ABSTRACT
The study examined the effects of pre-slaughter gas stunning and slaughter without stunning on meat quality and skeletal muscle proteome of broiler chickens. Fifty Cobb broiler chickens were randomly assigned to either a neck cut without pre-slaughter stunning (Halal slaughter) or pre-slaughter gas stunning followed by a neck cut. Samples of Pectoralis major muscle at 7 min, 4 h and 24 h postmortem were analyzed for pH, shear force, color, drip and cooking losses. Proteome profile of the 7 min samples was examined by two-dimensional polyacrylamide gel electrophoresis. Birds subjected to Halal slaughter had higher (P < 0.05) redness than those gas stunned at 4 and 24 h postmortem. Gas-stunned birds had lower (P < 0.05) muscle pH and shear force and higher (P < 0.05) drip and cooking losses compared with those subjected to Halal slaughter throughout postmortem storage. Gas stunning up-regulated (P < 0.05) the expression of beta-enolase, pyruvate kinase and creatine kinase compared with Halal slaughter. Results indicate that pre-slaughter gas stunning hastened postmortem energy metabolism and had detrimental effects on the water holding capacity and redness of broiler breast muscles.
RÉSUMÉ
Este estudio examinó los efectos de la matanza con previo aturdimiento por gas y la matanza sin aturdimiento en la calidad de la carne y el proteoma del músculo esquelético en pollos de engorde. Cincuenta pollos de engorde de Cobb fueron asignados de forma aleatoria para cortarles el cuello sin previo aturdimiento (matanza Halal) o con aturdimiento con gas previo a la matanza antes de cortarles el cuello. Se analizaron las muestras del músculo pectoral mayor a 7 min, 4 h y 24 h postmortem para el pH, la fuerza cortante, el color, la pérdida por goteo y de volumen en el cocinado. Se examinó el perfil del proteoma de las muestras de 7 min mediante dos electroforesis en gel poliacrilamida dimensionales. Las aves que estuvieron sujetas a matanza Halal tuvieron un color rojizo más fuerte (P < 0,05) que aquellas a las que aturdieron con gas después de 4 y 24 h postmortem. Las aves que aturdieron con gas tuvieron menor pH y fuerza cortante (P < 0,05) en el músculo y mayores (P < 0,05) pérdidas por goteo y de volumen en el cocinado en comparación con aquellas sujetas a matanza Halal mediante almacenamiento postmortem. El gas de aturdimiento reguló a la alta (P < 0,05) la expresión enolasa beta, la piruvatocinasa y la creatina quinasa en comparación con la matanza Halal. Los resultados indicaron que el aturdimiento por gas previo a la matanza aceleró el metabolismo de la energía postmortem y tuvo efectos perjudiciales en la capacidad de retención de agua y el color rojizo de los músculos de pechuga del pollo de engorde.
PALABRAS CLAVE:
1. Introduction
Slaughtering plays a crucial role in animal welfare and meat quality and safety in the production chain of livestock (Sabow et al., Citation2015a). Notwithstanding its short duration, slaughtering is a delicate step in meat production and its mishandling could render livestock production efforts useless (Farouk et al., Citation2014). Rendering birds unconscious and insensible to pain prior neck cut minimize struggling through a more accurate and speedy slaughtering process (Aberle et al., Citation2010) without adversely affecting meat quality (Bilgili, Citation1999; Huang, Huang, Wang, et al., Citation2014). The application of electrical water bath stunning is a common practice in poultry industry especially in Malaysia. In spite of the merits of electrical stunning, there are still unresolved issues relating to meat quality such as carcass downgrading due to the presence of hemorrhages and broken bones (Farouk et al., Citation2014). Gas stunning and killing have been adopted as an alternative to electrical stunning as it renders rapid unconsciousness with fewer incidences of hemorrhages and broken bones (Raj, Citation1998). Hence, the use of anoxic gases such as argon, carbon dioxide and nitrogen has been recommended for better meat quality and welfare in the Europe and USA (Gregory, Citation1994).
From animal welfare perspective, the gas-stunning system was believed to be a better alternative to electrical stunning as it is less stressful to the birds particularly when the procedures of uncrating and manual shackling are removed from the processing line (Gerritzen, Lambooij, Reimert, Stegeman, & Spruijt, Citation2004). The modified gas atmosphere induces euthanasia conditions to stimulate rapid unconsciousness, which has been classified as more humane procedure when compared with the electrical stunning method (Gerritzen et al., Citation2004). Nonetheless, there are resistance to the use of gas stunning from Muslim consumers particularly due to the uncertainty about the reversibility of the procedure, thereby casting doubt on the Halal status of such products (Farouk et al., Citation2014). In addition, inhalation of high concentration of carbon dioxide was found to be painful through unpleasant breathing sensations (Lambooij, Pieterse, Hillebrand, & Dijksterhuis, Citation1999) and triggered emotional aspects of pains (Hari, Portin, Kettenmann, Jousmäki, & Kobal, Citation1997).
