Abstract
Response surface methodology (RSM) was used to optimise the parameters for phenolic extraction from apple pomace using water, extraction time, extraction temperature and pomace to water ratio. The responses from these parameters were evaluated by measuring the total phenolic content and antioxidant activity of extracts. The optimum extraction parameters found in this study were 30 min extraction time, 85°C extraction temperature and 0.05 pomace to water ratio. A verification experiment of these extraction parameters was performed, along with three other corroborative sets of parameters. There was no significant difference between the predicted and actual values, confirming that the predictions using the models obtained through RSM were valid.
La metodología de respuesta de la superficie (RSM) fue empleada para optimizar los parámetros para la extracción fenólica del orujo de la manzana utilizando agua, tiempo de extracción, temperatura de extracción y ratio de agua en el orujo. Las respuestas de estos parámetros fueron evaluadas calculando el total de contenido fenólico y la actividad antioxidante de los extractos. Los parámetros óptimos de extracción que se encontraron en este estudio fueron: 30 min tiempo de extracción, 85°C temperatura de extracción y 0,05 ratio de agua en el orujo. Se realizó un experimento de verificación de estos parámetros de extracción, además de otros tres conjuntos corroborativos de parámetros. No se encontró una diferencia significativa entre los valores previstos y los reales, confirmando que las previsiones que utilizaron los modelos obtenidos mediante la RSM eran válidas.
1. Introduction
Phenolic compounds are one of the primary groups of phytochemicals found in fruits and vegetables. They are naturally occurring compounds that serve as a pollination aid (through pigmentation) and a self-defence mechanism against environmental stresses such as radiation, infection or injury (Lattanzio, Lattanzio, & Cardinali, Citation2006).
These compounds have been reported by numerous studies to have several biological effects towards human health such as antioxidant and antimicrobial activities. The antioxidant activity of the phenolic compounds has been linked to the prevention of some prevalent chronic diseases such as coronary heart disease and cancer. Some studies have also proposed that the compounds may help to slow down the ageing process (Barth et al., Citation2005; Boyer & Liu, Citation2004; Clifford, Citation2004; Cutler et al., Citation2008; Hollman & Katan, Citation1999; Jedrychowski & Maugeri, Citation2009; Jung et al., Citation2009). These desirable health effects are considered to be caused by the ability of the phenolic compounds to help reduce the imbalance between oxidative stress and antioxidants in the body (Roupas & Noakes, Citation2010; Scalbert, Manach, Morand, Rémésy, & Jiménez, Citation2005; Schaefer et al., Citation2006; Sesso, Gaziano, Liu, & Buring, Citation2003; Suárez et al., Citation2010; Sun, Chu, Wu, & Liu, Citation2002).
Apples are one of the most popular and widely consumed fruits in the world and are the major contributor of dietary phenolics in the Western diet (Boyer & Liu, Citation2004). The consumption of apples has been strongly related with the reduced risk of several diseases (Liu, Liu, & Chen, Citation2005; Roupas & Noakes, Citation2010; Sembries et al., Citation2003; Suárez et al., Citation2010). As with most popular fruits, apples are not just consumed fresh, they are processed into numerous commercial products, apple juice being the most prominent. However, when apples are processed into juice, there is a significant reduction in the total phenolic content (TPC) and antioxidant activity. Apple juice produced by a conventional pressing method loses 58% of its phenolic compounds (Guyot, Marnet, Sanoner, & Drilleau, Citation2003). Other studies found that up to 90% of the antioxidant activity is lost during the juicing process and the levels of flavonoids and chlorogenic acid in the juice were reduced by between 50% (chlorogenic acid) and 3% (catechins) (Van Der Sluis, Dekker, Skrede, & Jongen, Citation2002). This is because the majority of the phenolics are retained in the pomace (Carson, Collins, & Penfield, Citation1994; Van Der Sluis et al., Citation2002; Van Der Sluis, Dekker, Skrede, & Jongen, Citation2004).
