Abstract
The levels of thiamine were compared between different types of milk, fermented milk products and rice milk. The analysed products were mainly from the markets, so vitamin analysis may be a good parameter for the quality of commercial food. Thiamine concentrations were determined by high performance liquid chromatography (HPLC) using a reversed-phase C-18 column with fluorescence detector. The average thiamine contents were 0.46 µg/mL for ultra high temperature (UHT), 0.39 µg/mL for pasteurized and 0.38 µg/mL for chocolate milk. Unprocessed cow milk had average thiamine concentration of 0.47 µg/mL. The highest thiamine content (11.95 µg/mL) was found in rice milk. Sour cream samples had higher concentration of thiamine (0.54 µg/mL) than yogurt (0.45 µg/mL) and sour milk (0.47 µg/mL). In the opened UHT milk packages, which were stored in refrigerator for 10 days, significant thiamine loss was not observed. Investigated products contained appropriate amounts of thiamine and had satisfactory nutritional value with respect to vitamin B1.
Se compararon los niveles de tiamina presentes en distintos tipos de leche, en productos de leche fermentada y en la leche de arroz. En general, los productos valorados fueron obtenidos de los mercados tomando en cuenta que el análisis vitamínico puede ser un buen parámetro para definir la calidad de los alimentos comerciales. En este sentido, se determinaron las concentraciones de tiamina por cromatografía líquida de alta resolución (HPLC) utilizando una columna C-18 de fase invertida con detector fluorescente. El contenido de tiamina promedio fue 0,46 µg/mL en la temperatura ultra alta (UHT), 0,39 µg/mL en la pasteurizada y 0,38 µg/mL en la leche de sabor a chocolate. La leche de vaca no procesada presentó una concentración promedio de tiamina de 0,47 µg/mL. El contenido de tiamina más alto (11,95 µg/mL) se encontró en la leche de arroz. Mientras que las muestras de crema agria mostraron concentraciones de tiamina (0,54 µg/mL) superiores a las de yogurt (0,45 µg/mL) y a las de leche agria ((0,47 µg/mL). En los contenedores de leche UHT abiertos y almacenados en el refrigerador durante 10 días, se detectó que no se produjo una pérdida significativa de tiamina. Los productos valorados contenían una cantidad de tiamina apropiada y su valor nutricional con respecto a la vitamina B1 fue satisfactorio.
Palabras claves: tiamina; leche; productos lácteos; determinación HPLC
Introduction
Throughout the world, milk and dairy products have always been used in human nutrition. Milk is a food with high nutritional benefits and is therefore considered as an important natural product that comprises a healthy, balanced diet for all age groups. Proteins, minerals and vitamins are an integral part of milk nutritional profile. Consuming milk and dairy products is a quick and convenient way of obtaining significant amounts of proteins and most micronutrients including B-group vitamins (Akalin, Gönç, & Dinkçi, Citation2004; Walstra, Wouters, & Geurts, Citation2005). B-group vitamins have significant role in human vitality and body functioning. Thiamine is one of the B-vitamins present in milk, essential for normal function of muscles, heart, nervous system and mental activity that converts carbohydrates in the body to produce energy. The biologically active form, thiamine pyrophosphate (TPP), is a cofactor for a number of key enzymes in tricarboxylic acid cycle (Krebs cycle) and in pentose phosphate pathway. Also, it is involved in lipid and protein metabolism, blood-cell formation and biosynthesis of acetylcholine for nerve transmission. Recommended daily allowance (RDA) is 1.5 mg for men and 1.1 mg for women per day, but increases during pregnancy and lactation (Akalin et al., Citation2004; Fox & McSweeney, Citation1998; Lynch & Young, Citation2000). Nowadays people around the world increasingly consume factory-produced milk. During the processing of milk, some chemical changes in the composition of milk occur, so it is interesting to evaluate the content of biomolecules in industrial dairy products.
The demands of modern consumers, especially young population is for chocolate milk. It is known that chocolate milk is cocoa-flavoured milk with many nutrients including vitamins, high-quality proteins, carbohydrates, electrolytes and fat, which makes it an effective post-workout recovery and rehydration drink for adults engaged in all types of physical activity. The industrial processes used in the production of chocolate milk may affect the levels of nutrients in the final product so the influence of cocoa addition on the content of biomolecules should be evaluated.