Although numerous studies have investigated the impacts of gas stunning on welfare indicator and meat quality in poultry (Coenen, Lankhaar, Lowe, & McKeegan, Citation2009; McKeegan et al., Citation2007; Savenije et al., Citation2002; Xu et al., Citation2011), the relationships between gas stunning and muscle proteome in broilers are poorly documented. Proteomics offers platform for understanding the biochemical basis of meat quality in relation to postmortem muscle metabolism and pre-slaughter handlings (Amid, Samah, & Yusof, Citation2012; Morzel et al., Citation2004). Thus, the present study was carried out to examine and compare the effects of gas stun-to-kill prior to neck cut and slaughtering without stunning (Halal slaughter) on quality traits and proteome profile of breast muscles in broiler chickens.
2. Materials and methods
2.1. Animal welfare
The study was conducted according to the ethics and guidelines stipulated by the Research Policy of Universiti Putra Malaysia.
2.2. Experiment birds, slaughtering and muscle sampling
A total of 50 Cobb broilers (42 days’ old; mixed sex; 2.60 ± 0.2 kg body weight) were obtained from a commercial farm and transported to the research slaughterhouse at the Department of Animal Science, Universiti Putra Malaysia. Following a 3 h lairage, the birds were randomly allotted (n = 25 in each group) to two types of slaughtering methods. In the first group, the birds were slaughtered without stunning (Halal slaughter) as outlined in the MS1500: 2009 (Department of Standards Malaysia, Citation2009). The Halal slaughter involves subjecting the birds to a transverse neck cut by using a sharp knife severing the esophagus, trachea, jugular veins and carotid arteries. The second group were stun-to-kill under modified atmosphere containing a mixture of gases (40% CO2, 30% O2, and 30% N2) in an air-tight chamber with a dimension of 1.0 × 1.0 × 0.5 m. The choice of the gas mixture used in the current study was premised on the reports of earlier studies (McKeegan et al., Citation2007; Savenije et al., Citation2002; Xu et al., Citation2011) which espoused its ability to effect an adequate stun while optimizing animal welfare and meat quality in poultry. The gas chamber was equipped with four units of small fans placed at each corner to ensure uniform distribution and circulation of the gas mixture. Individual birds were randomly selected and placed in the chamber for a duration of 5 min. The birds were removed from the chamber and checked for the presence of any vital signs such as breathing and somatosensory reflexes to confirm death. The birds were slaughtered (as done for the Halal birds) within 10 s. In both slaughter groups, bleeding was allowed for 1 min. Scalding (60 °C for 30 s), feather removal and evisceration were carried out on both groups of birds.
The right Pectoralis major muscle was dissected from the carcass (n = 24 for each slaughter group) and divided along the long axis into three parts of approximately 40 g each. At 7 min postmortem, the first part was snap frozen in liquid nitrogen, stored at −80 °C and assigned for the proteomic analysis and pH. The two other parts were kept in the chiller (4 °C) and assigned for the determination of pH at 4 and 24 h postmortem. After the completion of each postmortem period, the samples were snap frozen in liquid nitrogen and stored at −80 °C until analysis. The left Pectoralis major muscle (n = 24 for each slaughter group) was dissected along the long axis and divided into four parts of approximately 30 g each. The first part (30 g) was labeled, vacuum packaged and stored in a 4 °C chiller for the determination of drip loss at 1, 4 and 24 h postmortem. The second part was assigned for the determination of color coordinates, cooking loss and shear force at 7 min postmortem. The two other parts were kept in the chiller (4 °C) and assigned for the determination of color, cooking loss and shear force at 4 and 24 h postmortem.
2.3. Muscle pH
Approximately 0.5 g of pulverized muscle samples (n = 25 for each slaughter group, at 7 min, 4 h and 24 h postmortem) was homogenized in 2 ml of deionized water in the presence of 5 mM sodium iodoacetate (Merck Schuchardt OHG, Germany) to prevent further glycolysis. The pH of the resultant homogenate was read using a bench pH meter (Mettler Toledo, USA).
2.4. Drip loss
Drip loss was determined as described by Sabow et al. (Citation2015b). Approximately 30 g of muscle sample (n = 25 for each slaughter group) was weighed (W1) at 7 min postmortem, vacuum packed and stored in a chiller (4 °C). At 1, 4 and 24 h postmortem, the samples were gently dabbed dry with clean tissue paper, reweighed and recorded as W2. The percentage drip loss was calculated using the following formula:
2.5. Color coordinates
The colors of muscle samples (30 g, 12 mm thickness, n = 24 for each slaughter group) were determined by measuring L* (lightness), a*(redness/greenness) and b* (yellowness/blueness) values at 7 min, 4 h and 24 h postmortem using a Color Flex spectrophotometer (Hunter Lab Reston, Reston, VA, USA). The colorimeter was calibrated against black and white reference tiles prior use. Duplicate measurements were taken for each sample and the average was used for analysis.