Pomace is the solid remains of an apple after the juice has been extracted. It is a heterogeneous mixture of flesh and skin (94.5%), seeds (4.1%) and stems (1.1%) (Linskens & Jackson, Citation1999). Presently, apple pomace is considered as waste by the apple juice industry and processors must allocate funds to properly dispose of it. Given that apple juice is one of the most popular juices, the amount of pomace generated by worldwide apple juice manufacturers is estimated to be several million tonnes per year (Appledale Processors Co-op, Citation2012; Çam & Aaby, Citation2010).
There are many opportunities to utilise this waste product, such as: as animal feed, for ethanol production and as a source of fibre and pectin (Williams, Citation1987). In recent years, interest in extracting phenolic compounds from apple pomace has increased. Apple pomace is viewed as an alternative source of strong antioxidants with a low cost (Bhushan, Kalia, Sharma, Singh, & Ahuja, Citation2008; Cetkovic et al., Citation2008; McCann et al., Citation2007; Soler, Soriano, & Mañes, Citation2009). Several studies have shown that the phenolic compounds from the pomace are extractable using several types of organic solvents, namely methanol, acetone and ethanol (Ajila, Brar, Verma, Tyagi, & Valéro, Citation2011; Hayat et al., Citation2010; Mayya, Bhattacharyya, & Argillier, Citation2003; Reis, Rai, & Abu-Ghannam, Citation2012; Suárez et al., Citation2010; Van Der Sluis et al., Citation2004). However, the use of organic solvents may not be practical to adopt by the industry due to their toxicity. Several environmentally friendly methods of extracting phenolics from plant materials such as fermentation, pressurised liquid extraction and super critical carbon dioxide have been evaluated (Bazhal, Lebovka, & Vorobiev, Citation2003; Bhushan et al., Citation2008; Corrales, Toepfl, Butz, Knorr, & Tauscher, Citation2008; Elez-Martínez, Soliva-Fortuny, & Martín-Belloso, Citation2009). Despite being environmentally friendly, from the manufacturer’s point of view there are still potential problems with these advanced techniques, such as cost and personnel training.
Previous studies have shown that water is a suitable extraction solvent able to liberate phenolic compounds from apple pomace (Çam & Aaby, Citation2010; Reis et al., Citation2012). It is cheap, non-toxic, easily accessible and appealing to consumers. The optimisation step of the extraction process is a crucial step in the development of a feasible process (Ghosalkar, Sahai, & Srivastava, Citation2008). Çam and Aaby (Citation2010) investigated the use of water to extract phenolics from apple pomace. They found that the optimised conditions for the extraction were at 100°C for 37 min and a 0.01 pomace to water ratio. However, a high temperature may not be a desired condition for manufacturers as it requires energy which in turn increases production costs. Additionally, achieving and maintaining water temperature at such a high level may be a problem.
The central composite rotatable design (CCRD) is an experimental design used to allocate affecting operation variables into a range of evaluation. In this case, extraction temperature, extraction time and pomace to water ratio are the evaluated parameters in a defined range. The response surface methodology (RSM) provides the statistical elements required for the evaluation of those parameters. This methodology focuses on the construction of models that can predict the effect of the parameters in the area of evaluation (Fabian, Citation2012).
Therefore the aim of this study is to optimise the extraction of phenolic compounds from apple pomace with water using RSM based on the effects of three parameters (temperature, time and pomace to water ratio) and to analyse the antioxidant activity of the extracts.
2. Materials and methods
2.1. Apple pomace
Apple pomace was sourced from a local commercial juice manufacturer (Appledale Processors Co-op. Ltd., Orange, NSW, Australia). The pomace was homogenised and stored at −15°C until use.
2.2. Chemicals
Chemical reagents were all of analytical grade and were purchased from Sigma Aldrich Laboratory Chemicals (Castle Hill, NSW, Australia). Deionised water was freshly prepared with a Millipore Milli-Q water purification system (Millipore Australia, North Ryde, NSW, Australia).
2.3. Extraction of phenolic compounds from apple pomace
Pomace extracts for the analyses were obtained by adding 5 g of apple pomace into a given amount of deionised water at an assigned temperature. The mixtures were placed into a shaking water bath for the allocated extraction lengths. Following the extraction, the mixtures were placed into an ice bath until they cooled down to 15°C (Hirun & Roach, Citation2011) in order to stabilise the polyphenolics. The mixtures were then vacuum-filtered using a double-layer cheesecloth, followed by centrifugation at 12,100 × g (Beckman J2-AC centrifuge, Beckman Instruments Inc., Brea, California, USA) (Candrawinata, Blades, Golding, Stathopoulos, & Roach, Citation2012). The filtrate obtained would thereafter be referred to as the aqueous extract of the pomace. Details of the extraction parameters will be described later.