Dairy products obtained by fermentation of milk (yogurt, sour milk and sour cream) have been produced for a long period of time in the Middle East. Today, yogurt is probably the most popular fermented milk product in the human diet. The essential flora of yogurt consists of the thermophiles Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus. In recent times, probiotic yogurt which contains live, active bacterial cultures has become a very popular dairy product. Probiotics are defined as cultured strains of lactic acid bacteria, which have been isolated from human intestinal flora, e.g. certain lactobacilli and bifidobacteria (Walstra et al., Citation2005). Health benefits of probiotic bacteria include antimicrobial properties, improvement in lactose metabolism, antidiarrhoeal properties, immune system stimulation, and suppression of Helicobacter pylori infection (McKinley, Citation2005). Sour milk is the product obtained by the fermentation of milk, which occurs either by spontaneous souring caused by various lactic-acid-producing bacteria or after addition of mesophilic microorganisms (Lactococcus lactis, L. cremoris, L. diacetylactis, Leuconostoc cremoris) to heated milk at 20°C. Sour cream is produced by the fermentation of high-pasteurized milk cream with a fat percentage of 18–20 (Walstra et al., Citation2005).
The requirements of modern consumers, especially in the developed countries, are for a healthy food, so in the recent decades vegetarian diet became very popular. Rice plays an important role in this type of diet. One of the most favourable rice products is rice milk, which is mostly made from brown rice. Rice seems to be nonallergenic and rice milk has been fed to infants allergic to cow’s milk. Also, it is good for people who are lactose intolerant and those with soy or nuts allergies. Rice and its products are an important source of B vitamins, so it is interesting to determine thiamine content in rice powder milk.
Thiamine exists in many forms, but the most common synthetically form is thiamine hydrochloride. Regulation (EC) No 1925/2006 of the European Parliament and of the Council allows addition of thiamine hydrochloride and thiamine mononitrate in food products. To assure an adequate intake of thiamine, it is essential to know its levels in the final products. The aim of this article was to estimate and compare thiamine content in different types of unsupplemented dairy and non-dairy products on the Serbian market in order to assess influence of technological procedures on these products quality and their nutritive value with respect to water-soluble vitamin B1.
Materials and methods
Chemicals and reagents
Standard thiamine (analytical grade) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Potassium ferricyanide (K3[Fe(CN)6]) and ammonium acetate (NH4Ac) were also purchased from Sigma-Aldrich. Hydrochloric and perchloric acids, absolute ethanol, methanol (MeOH) and sodium hydroxide were obtained from Merck (Darmstadt, Germany). All solvents were of HPLC grade. Solutions were filtered through a 0.22-µm filter before use. Deionized water was generated using a Smart2Pure Ultrapure water purification system from Thermo Electron LED GmbH (Niederelbert, Germany).
Apparatus
The Agilent Technologies 1200 Series apparatus (Santa Clara, CA, USA) with fluorescence detector, binary pump, solvent degasser system and the Agilent Chem Station programme was used for the chromatographic analysis, monitoring and data acquisition.
Chromatographic conditions
Chromatographic determination of thiamine was achieved at 30°C using RESTEK Ultra IBD C18 column (5 µm, 150 mm × 4.6 mm) with the mobile phase consisted of 50.0 (%, v/v) methanol and 50.0 (%, v/v) 0.005M NH4Ac solution (pH 5.0) by isocratic elution. The flow rate was kept at 1.0 mL/min and 20 µl of the sample was injected. The fluorescence detector was programmed to an excitation wavelength of 370 nm and emission wavelength of 435 nm (optimum wavelengths for thiochrome). The solutions were injected immediately after thiochrome formation, because derivatization product is unstable and influences on intensity of fluorescence signal.
Standard solutions and calibration curve
Concentrations of thiamine in analysed samples were determined by calibration curve method. The stock standard solution of thiamine (1 µg/ml) was prepared by dissolving standard of thiamine in hydrochloric acid (5%, w/w) and stored at 4°C in glass bottles. The working calibration solutions of thiamine were freshly prepared for each run by diluting the stock standard solution with 5% hydrochloric acid. The same volume of 1% K3[Fe(CN)6] in 15% NaOH was added in all standard thiamine solutions. The concentrations of these solutions were in the expected range for thiamine in milk products. Twenty microlitres of these solutions was injected into column and each solution was injected 5 times. The calibration curve was constructed by plotting the peak area of analyte against its concentration. Linearity of the calibration curve was confirmed in the range of analytical interest (R > 0.9998).