2.6. Cooking loss
Cooking loss was determined as described by Sabow et al. (Citation2015b). Chunked muscle samples (n = 25 for each slaughter group at each of 7 min, 4 h and 24 h postmortem) of approximately 30 g were weighed and recorded as W1, held in polyethylene bags, vacuum packaged and subsequently submerged in 80 °C water bath until the core temperature of the muscles reached 80 °C. Samples were removed from the water bath and allowed to cool down to room temperature prior reweighing and recorded as W2. The percentage cooking loss was calculated using the following formula:
2.7. Shear force analysis (Volodkevitch bite jaw)
The samples used for the cooking loss analysis were used for the shear force analysis. The samples were cut into blocks (two blocks per sample) of 10 mm (width), 10 mm (height) and 15 mm (length). The shear force values were determined using a Volodkevitch bite jaw fitted to a texture analyzer (TA-HD plus®, Stable Micro Systems, Surrey, UK). The shearing was consistently conducted perpendicular to the orientation of muscle fibers of each sample block.
2.8. Protein expression and identification
Approximately 0.5 g of initially pulverized Pectoralis major tissues were homogenized (Wiggen Hauser, Germany) in 5 ml of ice-cold extraction buffer containing 40 Mm tris, 7 M urea, 2 M thiourea, 50 mM DTT, 4% CHAPS, 0.25% Bio-Lyte® 3/10, 40% (Bio-Rad, USA) and 1 µl/ml of ProteoBlock™ protease inhibitor (Thermo Scientific, USA) for approximately 20 s and were vortexed for 5 min prior to centrifugation at 12,000 g for 15 min at 4 °C. The resultant supernatants were collected and cleaned with 2D clean up kit (BioRad, USA). Extracted protein samples were diluted in freshly prepared rehydration buffer containing 7 M urea, 2 M thiourea, 50 mM DTT, 4% CHAPS, 0.2% Bio-Lyte® 3/10, 40% (Bio-Rad, USA), and 0.0002% bromophenol blue for a final concentration of 100 µg protein in 125 µl volume. Initially thawed IPG strips (7 cm, pH 3–10) (Bio-Rad, USA) were rehydrated with the samples using a three-step focusing condition (Step 1–250 V, 15 min, rapid ramp at 20 °C; Step 2–4000 V, slow ramp, 1 h, at 20 °C; Step 3–4000 V, 10,000 Vh, rapid ramp at 20 °C) was programmed in the PROTEAN IEF cell (Bio-Rad, USA).
After completing the Isoelectric focusing (IEF), the IPG strips were then equilibrated with 2.5 ml of equilibration buffer 1 (containing 6 M urea, 2% SDS, 0.375 M tris-HCl, 20% glycerol and 2% DTT) for 10 min followed by second equilibration (containing 6 M urea, 2% SDS, 0.375 M tris-HCl, 20% glycerol, 2.5% IAA) for further 10 min with occasional and gentle shaking. Upon completion, the IPG strips were removed and separated on 10% SDS polyacrylamide gel using the Mini Protean tetra-cell electrophoresis system (Bio-Rad, USA) at running condition of 120 V for 100 min. The resultant gels were stained with coomassie blue solution overnight with subsequent destaining. Gels were viewed using a gel densitometer (GS-800 Calibrate Imaging Densitometer, Bio-Rad, USA). The images on gels were compared, normalized and subjected to background subtraction prior to spots detection and quantification using the QuantityOne software (Bio-Rad, USA). The spots analysis was carried out using the PDQuest software (Bio-Rad, USA) for the detection of proteins optical densities at two-fold differences. The whole procedure for 2D gel electrophoresis followed BioRad’s product manual with slight modification (http://www.bio-rad.com/LifeScience/pdf/Bulletin_2651.pdf).
Proteins which were differentially expressed at two-fold differences were excised, purified and sequenced using MALDI TOF/TOF analysis according to the manufacturer’s protocol (Morzel et al., Citation2004). The resultant peptide sequences were compared to the ones in the database (http://www.matrixscience.com/cgi/). The best alignment score (Mowse score) of more than 67% between experimental and theoretical protein sequences were considered significant at P < 0.05.
2.9. Statistical analysis
Data obtained for meat quality parameters were subjected to the generalized linear model (GLM) procedure of SAS (SAS, Citation2007) in which slaughter method (Halal slaughter and gas stunning), postmortem storage time (7 min, 4 h and 24 h) and the interaction between slaughter method and postmortem storage time were fitted as fixed effects in a repeated measure analysis of variance. Differences between means were determined based on Duncan multiple range test at P < 0.05. Proteomics analysis was conducted using the PDQuest software (Bio-Rad, USA).