As a control, apple pomace was extracted using analytical grade methanol, adapted from a procedure developed by Golding, McGlasson, Wyllie, and Leach (Citation2001). The pomace (5 g) was added into 20 mL methanol and sonicated for 20 min with an UltraSONIK 57X NEY sonifier (Extech Equipment Pty. Ltd., Melbourne, VIC, Australia) before it was vacuum-filtered through a double-layer cheesecloth followed by centrifugation at 12,100 × g (Beckman J2-AC centrifuge, Beckman Instruments Inc., California, USA). The filtrate obtained would thereafter be referred to as the methanol extract of the pomace.
2.4. Analytical procedure
The extracts were subsequently analysed for their TPC and antioxidant activity. All extractions and measurements were performed in triplicate.
2.4.1. TPC assay
TPC was measured by the assay based on a method established by Folin and Ciocalteu (Citation1927). This assay was adapted from Swain and Hillis (Citation1959) and Thaipong, Boonprakob, Crosby, Cisneros-Zevallos, and Byrne (Citation2006) with minor modifications. Briefly, 150 μL of extract was mixed with 150 μL of 0.25 N Folin Ciocalteu reagent. The mixture was allowed to react for 2 min before adding 2400 μL of 5% (w/v) sodium carbonate solution. The incubation time was set for 1 h. The results were expressed in gallic acid equivalents (GAE, μg/g fresh pomace).
2.4.2. Antioxidant activity assays
Antioxidant activity of the extracts was measured using three different assays, namely DPPH (2,2-diphenyl-1-picrylhydrazyl), FRAP (Ferric Reducing Ability of Plasma) and ABTS (2,2ʹ-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)) assays.
For the DPPH assay, the method was adapted from Brand-Williams, Cuvelier, and Berset (Citation1995) and Thaipong et al. (Citation2006) with some modifications. The incubation time was set for 30 min. The FRAP assay was performed according to Benzie and Strain (Citation1996) and Thaipong et al. (Citation2006). The ABTS assay was conducted based on the method described by Thaipong et al. (Citation2006). The results of all the antioxidant activity assays are expressed in Trolox equivalents (TE, μg/g fresh pomace).
2.5. Experimental design
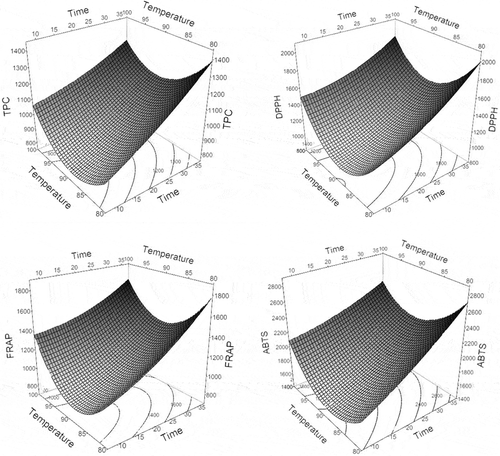
Extraction temperature, extraction time and pomace to water ratio at the determined range were evaluated using a CCRD based on TPC and antioxidant activity. The RSM using CCRD consists of a factorial design axial point and replicates at a central point. As shown in , the independent variables in this study were time, temperature and pomace to water ratio. Previous work on the effect of extraction time, extraction temperature and pomace to water ratio shows that the highest total phenolic yield was obtained within the range of 15 to 30 min, at an extraction temperature ranged from 85°C to 95°C with pomace to water ratio 0.05–0.08 (g/mL) (data unpublished).
Table 1. Independent variables and their ranges.
Tabla 1. Variables independientes y sus intervalos.
