in California . Crop Protection , 26 : 647 – 656 .
- Kinay , P. and Yildiz , M. 2008 . The shelf life and effectiveness of granular formulations of Metschnikowia pulcherrima and Pichia guilliermondii yeast isolates that control postharvest decay of citrus fruit . Biological Control , 45 : 433 – 440 .
- Lahlali , R. , Massart , S. , Serrhini , M.N. and Jijakli , M.H. 2008 . A box-behnken design for predicting the combined effects of relative humidity and temperature on antagonistic yeast population density at the surface of apples . International Journal of Food Microbiology , 122 : 100 – 108 .
- Leibinger , W. , Breuker , B. , Hahn , M. and Mendgen , K. 1997 . Control of postharvest pathogens and colonization of the apple surface by antagonistic microorganisms in the field . Phytopathology , 87 : 1103 – 1110 .
- Liu , X. , Fang , W. , Liu , L. , Yu , T. , Lou , B. and Zheng , X. 2010 . Biological control of postharvest sour rot of citrus by two antagonistic yeasts . Letters in Applied Microbiology , 51 : 30 – 35 .
- Long , C. , Zheng , W. and Deng , B. 2005 . Biological control of Penicillium italicum of citrus and Botrytis cinerea of grape by strains 34-9 of Kloeckera apiculata . European Food Research and Technology , 221 : 197 – 201 .
- Macarisin , D. , Droby , S. , Bauchan , G. and Wisniewski , M. 2010 . Superoxide anion and hydrogen peroxide in the yeast antagonist–fruit interaction: A new role for reactive oxygen species in postharvest biocontrol? . Postharvest Biology and Technology , 58 : 194 – 202 .
- Mari , M. and Guizzardi , M. 1998 . The postharvest phase: Emerging technologies for the control of fungal diseases . Phytoparasitica , 26 : 59 – 66 .
- Medina , U.V.M. , Robles , M.M. , Becerra , S.R. , Orozco , J.R. , Orozco , M.S. , Garza , J.G.L. and Felix , F.A. … . 2001 . El cultivo del limón mexicano. Libro técnico No. 1 , Mexico City , , Mexico : Instituto Nacional de Investigaciones Forestales y Agropecuarias (INIFAP) .
- Miedes , E. and Lorences , E.P. 2006 . Changes in cell wall pectin and pectinase activity in apple and tomato fruits during Penicillium expansum infection . Journal of the Science of Food and Agriculture , 86 : 1359 – 1364 .
- Ochoa , J.L. , Hernández-Montiel , L.G. , Latisnere-Barragán , H. , Leónde La Luz , J.L. and Larralde-Corona , C.P. 2007 . Aislamiento e identificación de hongos patógenos de naranja Citrus sinensis L. Osbeck cultivada en Baja California Sur, Mexico . Ciencia y Tecnología Alimentaria , 5 : 352 – 359 .
- Oelofse , D. , Dubery , I. and Berger , D.K. 2008 . Exo-β-1,3-Glucanase from yeast inhibits Colletotrichum lupini and Botrytis cinerea spore germination . Journal of Phytopathology , 157 : 1 – 6 .
- Prusky , D. and Lichter , A. 2008 . Mechanisms modulating fungal attack in post-harvest pathogen interactions and their control . European Journal of Plant Pathology , 121 : 281 – 289 .
- Qin , G. , Tian , S. , Chan , Z. and Li , B. 2007 . Crucial role of antioxidant proteins and hydrolytic enzymes in pathogenicity of Penicillium expansum . Molecular and Cellular Proteomics , 6 : 425 – 438 .
- Santos , A. , Mauro , M.S. , Bravo , E. and Marquina , D. 2009 . PMKT2, a new killer toxin from Pichia membranifaciens, and its promising biotechnological properties for control of the spoilage yeast Brettanomyces bruxellensis . Microbiology , 155 : 624 – 634 .
- Smilanick , J.L. and Mansour , M.F. 2008 . Influence of temperature and humidity on survival of Penicillium digitatum and Geotrichum citri-aurantii . Plant Disease , 91 : 990 – 996 .
- Smilanick , J. , Sorenson , D. , Mansour , M. , Aieyabei , J. and Plaza , P. 2003 . Impact of a brief post-harvest hot water drench treatment on decay, fruit appearance, and microbial populations of California lemons and oranges . HortTechnology , 13 : 333 – 338 .
- Taqarort , N. , Echairi , A. , Chaussod , R. , Nouaim , R. , Boubaker , H. , Benaoumar , A.A. and Boudyach , E. 2008 . Screening and identification of epiphytic yeasts with potential for biological control of green mold of citrus fruits . World Journal of Microbiology and Biotechnology , 24 : 3031 – 3038 .
- Torres , R. , Teixidó , N. , Viñas , I. , Mari , M. , Casalini , L. , Giraud , M. and Usall , J. 2006 . Efficacy of Candida sake CPA-1 formulation for controlling Penicillium expansum decay on pome fruit from different Mediterranean Regions . Journal of Food Protection , 69 : 2703 – 2711 .
- Usall , J. , Teixido , N. , Torres , R. , Ochoa , X. and Viñas , I. 2001 . Pilot test of Candida sake (CPA) applications to control postharvest blue mold on apple fruit . Postharvest Biology and Technology , 21 : 147 – 156 .
- Wilson , C.L. and Chalutz , E. 1989 . Postharvest biological control of Penicillium rots of citrus with antagonistic yeasts and bacteria . Scientia Horticulturae , 40 : 105 – 112 .
- Yao , H. , Tian , S. and Wang , Y. 2004 . Sodium bicarbonate enhances biocontrol efficacy of yeast on fungal spoilage of pears . International Journal of Food Microbiology , 93 : 297 – 304 .
- Zhang , H. , Zheng , X. and Xi , Y. 2005 . Biological control of postharvest blue mold of oranges by Cryptococcus laurentii (Kufferath) Skinner . BioControl , 50 : 331 – 342 .
- Zheng , X.D. , Zhang , H.Y. and Sun , P. 2005 . Biological control of postharvest green mold decay of oranges by Rhodotorula glutinis . European Food Research and Technology , 220 : 353 – 357 .
- Zhu , J.W. , Xie , Q.Y. and Li , H.Y. 2006 . Occurrence of imazalil-resistant biotype of Penicillium digitatum of postharvest decay of arbutus berries . Botanical Bulletin Academic Sinica , 45 : 55 – 60 .
- Zong , Y. , Liu , J. , Li , B. , Qin , G. and Tian , S. 2010 . Effects of yeast antagonists in combination with hot water treatment on postharvest diseases of tomato fruit . Biological Control , 54 : 316 – 321 .