Sample collection and preparation
Raw, UHT, pasteurized, chocolate, sour milk and yogurt samples
Pasteurized, ultra high temperature (UHT), chocolate, sour milk and yogurt of most representative producers in Serbia with a different fat content (0.5–3.5%) were collected from local shops. All samples were prepared and analysed immediately after opening. Additionally, some samples of UHT milk were analysed before and after refrigerated storage at 4°C for 10 days. Samples of raw cow milk were obtained from local farms in the same mountainous area of Nis Region (Southeast Serbia) in the winter period. Cows were fed only with natural food (grass, forages, straw, hay) without any vitamin supplements. Milk was kept refrigerated at 4°C until it was processed. Additionally, for the raw milk measurement of the milk fat according to standard Gerber method (AOAC Official Method, Citation2004) was done.
For the raw, UHT, pasteurized, chocolate, sour milk and yogurt, the sample preparation procedure was as follows: 2 mL aliquot of the sample, in a test tube, was treated with 2 mL of perchloric acid (12%, w/v), vortexed vigorously for 1 min and centrifuged at 4000 rpm for 15 min at 4°C. The formed protein precipitate was removed. A 1 mL aliquot of obtained supernatant was derivatized by 0.5 mL 1% potassium ferricyanide in 15% sodium hydroxide. After vortex mixing for 1 min, the insoluble precipitate was removed by centrifugation (5 min, 4000 rpm at 4°C). The obtained supernatant was filtered through Econofilter 25 mm/0.45 µm membrane syringe filter (Agilent Technologies) in vial and 20 µl was injected into HPLC column. All samples were analysed in five replicates.
Sour cream samples
Sour cream samples with 20% fat content were purchased from local shops. In order to remove milk fat 2 g of sample was treated with 2 mL of absolute ethanol, vortex-mixed in a test tube and centrifuged for 15 min at 4000 rpm at 4°C. Then, 2 mL of the obtained supernatant was treated with 2 ml of 12% perchloric acid and further preparation and derivatization procedure was as described above.
Rice milk samples
The powder rice milk was obtained from the local store. Amount of 1 g of powder sample was weighted in test tube, dissolved in 10 mL of deionized water and kept in a warm water bath for 10 min. After complete dissolving, the test tube was cooled and 2 mL of the sample was treated with 12% perchloric acid and K3[Fe(CN)6] according to the above-mentioned procedure for the milk products.
Statistical analysis
The data were processed using the SPSS 18.0 commercial statistical program software (IBM Corporation, Armonk, NY). t-Test was used to compare the thiamine content between different types of milk. The effect of refrigerated storage on thiamine content in opened milk packages was examined by paired t-test. All samples were analysed at least in five replicate. The results are presented as mean value ± standard deviation (SD). Statistical significance was assigned to p < 0.05.
Results and discussion
Different methods for the determination of thiamine in dairy products have been described, but the most popular are those employing liquid chromatography. HPLC technique allows rapid separation and quantification of vitamin B1 in milk using reversed-phase columns either by UV or fluorescence detection (Agostini-Costa et al., Citation2007; Albalá-Hurtado, Veciana-Nogués, Izquierdo-Pulido, & Mariné-Font, Citation1997; Bognar, Citation1992; Ching-Hung et al., Citation2011; Jakobsen, Citation2008; Jedlicka & Klimes, Citation2005; Lizhi, Lin, & Hongwei, Citation2009; Lynch & Young, Citation2000; Ollilainen et al., Citation1993; Rychlik, Bitsch, & Bitsch, Citation2011; Ujiie, Tsutake, Morita, Tamura, & Kodaka, Citation1990; Vidal-Valverde & Diaz-Pollan, Citation2000; Woollard & Indyk, Citation2002). Fluorescence detection is more sensitive; therefore, thiamine is usually precolumn derivatized. Derivatization of thiamine is based on the oxidation of thiamine with potassium hexacyanoferrate (III) in an alkaline medium which leads to the formation of the fluorescent thiochrome derivative (Jansen, Citation1936). Tiochrome is further separated on a HPLC column and detected at ex 370 nm and em 435 nm. In this article, a modified HPLC method by Lizhi et al. (Citation2009) and Rodríguez-Bernaldo de Quirós, López-Hernández and Simal-Lozano (Citation2004) was used for the determination of vitamin B1 in the analysed products. First, optimization of the chromatographic conditions and then validation of HPLC method were performed.