The CCRD enables an experimental design that addresses these independent variables at different levels as seen in . The number of experiments (N) was calculated from Equation (1):
The response variables were TPC, DPPH, FRAP and ABTS (). The data were fitted to a second order polynomial regression model, expressed by Equation (2):
Table 2. Response surface design and corresponding response values for extraction with X1 = Temperature (°C), X2 = Time (min) and X3 = Pomace to water ratio. Total phenolic content (TPC) in GAE, μg/g fresh pomace, DPPH, FRAP and ABTS assay (Antioxidant activity) in TE, μg/g fresh pomace.
Tabla 2. Diseño de respuesta de la superficie y valores correspondientes de respuesta en la extracción con X1 = Temperatura (°C), X2 = Tiempo (min) y X3 = Ratio de agua en el orujo. Total de contenido fenólico (TPC) en GAE, μg g−1 orujo fresco, ensayo DPPH, FRAP y ABTS (Actividad antioxidante) en TE, μg g−1 orujo fresco.
The experimental runs were coded and randomised to minimise the effects of unexpected variability or bias in the observed responses.
The quality of the fitted model was expressed by the coefficient of determination R2 value and the statistical significance was determined by an F-test (p < 0.05).
3. Results and discussion
3.1. Fitting of the models
The extraction procedures conducted based on the set parameters resulted in the TPC ranging between 832.5 and 1257.6 µg GAE/g of pomace. The antioxidant activity, the values ranged from 1006.7 to 1654.3 µg TE/g of pomace (DPPH), from 895.6 to 1452.6 µg TE/g of pomace (FRAP) and from 1592.9 to 2416.5 µg TE/g of pomace (ABTS) ().
The experimental data were fitted into the second order polynomial model and the regressions of the obtained equations were evaluated from variance analysis using ANOVA (). The models were statistically significant with p values were lower than 0.05 at 95% confidence, while the lack of fit values were not significant. The values of R2 > 0.85 indicated a good fit of the models for responses, which is also shown by the lower residue values.
Table 3. Regression coefficients of significant terms, the coefficients if determination (R2 value) and lack of fit values of the model.
Tabla 3. Coeficientes en regresión de términos significativos, los coeficientes según determinación (valor R2) y carencia de valores de ajuste del modelo.
3.2. Extraction parameters and model responses
The linear term of the extraction time shows significant influence on the polynomial model for the responses of all assays. Since the values of the regression coefficients for the extraction time were high, they indicate that extraction time had a major influence in all four models, especially in the ABTS model. The positive values show that an increase of extraction time tends to increase the TPC and antioxidant activity extracted from the pomace (Seker & Tercan, Citation2012).
The linear term of the extraction temperature did not seem to significantly affect the responses from all models, however in the quadratic term, extraction temperature showed significant effect on the responses. This is likely due to the range of the extraction temperature which was pre-selected based on the preliminary experiment, and had already been in the optimum range, therefore changes within this range did not significantly affect the extraction results.
The linear term of the pomace to water ratio was significant for all responses, except for the FRAP assay, with negative values. This indicates that an increase in this parameter has a tendency to decrease the TPC and antioxidant activity extracted from the pomace (). The difference in results between DPPH and ABTS assays and FRAP assay may be due to the different chemistry in the assays. The relationships between parameters for every assay can be seen in the contour plots presented in .
3.3. Validation of the models and comparison with methanol extracts
The optimal conditions predicted by the models and three other sets of independent variables were tested to confirm their validity as well as the robustness of the extraction method, in terms of reliability and reproducibility. According to the results (), the proposed models and method are reliable and reproducible with relatively low values of standard deviations and variation coefficients.
Table 4. Comparison between predicted results and real experimental results for the optimum conditions (OP) and three corroborative points (CP).
Tabla 4. Comparación entre los resultados previstos y los resultados reales del experimento para unas condiciones óptimas (OP) y tres puntos corroborativos (CP).