3. Results and discussion
3.1. Muscle pH values
Muscle pH is an important predictor of meat quality. Changes in postmortem muscle pH resulted from the conversion of muscle glycogen to lactate through anaerobic glycolysis (Sabow et al., Citation2015b). Hence, the amount of glycogen at death may influence the rate and extent of anaerobic glycolysis, which would in turn determine the ultimate pH and other meat quality traits (Adeyemi & Sazili, Citation2014). As shown in , muscle pH of non-stunned birds (Halal) was consistently higher (P < 0.05) throughout postmortem storage compared with those gas stunned. The lower pH values in gas-stunned birds could be due to rapid postmortem glycolysis. High concentration of CO2 has been implicated in hypercapnic state (McKeegan et al., Citation2007) which usually resulted in major respiratory disturbance at the point of gas induction (Coenen et al., Citation2009). Similarly, Savenije et al. (Citation2002) found a higher rate of glycolysis and metabolic degradation in Ar/CO2 gas-stunned birds compared with electrically stunned birds. The mechanism for rapid glycolysis following gas stunning or killing method could be explained by the incident of brain convulsions caused by the undesirable changes in atmospheric gas composition (Raj, Citation1998; Savenije et al., Citation2002). Furthermore, the gas stunning might have resulted in a phase of discomfort which might have increased the utilization of the glycogen reserves prior to bleeding. Convulsion resulting from a mild concentration of gas stunning can cause muscle fibrillation and promote faster muscle acidification (Raj, Citation1998).
Table 1. Mean± S.E. of pH, cooking and drip losses, color and shear force of Pectoralis major muscles in broiler chickens as influenced by slaughter method and postmortem storage.
Tabla 1. Promedio± S.E del pH, la pérdida en el cocinado, la pérdida por goteo, el color y la fuerza cortante del músculo pectoral mayor en pollos de engorde influenciados por el método de matanza y el almacenamiento postmortem.
Postmortem storage influenced (P < 0.05) pH of broiler breast muscles (). Regardless of slaughter method, the pH at 7 min postmortem was higher (P < 0.05) compared with those measured on other postmortem periods. The decline in pH could be due to the postmortem acidification of muscle (Adeyemi & Sazili, Citation2014). However, in both slaughter methods, the muscle pH values did not change beyond 4 h postmortem. This suggests that a completion of rigor was attained at 4 h postmortem. This finding is consistent with those of Savenije et al. (Citation2002) who observed that the pH of breast muscle measured at 4 h postmortem was similar to those observed at 8, 24 and 48 h postmortem in broiler chickens subjected to pre-slaughter gas stunning (CO2/O2/N2 and Ar/CO2).
3.2. Drip and cooking losses
Data on cooking and drip losses are presented in . Gas-stunned birds exhibited higher (P < 0.05) cooking and drip losses than their unstunned counterparts throughout storage.
This could be due to the low pH of meat from the gas-stunned birds. A low pH resulting from rapid glycolysis increased the denaturation of myofibrillar proteins, thereby reducing water holding capacity (Adeyemi & Sazili, Citation2014; Savenije et al., Citation2002). In addition, inhalation of CO2 during gas stunning increased anoxic convulsions which resulted in rapid utilization of adenosine triphosphate (ATP) (Raj & Gregory, Citation1994; Raj, Citation1998) causing low pH and water holding capacity. Regardless of slaughter method, drip loss increased (P < 0.05) as postmortem storage continued. This observation could be due to the degradation of myofibrillar proteins by endogenous proteases, thus affecting the ability of muscle to hold water (Adeyemi & Sazili, Citation2014; Sabow et al., Citation2015b). Similar finding was observed during postmortem conditioning of broiler chickens (Huang, Huang, Wang, et al., Citation2014; Savenije et al., Citation2002).
The impact of postmortem ageing on cooking loss depends on the slaughter method. Ageing had no effect (P > 0.05) on cooking loss of breast muscle from the Halal birds. However, cooking loss in gas-stunned birds decreased (P < 0.05) over storage. This could be attributed to the postmortem muscle structural breakdown caused by the disruption of the channels through which water is lost, resulting in the formation of a ‘sponge effect’ that traps the water and prevents it from getting lost (Farouk et al., Citation2014). The present observation corroborates the findings of Sabow et al. (Citation2015b) who observed a decrease in the cooking loss of chevon as postmortem storage progressed.