Optimization of the chromatographic conditions
Scan analysis of vitamin standard and sample solutions after derivatization were performed to check the optimum conditions for separation and quantification of thiamine. During the method development, several mobile phases (5.0 mM NH4Ac, pH 5.0 and MeOH) with various proportions of organic solvents (25:75, 35:65 and 50:50, v/v) were tested in order to minimize the run-time and to optimize peaks separation and retention parameters. The best resolution was obtained with the 50:50 volume ratio of NH4Ac:MeOH. The optimal working temperature for chromatographic column was found to be 30°C. Of the several flow rates (0.8, 1.0 and 1.2 mL/min), 1.0 mL/min was selected to reduce analysis time and achieved better resolution.
The final chromatographic system comprising a reverse-phase C18 column (5 µm, 150 mm × 4.6 mm) with a mobile phase consisting of 50.0% methanol and 50.0% 5.0 mM NH4Ac solution (pH 5.0) in an isocratic elution mode at the flow rate of 1.0 mL/min. The temperature of the column was 30°C and fluorescence detection was performed at ex 370 nm and em 435 nm. The retention time for thiamine was 2.9 min and the run-time was below 5 min.
Validation of HPLC method
The method was validated by means of following parameters: accuracy, precision, linearity, detection and quantification limits.
The precision of the analytical method, estimated as the relative standard deviation (RSD) for three replicates of three standard solutions, was found to be 0.94%. Linearity of the method was evaluated by regression analysis after construction of calibration curve. For the calibration curve, a series of vitamin standards (concentration levels in the range of analytical interest) were used. Linear calibration curve of thiamine with correlation coefficient (R) of 0.9998 was obtained (). The limit of detection (LOD) was estimated following International Conference on Harmonisation (ICH) Guidelines (2005). LOD determined using the equation: LOD = 3.3SEa/b (SEa – the standard error of the intercept, b – the slope of the calibration line) was 8.4 ng/mL. The limit of quantification (LOQ) was found to be 25.37 ng/mL, and was also calculated according to ICH guidelines using the equation: LOQ = 10SEa/b (). Accuracy of the method was expressed as recovery of the extraction procedure. Recoveries were determined by the standard addition method at three concentration levels. The obtained values for the spiked milk samples were 98.5%, 98.8% and 99.33%.
Table 1. Characteristic parameters for the linear regression equations.
Parámetros característicos para las ecuaciones de regresión lineal.
Optimization of the extraction procedure for thiamine
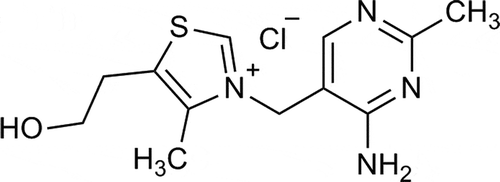
In order to develop an appropriate extraction method, we tried several sample preparation procedures. Physico-chemical characteristics of thiamine molecule are important for quality of extraction. Thiamine consists of two heterocyclic rings, thiazole and pyrimidine, connected by methylene bridge (). In milk, it is found not only as free thiamine but also as phosphorylated or protein-bound derivatives. Thiamine is more stable under slightly acidic conditions and OH– ions in foods can cause its degradation. Considering that milk contains large amount of proteins (30–35 g/L), the sample preparation must include deprotenization step with strong acid. In literature, extraction with trichloroacetic (TCA), trifluoroacetic (TFA) or acetic acid was recommended (Ball, Citation2006; Fox & McSweeney, Citation1998). These organic acids are very toxic and environmental harmful so we tried to use less toxic reagent, for example, perchloric or hydrochloric acid. In our previous work, deproteinization with perchloric acid was a good choice for the determination of water soluble vitamin B2 (riboflavin) in some dairy products (Sunaric, Denic, & Kocic, Citation2012). Because thiamine has ability for protein binding, extraction with high concentration of perchloric acid needs to be performed. The 6%, 12% and 20% (w/v) solutions of perchloric acid were tested for sample pretreatment. By using 6% perchloric acid cloudy sample solution was obtained, which indicates that precipitation was incomplete. In addition, intensive endogenous peaks appeared at lower retention times. Perchloric acid solutions of higher concentrations (12% and 20%, w/v) provided chromatograms clean enough to ensure accurate and precise identification and quantification of thiamine. Also, the precipitation of proteins in the sample was complete. For these reasons, 12% perchloric acid was selected for further work. Since the milk lipids were removed as upper solid layer after first centrifugation at 4°C, the use of weakly polar organic solvents (methanol, ethanol) was not necessary. After deproteinization step, derivatization reagent was added in the supernatant and a cloudy solution was appeared, so it was necessary to centrifuge the sample again. Obtained derivatization product tiochrome showed unstability in the intensity of the fluorescent signal with time. For that reason, adjustment of pH value of the sample to pH 5–6 before adding of derivatization reagent using a mixture 2M K2CO3/6M KOH was tried, but this did not provide a significant improvement. Furthermore, the colour of the solution was changed in the first 15 min of centrifugation, which indicates that tiochrome was further degraded, leading to a reduction in the intensity of the fluorescent signal. The most optimal time for the second centrifugation was 5 min, because there were no significant changes in fluorescence for this time interval. Finally, sample preparation procedure as described in the Materials and methods section was adopted. The great advantage of this procedure is that it is simple and fast, unlike to other authors who used more extensive treatment including hot mineral acid digestion followed by enzymatic hydrolysis (Akalin et al., Citation2004; Jakobsen, Citation2008; Lloyd, Zou, Ogden, & Pike, Citation2004; Sierra & Vidal-Valverde, Citation2000). In addition, our sample pretreatment gave accurate and precise results for thiamine determination in dairy products, which was confirmed by literature data.