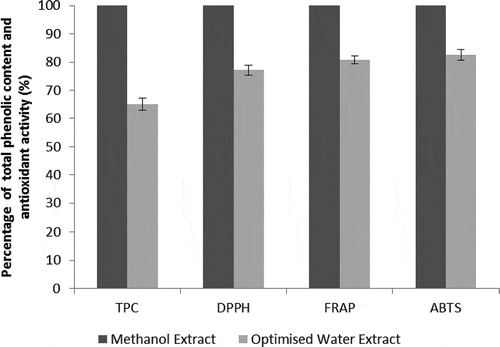
As a comparison to extraction using an organic solvent, methanol extract of the pomace was also evaluated. The TPC of the methanol extract was 722.4 mg GAE/100 g DW and its antioxidant activity was 796.9 mg TE/100 g DW, 662.7 mg TE/100 g DW and 1096.5 mg TE/100 g DW, as measured by DPPH, FRAP and ABTS assays, respectively. A study by Yan et al. (Citation2010) measured a TPC of 870 mg GAE/100 g DW and antioxidant activity of 696 mg TE/100 g DW (using DPPH assay) from methanol extract. As seen in , when compared with the methanol extract, aqueous extract with the optimised parameters contained less phenolic compounds and exhibited lower antioxidant activity. These discrepancies were expected since phenolic extraction depends on the solvent polarity. Phenolic compounds tend to have higher solubility in the less polar solvent (methanol) than water (Alo, Anyim, Igwe, Elom, & Uchenna, Citation2012).
Figure 2. Comparison between the total phenolic content and antioxidant activity (DPPH, FRAP and ABTS) of the methanol extracts and water extracts with optimum extraction parameters.
Figura 2. Comparación entre el total de contenido fenólico y la actividad antioxidante (DPPH, FRAP y ABTS) de los extractos de metanol y los extractos de agua con parámetros óptimos de extracción.
Chaovanalikit et al. (Citation2012) conducted another study to replace the use of methanol with acetone and ethanol to extract phenolics from apple pomace. They also found that even at the optimum extraction conditions, the acetone and ethanol extracts had lower antioxidant activity compared to extracts obtained by using methanol, 436 mg TE/100 g DW and 444 mg TE/100 g DW respectively (using DPPH). These values were lower when compared to the antioxidant activity of the optimum water extraction in this study (614.6 mg TE/100 g DW, measured by DPPH). This indicates that extraction using water is as good, if not better, than using acetone and ethanol. Furthermore, the use of water in place of organic solvent, even those which are considered GRAS (generally recognised as safe) such as ethanol, eliminates the need for solvent removal from the extract.
3.4. Correlation between the TPC and antioxidant activity
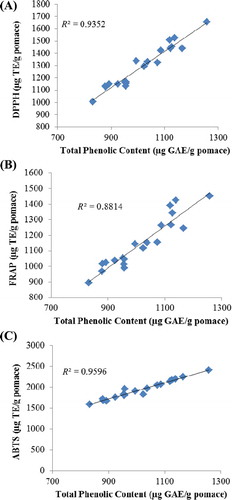
The contribution of phenolics to the total antioxidant activity can be evaluated from the correlation between the TPC and the measured antioxidant activity (Chirinos et al., Citation2008; Kwok, Hu, Durance, & Kitts, Citation2004; Wang & Xu, Citation2007). The high values of linear correlation coefficients (TPC × DPPH = 0.93, TPC × FRAP = 0.88 and TPC × ABTS = 0.96) indicate that the phenolics were indeed the major contributor of the antioxidant activity (). Based on the R2 values, DPPH and ABTS assays appeared to be the most suitable assays to measure antioxidant activity in apple samples, including pomace. Harwood and Moody (Citation1989) and Wang and Xu (Citation2007) also found a high correlation between TPC and antioxidant activity measured by DPPH assay.
4. Conclusion
The optimisation of phenolics extraction from apple pomace using only water showed a high feasibility of replacing the use of organic solvents. Extraction time was found to be a significant parameter with major influence on the TPC and antioxidant activity of the extract (DPPH, FRAP and ABTS). The pomace to water ratio was also significant for TPC and antioxidant activity (DPPH and ABTS). Extraction temperature was found to be insignificant in this range (85–95°C). The optimum extraction parameters found in this study were 30 min extraction time, 85°C extraction temperature and 0.05 pomace to water ratio. The verification of these extraction parameters was performed, along with three other corroborative sets of parameters, to ensure the validity of the models.