3.3. Shear force values
The results for the shear force value are presented in . The application of gas stunning reduced (P < 0.05) shear force values of broiler breast muscle throughout postmortem storage. This observation could be due to the low pH induced by the gas stunning. A low pH value is often associated with a fast postmortem glycolysis which caused a greater rate of autolysis of calpain, thus accelerating the degradation of myofibrillar protein resulting in a tender meat (Huang, Huang, Yang, et al., Citation2014; O’Halloran, Troy, Buckley, & Reville, Citation1997). As found in the current study, irreversible gas stunning decreased shear force values in lambs (Linares, Bórnez, & Vergara, Citation2007; Vergara, Linares, Berruga, & Gallego, Citation2005) and poultry (Veeramuthu & Sams, Citation1993). In addition, Savenije et al. (Citation2002) reported an accelerated rigor development following gas stunning indicated by an improvement in chicken meat tenderness. Carbon dioxide is highly soluble in meat (Gill, Citation1988). Thus, through inhalation during gas stunning or killing procedure, CO2 could remain at high level in the muscle tissue (Gill, Citation1988). During cooking, CO2 binds to collagen triple helix (Mitsuda et al., Citation1977) and also accumulate at the perimysial seams (weakest point of the muscle) producing pores and fissures associated with an increase in meat tenderness (Bruce, Wolfe, Jones, & Price, Citation1996). Regardless of slaughter method, shear force decreased (P < 0.05) as postmortem ageing continued. This observation could be due to the postmortem weakening of myofibrillar proteins by endogenous proteases (Adeyemi & Sazili, Citation2014).
3.4. Color values (L*, a*, b*)
Color is a major factor influencing consumers’ acceptability of meat and it is a useful tool for assessing meat at purchase (Sabow et al., Citation2015b). The color coordinates of broiler breast muscles is shown in . Slaughter method had no effect (P > 0.05) on the L* and b* values of breast muscles of broiler chickens throughout storage. Similarly, Northcutt, Buhr, and Young (Citation1998) did not observe differences in color of breast muscle in turkey subjected to pre-slaughter electrical stun, gas stun or no stun. Slaughter method had no effect (P > 0.05) on a* value at 7 min postmortem. In contrast, gas-stunned birds had lower a* value compared with Halal birds at 4 and 24 h postmortem. This could be attributed to the low pH associated with fast postmortem glycolysis in gas-stunned birds compared with their Halal counterpart. Muscles having low pH are characterized by paleness (lower a* value) and low-water holding capacity (Huang, Huang, Wang, et al., Citation2014). Postmortem ageing had a significant impact (P < 0.05) on L*, a* and b* values of breast muscle of broiler chickens. Regardless of slaughter method, the L* value obtained at 7 min postmortem was lower than those obtained at 4 and 24 h postmortem. Similarly, the b* increased (P < 0.05) over storage. The impact of postmortem ageing on a* of broiler breast muscle depends on the slaughter method. In gas-stunned birds, the a* value observed at 7 min postmortem was higher (P < 0.05) than those observed at 4 and 24 h postmortem. Contrarily, in the Halal birds, the a* value observed at 7 min and 4 h postmortem were similar but lower than that observed at 24 h postmortem.
3.5. Muscle proteome
The two-dimensional polyacrylamide gel electrophoresis (2D PAGE) of the extracted proteins from skeletal muscles revealed an average of 128 spots regardless of slaughter methods. However, only three proteins were expressed by two-fold differences between the treatments. The proteins were identified as pyruvate kinase, creatine kinase and beta-enolase (, ). The comparable experimental and theoretical isoelectric points (pI) and molecular masses (Mw) suggest that gas-stunning method employed in this study did not alter post-translational modification (such as the pI or molecular mass) of the enzymes but changed the rate of synthesis of the enzymes.
Table 2. Mean± S.E. of differentially expressed proteins in Pectoralis major muscle of broiler chickens subjected to gas stunning or Halal slaughter.
Tabla 2. Promedio± S.E de proteínas expresadas de manera diferenciada en el músculo pectoral mayor de pollos de engorde sujetos a aturdimiento por gas o matanza Halal.
Figure 1. Differences in protein expression of Pectoralis major muscles in broiler chickens subjected to gas stunning (GS) and slaughter without prior stunning (Halal). a = beta-enolase, b = creatine kinase M type, and c = pyruvate kinase isozyme.
Figura 1. Diferencias en la expresión proteínica del músculo pectoral mayor en pollos de engorde sujetos a aturdimiento por gas (GS) y matanza sin previo aturdimiento (Halal). a = enolasa beta, b = creatina quinasa tipo M, c = isoenzima piruvatocinasa.