Determination of thiamine in selected dairy products and rice milk
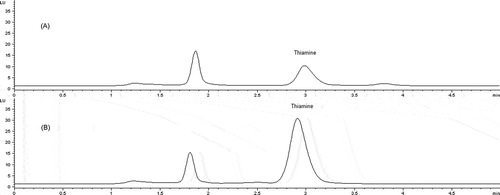
The chromatograms of unspiked and milk spiked with thiamine standard solution are shown in . Obtained chromatographic peaks for the samples were identified by comparing their retention times with those of thiamine standard. The thiamine concentrations in the injected samples were found by interpolation of the peak area from the standard calibration curve. Considering all steps in sample preparation, concentration of thiamine was finally calculated and expressed per millilitre or gram of the product.
Figure 2. Chromatograms of thiamine in unspiked (A) and spiked UHT milk (B).
Cromatogramas de tiamina en leche UHT no intervenida (A) e intervenida (B).
The results of thiamine determination in different types of milk are summarized in and reported as mean value ± standard deviation for five determinations. The precision of the determination, estimated as relative standard deviation (RSD) for five replicates of products samples was below 6.2%. The average content of thiamine in raw cow milk (0.47 µg/mL) was slightly higher in comparison with that reported in most of the literature (0.20–0.40 µg/mL) (Echols, Miller, & Foster, Citation1986; Fox & McSweeney, Citation1998; Gaucheron, Citation2011; Schönfeldt, Hall, & Smit, Citation2012; Sierra & Vidal-Valverde, Citation2000; Zagorska & Ciprovica, Citation2008). These values partially depend on the country and geographical area. According to Schönfeldt et al., the raw cow milk from the United States, the United Kingdom and Denmark had 0.40 µg/mL, from Australia and New Zealand had 0.30 µg/mL, while South Africa’s milk had 0.20 µg/mL of thiamine. On the other hand, Alm (Citation1982), Oupadissakoon, Chambers, and Chambers (Citation2009) and Pandya and Ghodke (Citation2007) found a similar content of thiamine (0.43 µg/mL, 0.45 µg/mL and 0.44 µg/mL, respectively) in raw cow milk. It is believed that most of the thiamine in bovine milk is produced by micro-organisms in the rumen; therefore, animal nutrition has no major impact (Ball, Citation2006; Fox & McSweeney, Citation1998). However, the breed of cow and particularly, the stage of lactation may influence the concentration of thiamine in milk (Akalin et al., Citation2004).
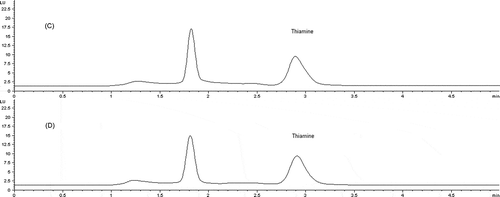
Analysed UHT and pasteurized milk samples with different fat content (0.5–3.5%) had average thiamine concentration of 0.46 µg/mL and 0.39 µg/mL, respectively (). In general, the contents of thiamine in the pasteurized and UHT milk were higher compared to those found in Turkey, Pakistan, Romania, the United States and Spain (Akalin et al., Citation2004; Asadullah, Tarar, Ali, Jamil, & Begum, Citation2010; Constantin & Csatlos, Citation2010; Knott, Citation1942; Zafra-Gómez, Garballo, Morales, & Garciaí-Ayuso, Citation2006). Pasteurized milk from Channel Island breeds of cow contains about 0.40 µg/mL (Fox & McSweeney, Citation1998). The results of our assessment showed no significant differences in thiamine concentration in the same type of UHT and pasteurized milk from the same producer with different fat content (p > 0.05). The observed small differences are more related to the producers than to the percentage of the milk fat. Loss of vitamin can be related to the intensity of food processing and to the duration of food storage. In addition, we examined the effect of refrigerated storage (+4°C) on the thiamine content in opened UHT packages. Our results showed that opened UHT samples which were stored in refrigerator at +4°C for 10 days had average of 0.44 µg/mL of thiamine, while similar average thiamine content were found in freshly opened samples (, ). Although the opened packages have been exposed to oxygen, the significant thiamine degradation was not observed (p < 0.05). Bahman et al. (Citation2012) also found no appreciable loss of thiamine during storage of pasteurized milk at refrigerated temperature after 24 h.