References
- Ajila, C. M., Brar, S. K., Verma, M., Tyagi, R. D., & Valéro, J. R. (2011). Solid-state fermentation of apple pomace using phanerocheate chrysosporium – Liberation and extraction of phenolic antioxidants. Food Chemistry, 126(3), 1071–1080. doi:10.1016/j.foodchem.2010.11.129
- Alo, M. N., Anyim, C., Igwe, J. C., Elom, M., & Uchenna, D. S. (2012). Antibacterial activity of water, ethanol and methanol extracts of Ocimum gratissimum, Vernonia amygdalina and Aframomum melegueta. Advances in Applied Science Research, 3(2), 844–848.
- Appledale Processors Co-op. (2012). Personal communication.
- Barth, S. W., Fähndrich, C., Bub, A., Dietrich, H., Watzl, B., Will, F., & Rechkemmer, G. (2005). Cloudy apple juice decreases DNA damage, hyperproliferation and aberrant crypt foci development in the distal colon of DMH-initiated rats. Carcinogenesis, 26(8), 1414–1421. doi:10.1093/carcin/bgi082
- Bazhal, M., Lebovka, N., & Vorobiev, E. (2003). Optimisation of pulsed electric field strength for electroplasmolysis of vegetable tissues. Biosystems Engineering, 86(3), 339–345. doi:10.1016/S1537-5110(03)00139-9
- Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76. doi:10.1006/abio.1996.0292
- Bhushan, S., Kalia, K., Sharma, M., Singh, B., & Ahuja, P. S. (2008). Processing of apple pomace for bioactive molecules. Critical Reviews in Biotechnology, 28(4), 285–296. doi:10.1080/07388550802368895
- Boyer, J., & Liu, R. H. (2004). Apple phytochemicals and their health benefits. Nutrition Journal, 3, 1–15. doi:10.1186/1475-2891-3-5
- Brand-Williams, W., Cuvelier, M., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft und Technologie/Food Science and Technology, 28, 25–30. doi:10.1016/S0023-6438(95)80008-5
- Çam, M., & Aaby, K. (2010). Optimization of extraction of apple pomace phenolics with water by response surface methodology. Journal of Agricultural and Food Chemistry, 58(16), 9103–9111. doi:10.1021/jf1015494
- Candrawinata, V. I., Blades, B. L., Golding, J. B., Stathopoulos, C. E., & Roach, P. D. (2012). Effect of clarification on the polyphenolic compound content and antioxidant activity of commercial apple juices. International Food Research Journal, 19(3), 1055–1061.
- Carson, K. J., Collins, J. L., & Penfield, M. P. (1994). Unrefined, dried apple pomace as a potential food ingredient. Journal of Food Science, 59(6), 1213–1215. doi:10.1111/j.1365-2621.1994.tb14679.x
- Cetkovic, G., Canadanovicbrunet, J., Djilas, S., Savatovic, S., Mandic, A., & Tumbas, V. (2008). Assessment of polyphenolic content and in vitro antiradical characteristics of apple pomace. Food Chemistry, 109(2), 340–347. doi:10.1016/j.foodchem.2007.12.046
- Chaovanalikit, A., Mingmuang, A., Kitbunluewit, T., Choldumrongkool, N., Sondee, J., & Chupratum, S. (2012). Anthocyanin and total phenolics content of mangosteen and effect of processing on the quality of mangosteen products. International Food Research Journal, 19(3), 1047–1053.
- Chirinos, R., Campos, D., Costaa, N., Arbizuc, C., Pedreschi, R., & Larondelle, Y. (2008). Phenolic profiles of andean mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers: Identification by HPLC-DAD and evaluation of their antioxidant activity. Food Chemistry, 106, 1285–1298. doi:10.1016/j.foodchem.2007.07.024
- Clifford, M. N. (2004). Diet-derived phenols in plasma and tissues and their implications for health. Planta Medica, 70(12), 1103–1114. doi:10.1055/s-2004-835835
- Corrales, M., Toepfl, S., Butz, P., Knorr, D., & Tauscher, B. (2008). Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innovative Food Science and Emerging Technologies, 9(1), 85–91. doi:10.1016/j.ifset.2007.06.002
- Cutler, G. J., Nettleton, J. A., Ross, J. A., Harnack, L. J., Jacobs Jr, D. R., Scrafford, C. G. … Robien, K. (2008). Dietary flavonoid intake and risk of cancer in postmenopausal women: The Iowa Women’s Health Study. International Journal of Cancer, 123(3), 664–671. doi:10.1002/ijc.23564
- Elez-Martínez, P., Soliva-Fortuny, R., & Martín-Belloso, O. (2009). Impact of high-intensity pulsed electric fields on bioactive compounds in Mediterranean plant-based foods. Natural Product Communications, 4(5), 741–746.