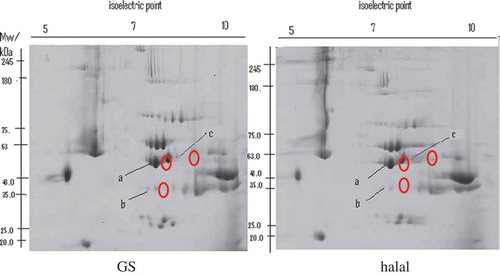
Gas stunning up-regulated (P < 0.05) the expression of beta-enolase. Beta-enolase (2-phospho-D-glycerate hydrolase) is a glycolytic enzyme which catalyzes the reversible dehydration of 2-phospho = D-glycerate (2-PG) to phosphoenolpyruvate (PEP) (Brewer, Zhu, Bidner, Meisinger, & McKeith, Citation2001). Beta-enolase encoded by ENO3 gene is one of the two forms of enolase isomers other than the alpha enolase. Beta-enolase is abundant in striated muscles particularly during the growth and developmental phase (Doherty et al., Citation2004). In a study on tumor cells, Sedoris, Thomas, and Miller (Citation2010) found increased expression of enolase protein in response to hypoxia (deprivation of oxygen supply). The authors also found that the increased enolase expression was influenced by differences in glucose levels. The increased expression of beta-enolase in gas-stunned broilers could be to their response to hypoxia and glucose metabolism. Gas stunning has been implicated in breathing discomfort (Nowak, Mueffling, & Hartung, Citation2007). This might have triggered physiological stress responses. In stress condition, broilers utilized more energy. Therefore, the lowered muscle pH values and muscle glycogen contents shown by the gas-stunned broilers could be a consequence of breathing stress.
The expression of creatine kinase M-type (G1:45382875) was higher (P < 0.05) in gas-stunned broilers compared with their Halal counterparts. Creatine kinase M-type is an enzyme found in sarcolemma and in sarcoplasmic reticulum of muscle cells where it is functionally involved in the calcium transport and ATPase activity (Rossi, Eppenberger, Volpe, Cotrufo, & Wallimann, Citation1990). It is involved in the transfer of high-energy phosphate between creatine and ATP molecule (Jia, Hollung, Therkildsen, Hildrum, & Bendixen, Citation2006) and it is found especially in the tissues that have high demand for energy such as skeletal muscle, brain and kidney. The higher expression of creatine kinase M-type observed in the gas-stunned broilers could possibly be associated with higher demand for energy during hypoxia. The finding coincides with the lower pH and higher tenderness in gas-stunned birds compared with their Halal counterparts. In line with the current finding, increased expression of creatine kinase has been associated with increased meat tenderness in cows (Bjarnadóttir et al., Citation2012; Zapata, Zerby, & Wick, Citation2009).
Gas stunning up-regulated (P < 0.05) pyruvate kinase isozyme. Pyruvate kinase is an enzyme involved in the final step of glycolytic pathway in generating the ATP. It catalyzes the removal of one phosphate molecule (transphosphorylation) from PEP to ADP (adenosine diphosphate) (Gupta & Bamezai, Citation2010). Other than muscle type (M-isozyme), pyruvate kinase also exists in different isoforms in the liver and erythrocyte. Similar to the other catalyst enzymes involved in the glycolysis metabolic pathway, the expression of pyruvate kinase is also dependent on the level of supply substrates such as PEP, fructose 1, 6 bisphosphate and ATP (Yamada & Noguchi, Citation1999). Low levels of substrate PEP and fructose 1:6 bisphosphate will switch to low production of pyruvate kinase while ATP molecules posed as negative allosteric inhibitor. Besides being regulated by concentration of substrates, the up-regulation of pyruvate kinase has also been associated with increased levels of adrenaline and insulin (Yamada & Noguchi, Citation1999). Hence, the physiological discomfort and stress by the gas stunning could be responsible for the higher expression of pyruvate kinase.
4. Conclusion
The results of the present study demonstrate that the application of the gas stunning significantly decreased the muscle pH, redness, shear force and increased cooking and drip losses of breast muscle of broiler chickens. The significantly higher expression of beta-enolase, pyruvate kinase and creatine kinase suggest that the gas stunning increased the rate of energy metabolism which could compromise meat quality in broilers.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aberle, E. D., Forrest, J. C., Gerrard, D. E., Mills, E. W., Hedrick, H. B., Judge, M. D., & Merkel, R. A. (2010). Principles of meat science (4th ed., p. 94). Dubuque, IA: Kendall/Hunt Publishing Company.