Table 2. Results of HPLC analysis of thiamine in cow milk.
Resultados del análisis HPLC de tiamina en leche de vaca.
Table 3. Thiamine content in chocolate, rice milk and fermented milk products.
Contenido de tiamina en leche de sabor a chocolate, en leche de arroz y en productos de leche fermentada.
Figure 3. Chromatograms of thiamine in UHT milk before (C) and after (D) refrigerated storage for 10 days.
Cromatogramas de tiamina en leche UHT antes de (C) y después de (D) su almacenamiento refrigerado durante 10 días.
Cocoa powder is a rich source of many nutrients like vitamins, minerals, amino acids and polyunsaturated fatty acids. It contains about 0.10 mg of thiamine per 100 g of the powder (National Food Institute-Technical University of Denmark, Citation2009). For chocolate milk samples, we found that average thiamine content was 0.38 µg/mL, which is slightly lower than the content in non-chocolate commercial and raw cow milk (). Also, thiamine concentrations in this type of dairy product were significantly different than the values found by other authors. For example, thiamine in Brazilian chocolate milk was found to be in the range of 0.06 to 0.2 µg/mL (Agostini-Costa et al., Citation2007), while in previous work from 1997, Agostini-Costa and Godoy reported that chocolate flavoured milk contained 0.8–1.8 µg/mL of thiamine.
Nutritional content of yogurt is similar to the nutritional content of milk (McKinley, Citation2005). However, variations in the quality of yogurt depend on the type of milk. In yogurt production, fresh cow’s milk and also sheep milk are usually used as a raw material. We found that thiamine average concentrations were 0.45 µg/mL in analysed yogurt, 0.47 µg/mL in sour milk and 0.54 µg/mL in sour cream purchased from local shops (). The thiamine content in yogurt was in agreement with concentration in the literature (0.29–0.50 µg/mL) (Akalin et al., Citation2004; Alm, Citation1982; Diaz Marquina, Orzaez Villanueva, & Matallana Gonzalez, Citation1991; Gaucheron, Citation2011; McKinley, Citation2005; Padovani, Lima, Colugnati, & Rodriguez-Amaya, Citation2007). Analysed probiotic yogurt had slightly lower average concentration of thiamine (0.41 µg/mL), probably because it is a cultured milk product that is soured and thickened by the action of specific lactic acid-producing and probiotic microbiological cultures. Probiotic culture strains may change thiamine content. Thus, Alm (Citation1982) reported that concentration of thiamine decreased after fermentation and he found 0.37 µg/mL of thiamine in yogurt. On the other hand, in sour cream, significantly higher thiamine concentration than in yogurt and sour milk was found (p < 0.05) ().
The analysed rice milk had significantly higher amount of thiamine (average 11.95 µg/mL) than all other examined products (p < 0.05) (). It is known that rice is rich in B-group vitamins; therefore, rice milk as non-dairy substitute has high nutritional value with respect to thiamine.
Conclusion
Today, a variety of different dairy products are available in the world market. Consumer’s increasing interest for maintaining or improving their health has led to the development of many new dairy products, e.g. the enrichment with vitamins and low-fat products. However, in technological processing of milk, especially care must be taken in order to minimize or to avoid vitamin loss.
In this work, thiamine concentration in different dairy products and rice milk was determined. Examined commercial milk and raw cow milk contain appropriate amounts of thiamine and have satisfactory nutritional value with respect to vitamin B1. It can be concluded that milk is one of the sources of thiamine for humans, because 250 ml of milk provides 11% and 15% of RDA for men and women, respectively. Since the rice milk contains large quantities of this vitamin, it can be considered that this product is important nutritional source of thiamine and good supplement for those on special diets either to lose weight and/or for other health conditions, as well as for vegetarians. Our results showed that other readily consumed dairy products (yogurt, sour cream, sour and chocolate milk) also had satisfactory amounts of thiamine.