- Fabian, F. M. (2012). Application of response surface methodology and central composite design for 5P12-RANTES expression in the Pichia pastoris system. Nebraska: University of Nebraska-Lincoln.
- Folin, O., & Ciocalteu, V. (1927). On tyrosine and tryptophane determinations in proteins. The Journal of Biological Chemistry, 73(2), 627–650.
- Ghosalkar, A., Sahai, V., & Srivastava, A. (2008). Optimization of chemically defined medium for recombinant Pichia pastoris for biomass production. Bioresource Technology, 99(16), 7906–7910. doi:10.1016/j.biortech.2008.01.059
- Golding, J. B., McGlasson, W. B., Wyllie, S. G., & Leach, D. N. (2001). Fate of apple peel phenolics during cool storage. Journal of Agricultural and Food Chemistry, 49(5), 2283–2289. doi:10.1021/jf0015266
- Guyot, S., Marnet, N., Sanoner, P., & Drilleau, J.-F. (2003). Variability of the polyphenolic composition of cider apple (Malus domestica) fruits and juices. Journal of Agricultural and Food Chemistry, 51(21), 6240–6247. doi:10.1021/jf0301798
- Harwood, L. M., & Moody, C. J. (1989). Experimental organic chemistry: Principles and practice (Illustrated ed.). New York: Wiley-Blackwell.
- Hayat, K., Zhang, X., Chen, H., Xia, S., Jia, C., & Zhong, F. (2010). Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Separation and Purification Technology, 73(3), 371–376. doi:10.1016/j.seppur.2010.04.026
- Hirun, S., & Roach, P. D. (2011). An improved solvent extraction method for the analysis of catechins and caffeine in green tea. Journal of Food and Nutrition Research, 50(3), 160–166.
- Hollman, P. C. H., & Katan, M. B. (1999). Dietary flavonoids: Intake, health effects and bioavailability. Food and Chemical Toxicology, 37(9–10), 937–942. doi:10.1016/S0278-6915(99)00079-4
- Jedrychowski, W., & Maugeri, U. (2009). An apple a day may hold colorectal cancer at bay: Recent evidence from a case-control study. Reviews on Environmental Health, 24(1), 59–74. doi:10.1515/REVEH.2009.24.1.59
- Jung, M., Triebel, S., Anke, T., Richling, E., Erkel, G., & Schrenk, D. (2009). Influence of apple polyphenols on inflammatory gene expression. Molecular Nutrition and Food Research, 53(10), 1263–1280. doi:10.1002/mnfr.200800575
- Kwok, B. H. L., Hu, C., Durance, T., & Kitts, D. D. (2004). Dehydration techniques affect phytochemical contents and free radical scavenging activities of saskatoon berries (Amelanchier alnifolia Nutt.). Journal of Food Science, 69, 122–126.
- Lattanzio, V., Lattanzio, V. M. T. & Cardinali, A. (2006). Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In: Imperato, F. (ed.) Phytochemistry: Advances in Research. Kerala, India: Research Signpost.
- Linskens, H. F., & Jackson, J. F. (1999). Analysis of plant waste materials. New York, Philadelphia: Springer.
- Liu, R. H., Liu, J., & Chen, B. (2005). Apples prevent mammary tumors in rats. Journal of Agricultural and Food Chemistry, 53(6), 2341–2343. doi:10.1021/jf058010c
- Mayya, K., Bhattacharyya, A., & Argillier, J.-F. (2003). Micro-encapsulation by complex coacervation: Influence of surfactant. Polymer International, 52, 644–647. doi:10.1002/pi.1125
- McCann, M. J., Gill, C. I. R., O’ Brien, G., Rao, J. R., McRoberts, W. C., Hughes, P., … Rowland, I. R. (2007). Anti-cancer properties of phenolics from apple waste on colon carcinogenesis in vitro. Food and Chemical Toxicology, 45(7), 1224–1230. doi:10.1016/j.fct.2007.01.003
- Reis, S. F., Rai, D. K., & Abu-Ghannam, N. (2012). Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chemistry, 135(3), 1991–1998. doi:10.1016/j.foodchem.2012.06.068
- Roupas, P., & Noakes, M. (2010). Apples, their antioxidants and benefits to human health (pp. 1–56). Adelaide, Australia: Commonwealth Scientific and Industrial Research Organisation (CSIRO).