- Adeyemi, K. D., & Sazili, A. Q. (2014). Efficacy of carcass electrical stimulation in meat quality enhancement: A review. Asian-Australasian Journal of Animal Sciences, 27, 447–456. doi:10.5713/ajas.2013.13463
- Amid, A., Samah, N. A., & Yusof, F. (2012). Identification of troponin I and actin, alpha cardiac muscle 1 as potential biomarkers for hearts of electrically stimulated chickens. Proteome Science, 10, 1–8. doi:10.1186/1477-5956-10-1
- Bilgili, S. F. (1999). Recent advances in electrical stunning. Poultry Science, 78, 282–286. doi:10.1093/ps/78.2.282
- Bjarnadóttir, S. G., Hollung, K., Hoy, M., Bendixen, E., Codrea, M. C., & Veiseth-Kent, E. (2012). Changes in protein abundance between tender and tough meat from bovine longissimus thoracis muscle assessed by iTRAQ and 2DE analysis. Journal of Animal Science, 90, 2035–2043. doi:10.2527/jas.2011-4721
- Brewer, M. S., Zhu, L. G., Bidner, L. G., Meisinger, D. J., & McKeith, F. K. (2001). Measuring pork color: Effects of bloom time, muscle, pH and relationship to instrumental parameters. Meat Science, 57, 169–176. doi:10.1016/S0309-1740(00)00089-9
- Bruce, H. L., Wolfe, F. H., Jones, S. D. M., & Price, M. A. (1996). Porosity in cooked beef from controlled atmosphere packaging is caused by rapid CO2 gas evolution. Food Research International, 29, 189–193. doi:10.1016/0963-9969(96)00057-9
- Coenen, A. M. L., Lankhaar, J., Lowe, J. C., & McKeegan, D. E. (2009). Remote monitoring of electroencephalogram, electrocardiogram, and behavior during controlled atmosphere stunning in broilers: Implications for welfare. Poultry Science, 88, 10–19. doi:10.3382/ps.2008-00120
- Department of Standards Malaysia. (2009). MS1500:2009 (1st revision) Halal food-production, preparation, handling and storage-general guideline. Cyberjaya, Selangor: Department of Standards Malaysia.
- Doherty, M. K., McLean, L., Hayter, J. R., Pratt, J. M., Robertson, D. H. L., El-Shafei, A., … Beynon, R. J. (2004). The proteome of chicken skeletal muscle: Changes in soluble protein expression during growth in a layer strain. Proteomics, 4, 2082–2093. doi:10.1002/pmic.200300716
- Farouk, M. M., Al-Mazeedi, H. M., Sabow, A. B., Bekhit, A. E. D., Adeyemi, K. D., Sazili, A. Q., & Ghani, A. (2014). Halal and Kosher slaughter methods and meat quality: A review. Meat Science, 98, 505–519. doi:10.1016/j.meatsci.2014.05.021
- Gerritzen, M. A., Lambooij, B., Reimert, H., Stegeman, A., & Spruijt, B. (2004). On-farm euthanasia of broiler chickens: Effects of different gas mixtures on behavior and brain activity. Poultry Science, 83, 1294–1301. doi:10.1093/ps/83.8.1294
- Gill, C. O. (1988). The solubility of carbon dioxide in meat. Meat Science, 22, 65–71. doi:10.1016/0309-1740(88)90027-7
- Gregory, N. G. (1994). Pre-slaughter handling, stunning and slaughter. Meat Science, 36, 45–56. doi:10.1016/0309-1740(94)90032-9
- Gupta, V., & Bamezai, R. N. (2010). Human pyruvate kinase M2: A multifunctional protein. Protein Science, 19, 2031–2044. doi:10.1002/pro.505
- Hari, R., Portin, K., Kettenmann, B., Jousmäki, V., & Kobal, G. (1997). Right-hemisphere preponderance of responses to painful CO2 stimulation of the human nasal mucosa. Pain, 72, 145–151. doi:10.1016/S0304-3959(97)00023-7
- Huang, J. C., Huang, M., Wang, P., Zhao, L., Xu, X., Zhou, G., & Sun, J. (2014). Effects of physical restraint and electrical stunning on plasma corticosterone, postmortem metabolism, and quality of broiler breast muscle. Journal of Animal Science, 92, 5749–5756. doi:10.2527/jas.2014-8195
- Huang, J. C., Huang, M., Yang, J., Wang, P., Xu, X. L., & Zhou, G. H. (2014). The effects of electrical stunning methods on broiler meat quality: Effect on stress, glycolysis, water distribution, and myofibrillar ultrastructures. Poultry Science, 93, 2087–2095. doi:10.3382/ps.2013-03248
- Jia, X., Hollung, K., Therkildsen, M., Hildrum, K. I., & Bendixen, E. (2006). Proteome analysis of early post-mortem changes in two bovine muscle types: M. longissimus dorsi and M. semitendinosus. Proteomics, 6, 936–944. doi:10.1002/pmic.200500249
- Lambooij, E., Pieterse, C., Hillebrand, S. J. W., & Dijksterhuis, G. B. (1999). The effects of captive bolt and electrical stunning and restraining methods on broiler meat quality. Poultry Science, 78, 600–607. doi:10.1093/ps/78.4.600
- Linares, M. B., Bórnez, R., & Vergara, H. (2007). Effect of different stunning systems on meat quality of light lamb. Meat Science, 76, 675–681. doi:10.1016/j.meatsci.2007.02.007
- McKeegan, D. E. F., McIntyre, J. A., Demmers, T. G. M., Lowe, J. C., Wathes, C. M., van den Broek, P. L. C., … Gentle, M. J. (2007). Physiological and behavioural responses of broilers to controlled atmosphere stunning: Implications for welfare. Animal Welfare, 16, 409–426.