Acknowledgement
This research was supported by grant TR 31060 from the Ministry of Education and Science of the Republic of Serbia.
References
- AOAC. (2004). Official method 2000.18, Fat Content of Raw and Pasteurized Whole Milk Gerber Method by Weight, First Action 2000, Final Action 2004, VA: Association of Official Analytical Chemists.
- Agostini, T.S., & Godoy, H.T. (1997). Simultaneous determination of nicotinamide, nicotinic acid, riboflavin, thiamin, and pyridoxine in enriched Brazilian foods by HPLC. Journal of High Resolution Chromatography, 20, 245–248.
- Agostini-Costa, T., Teixeira-Filho, J., Scherer, R., Kowalski, C.H., Prado, M.A., & Godoy, H.T. (2007). Determination of B-group vitamins in enriched flavored milk mixes. Brazilian Journal of Food and Nutrition, 18, 351–356.
- Akalin, A.S., Gönç, S., & Dinkçi, N. (2004). Liquid chromatographic determination of thiamin in dairy products. International Journal of Food Sciences and Nutrition, 55, 345–349.
- Albalá-Hurtado, S., Veciana-Nogués, M.T., Izquierdo-Pulido, M., & Mariné-Font, A. (1997). Determination of water-soluble vitamins in infant milk by high performance liquid chromatography. Journal of Chromatography A, 778, 247–253.
- Alm, L. (1982). Effect of fermentation on B-vitamin content of milk in Sweden. Journal of Dairy Science, 65, 353–359.
- Asadullah, K.U.N., Tarar, O.M., Ali, S.A., Jamil, K., & Begum, A. (2010). Study to evaluate the impact of heat treatment on water soluble vitamins in milk. Journal Pakistan Medical Association, 60, 909–912.
- Bahman, S., Yadav, N., Kumar, A., Ganguly, S., Garg, V., & Marwaha, S.S. (2012). Impact of household practices on the nutritional profile of milk. Indian Journal of Public Health, 56, 82.
- Ball, G.F.M. (2006). Vitamins in foods. Boca Raton, FL: CRC Press.
- Bognar, A. (1992). Determination of vitamin-B1 in food by high performance liquid-chromatography and postcolumn derivatization. Fresenius Journal of Analytical Chemistry, 343, 155–156.
- Ching-Hung, C., Ya-Hwei, Y., Chun-Ting, S., Shih-Ming, L., Chieh-Ming, J.C., & Chwen-Jen, S. (2011). Recovery of vitamins B from carbon dioxide-defatted rice bran powder using ultrasound water extraction. Journal of Taiwan Institute of Chemical Engineers, 42, 124.
- Constantin, A.M., & Csatlos, C. (2010). Research on the influence of microwave treatment on milk composition. Bulletin of the Transilvania University of Braşov, 3, 52.
- Diaz Marquina, A., Orzaez Villanueva, M.T., & Matallana Gonzalez, M.C. (1991). Variations of hydrosoluble vitamin contents in dairy products during storage. Le Lait, 71, 519–521.
- Echols, R.E., Miller, R.H., & Foster, W. (1986). Analysis of thiamine in milk by gas chromatography and the nitrogen-phosphorus detector. Journal of Dairy Science, 69, 1246–1249.
- Fox, P.F., & McSweeney, P.L.H. (1998). Dairy chemistry and biochemistry. London: Blackie Academic & Professional.
- Gaucheron, F. (2011). Milk and dairy products: A unique micronutrient combination. Journal of the American College of Nutrition, 30, 400S–409S.
- Jakobsen, J. (2008). Optimisation of the determination of thiamin, 2-(1-hydroxyethyl) thiamin, and riboflavin in food samples by use of HPLC. Food Chemistry, 106, 1209–1217.
- Jansen, B.C.P. (1936). A chemical determination of aneurin by the thiochrome reaction. Recueil Des Travaux Chimiques Des Pays-Bas, 55, 1046.
- Jedlicka, A., & Klimes, J. (2005). Determination of water- and fat-soluble vitamins in different matrices using high-performance liquid chromatography. Chemical Papers, 59, 202.
- Knott, E.M. (1942). Thiamin content of milk in relation to vitamin B1 requirement of infants. American Journal of Public Health, 32, 1013.