- Scalbert, A., Manach, C., Morand, C., Rémésy, C., & Jiménez, L. (2005). Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition, 45(4), 287–306. doi:10.1080/1040869059096
- Schaefer, S., Baum, M., Eisenbrand, G., Dietrich, H., Will, F., & Janzowski, C. (2006). Polyphenolic apple juice extracts and their major constituents reduce oxidative damage in human colon cell lines. Molecular Nutrition and Food Research, 50(1), 24–33. doi:10.1002/mnfr.200500136
- Seker, M., & Tercan, S. (2012). Kinetics of polyphenol losses and antioxidant activity of extracts from olive cake during evaporation. International Journal of Food Properties, 15(2), 438–449. doi:10.1080/10942912.2010.487968
- Sembries, S., Dongowski, G., Jacobasch, G., Mehrländer, K., Will, F., & Dietrich, H. (2003). Effects of dietary fibre-rich juice colloids from apple pomace extraction juices on intestinal fermentation products and microbiota in rats. British Journal of Nutrition, 90(3), 607–615. doi:10.1079/BJN2003925
- Sesso, H. D., Gaziano, J. M., Liu, S., & Buring, J. E. (2003). Flavonoid intake and the risk of cardiovascular disease in women. American Journal of Clinical Nutrition, 77(6), 1400–1408.
- Soler, C., Soriano, J. M., & Mañes, J. (2009). Apple-products phytochemicals and processing: A review. Natural Product Communications, 4(5), 659–670.
- Suárez, B., Álvarez, Á. L., García, Y. D., Barrio, G. D., Lobo, A. P., & Parra, F. (2010). Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chemistry, 120, 339–342. doi:10.1016/j.foodchem.2009.09.073
- Sun, J., Chu, Y.-F., Wu, X., & Liu, R. H. (2002). Antioxidant and antiproliferative activities of common fruits. Journal of Agricultural and Food Chemistry, 50(25), 7449–7454. doi:10.1021/jf0207530
- Swain, T., & Hillis, W. E. (1959). The phenolic constituents of Prunus domestica—The quantitative analysis of phenolic constituents. Journal of the Science of Food and Agriculture, 10(1), 63–68. doi:10.1002/jsfa.2740100110
- Thaipong, K., Boonprakob, U., Crosby, K., Cisneros-Zevallos, L., & Byrne, D. H. (2006). Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis, 19, 669–675. doi:10.1016/j.jfca.2006.01.003
- Van Der Sluis, A. A., Dekker, M., Skrede, G., & Jongen, W. M. (2002). Activity and concentration of polyphenolic antioxidants in apple juice. 1. Effect of existing production methods. Journal of Agricultural and Food Chemistry, 50(25), 7211–7219. doi:10.1021/jf020115h
- Van Der Sluis, A. A., Dekker, M., Skrede, G., & Jongen, W. M. (2004). Activity and concentration of polyphenolic antioxidants in apple juice. 2. Effect of novel production methods. Journal of Agricultural and Food Chemistry, 52(10), 2840–2848. doi:10.1021/jf0306800
- Wang, W.-D., & Xu, S.-Y. (2007). Degradation kinetics of anthocyanins in blackberry juice and concentrate. Journal of Food Engineering, 82, 271–275. doi:10.1016/j.jfoodeng.2007.01.018
- Williams, B. A. (1987). A waste utilization process for apple pomace. Brisbane, Australia: University of Queensland.
- Yan, W.-Q., Zhang, M., Huang, L., Tang, J., Mujumdar, A. S., & Sun, J.-C. (2010). Studies on different combined microwave drying of carrot pieces. International Journal of Food Science and Technology, 45, 2141–2148. doi:10.1111/j.1365-2621.2010.02380.x