- Mitsuda, H., Kawai, F., Yamamoto, A., Suzuki, F., Nakajima, K., & Yasumoto, K. (1977). Identification and properties of reactive sites in protein capable of binding carbon dioxide in a gas–solid phase system. Journal of Nutritional Science and Vitaminology, 23, 145–152. doi:10.3177/jnsv.23.145
- Morzel, M., Chambon, C. M., Hamelin, M., Santé-Lhoutellier, V., Sayd, T., & Monin, G. (2004). Proteome changes during Pork meat ageing following use of two different pre-slaughter handling procedures. Meat Science, 67, 689–696. doi:10.1016/j.meatsci.2004.01.008
- Northcutt, J. K., Buhr, R. J., & Young, L. L. (1998). Influence of pre-slaughter stunning on turkey breast muscle quality. Poultry Science, 77, 487–492. doi:10.1093/ps/77.3.487
- Nowak, B., Mueffling, T., & Hartung, J. (2007). Effect of different carbon dioxide concentrations and exposure times in stunning of slaughter pigs: Impact on animal welfare and meat quality. Meat Science, 75, 290–298. doi:10.1016/j.meatsci.2006.07.014
- O’Halloran, G., Troy, D., Buckley, D., & Reville, W. (1997). The role of endogenous proteases in the tenderisation of fast glycolysing muscle. Meat Science, 47, 187–210. doi:10.1016/S0309-1740(97)00046-6
- Raj, M. (1998). Welfare during stunning and slaughter of poultry. Poultry Science, 77, 1815–1819. doi:10.1093/ps/77.12.1815
- Raj, M., & Gregory, N. G. (1994). An evaluation of humane gas stunning methods for turkeys. Veterinary Record, 135, 222–223. doi:10.1136/vr.135.10.222
- Rossi, A. M., Eppenberger, H. M., Volpe, P., Cotrufo, R., & Wallimann, T. (1990). Muscle-type MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. Journal of Biological Chemistry, 265, 5258–5266.
- Sabow, A. B., Sazili, A. Q., Zulkifli, I., Goh, Y. M., Ab Kadir, M. Z. A., Abdulla, N., … Adeyemi, K. D. (2015a). A comparison of bleeding efficiency, microbiological quality and lipid oxidation in goats subjected to conscious halal slaughter and slaughter following minimal anesthesia. Meat Science, 104, 78–84. doi:10.1016/j.meatsci.2015.02.004
- Sabow, A. B., Sazili, A. Q., Zulkifli, I., Goh, Y. M., Ab Kadir, M. Z. A., & Adeyemi, K. D. (2015b). Physico-chemical characteristics of longissimus lumborum muscle in goats subjected to halal slaughter and anesthesia (halothane) pre-slaughter. Animal Science Journal. doi:10.1111/asj.12385
- SAS. (2007). User’s guide (9.2 ed.). Cary, NC: SAS.
- Savenije, B., Schreurs, F. J., Winkelman-Goedhart, H. A., Gerritzen, M. A., Korf, J., & Lambooij, E. (2002). Effects of feed deprivation and electrical, gas, and captive needle stunning on early postmortem muscle metabolism and subsequent meat quality. Poultry Science, 81, 561–571. doi:10.1093/ps/81.4.561
- Sedoris, K. C., Thomas, S. D., & Miller, D. M. (2010). Hypoxia induces differential translation of enolase/MBP-1. BMC Cancer, 10, 157–171. doi:10.1186/1471-2407-10-157
- Veeramuthu, G. J., & Sams, A. R. (1993). The effects of carbon dioxide and electrical stunning on rigor mortis and toughness development in early-harvested broiler breast fillets. Poultry Science, 72, 147.
- Vergara, H., Linares, M. B., Berruga, M. I., & Gallego, L. (2005). Meat quality in suckling lambs: Effect of pre-slaughter handling. Meat Science, 69, 473–478. doi:10.1016/j.meatsci.2004.09.002
- Xu, L., Ji, F., Yue, H. Y., Wu, S. G., Zhang, H. J., Zhang, L., & Qi, G. H. (2011). Plasma variables, meat quality, and glycolytic potential in broilers stunned with different carbon dioxide concentrations. Poultry Science, 90, 1831–1836. doi:10.3382/ps.2010-01330
- Yamada, K., & Noguchi, T. (1999). Nutrient and hormonal regulation of pyruvate kinase gene expression. Biochemical Journal, 337, 1–11. doi:10.1042/bj3370001
- Zapata, I., Zerby, H. N., & Wick, M. (2009). Functional proteomic analysis predicts beef tenderness and the tenderness differential. Journal of Agricultural and Food Chemistry, 57, 4956–4963. doi:10.1021/jf900041j