- Lizhi, L., Lin, Y.L., & Hongwei, Z. (2009). Determination of vitamin B1 and B2 in infant milk powder by HPLC fluorescence detector. Journal of Environment and Health, 26, 451–452.
- Lloyd, M.A., Zou, J., Ogden, L.V., & Pike, O.A. (2004). Sensory and nutritional quality of nonfat dry milk in long-term residential storage. Journal of Food Science, 69, 326–331.
- Lynch, P.L., & Young, I.S. (2000). Determination of thiamine by high-performance liquid chromatography. Journal of Chromatography A, 881, 267–284.
- McKinley, M.C. (2005). The nutrition and health benefits of yoghurt. International Journal of Dairy Technology, 58, 1–12.
- National Food Institute-Technical University of Denmark. (2009). Danish Food Composition Databank – ed. 7.01.
- Ollilainen, V., Vahteristo, V., Uusi-Rauva, A., Varo, P., Koivistoinen, P., & Huttunen, J. (1993). The HPLC determination of total thiamin (vitamin B1) in foods. Journal of Food Composition and Analysis, 6, 152–165.
- Oupadissakoon, G., Chambers, D.H., & Chambers, E.IV. (2009). Comparison of the sensory properties of ultra-high temperature (UHT) milk from different countries. Journal of Sensory Studies, 24, 427–440.
- Padovani, R.M., Lima, D.M., Colugnati, F.A.B., & Rodriguez-Amaya, D.B. (2007). Comparison of proximate, mineral and vitamin composition of common Brazilian and US foods. Journal of Food Composition and Analysis, 20, 733–738.
- Pandya, A.J., & Ghodke, K.M. (2007). Goat and sheep milk products other than cheeses and yoghurt. Small Ruminant Research, 68, 193–206.
- Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. Official Journal of the European Union, L 404/26–38.
- Rodríguez-Bernaldo de Quirós, A., López-Hernández, J., & Simal-Lozano, J. (2004). Simultaneous determination of thiamin and riboflavin in the sea urchin, Paracentrotus lividus, by high-performance liquid chromatography. International Journal of Food Sciences and Nutrition, 55, 259–263.
- Rychlik, M., Bitsch, R., & Bitsch, I. (2011). HPLC determination of thiamin in fortified foods. In M. Rychlik (Ed.), Fortified foods with vitamins: Analytical concepts to assure better and safer products (pp. 89–102). Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA.
- Schönfeldt, H.C., Hall, N.G., & Smit, L.E. (2012). The need for country specific composition data on milk. Food Research International, 47, 207–209.
- Sierra, I., & Vidal-Valverde, C. (2000). Influence of heating conditions in continuous-flow microwave or tubular heat exchange systems on the vitamin B1 and B2 content of milk. Le Lait, 80, 601–608.
- Sunaric, S., Denic, M., & Kocic, G. (2012). Evaluation of riboflavin content in dairy products and non-dairy substitutes. Italian Journal of Food Science, 24, 352–357.
- Ujiie, T., Tsutake, Y., Morita, K., Tamura, M., & Kodaka, K. (1990). Simultaneous determination of 2-(1-hydroxyethyl)thiamine and thiamine in foods by high performance liquid chromatography with postcolumn derivatization. Vitamins (Japan), 64, 379–385.
- Vidal-Valverde, C., & Diaz-Pollan, C. (2000). Comparison of capillary electrophoretic and high performance liquid chromatographic thiamin determination in milk. Milchwissenschaft, 55, 307–309.
- Walstra, P., Wouters, J.T.M., & Geurts, T.J. (2005). Dairy science and technology. Boca Raton, FL: CRC Press.
- Woollard, D.C., & Indyk, H.E. (2002). Rapid determination of thiamine, riboflavin, pyridoxine and niacinamide in infant formulas by liquid chromatography. Journal of AOAC International, 85, 945.
- Zafra-Gómez, A., Garballo, A., Morales, J.C., & Garciaí-Ayuso, L.E. (2006). Simultaneous determination of eight water-Soluble vitamins in supplemented foods by liquid chromatography. Journal of Agricultural and Food Chemistry, 54, 4531–4536.
- Zagorska, J., & Ciprovica, I. (2008). The chemical composition of organic and conventional milk in Latvia. In I. Ciprovica (Ed.), 3rd Baltic Conference on Food Science and Technology – FOODBALT Conference Proceedings (pp. 10–14). Jelgava: Faculty of Food Technology, Latvia University of Agriculture.