 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, small molecular (≤3 kDa) Coix glutelin hydrolysate (CGH) was obtained by the method of enzymatic hydrolysis and then immunomodulatory activity was evaluated in vitro and in vivo. Stimulation index of mice splenocytes was increased in a dose-dependent manner after treated with 3.125–25 μg/mL CGH. Acid phosphatase in mice peritoneal macrophages was significantly improved after stimulated with CGH at some concentration. The CGH stimulated the secretion of NO from Raw264.7 in a concentration-dependent manner and also significantly inhibited lipopolysaccharide-induced cell excessive activation at some extent. After successive administration of CGH for 21 days, the spleen index of ICR was significantly increased to 3.73 mg/g for the group administrated 800 mg/kg CGH. Malondialdehyde (MDA) assay displayed that 200 mg/kg CGH increased mice serum MDA concentration, while the variation was obsolete in the other CGH groups. All results demonstrated that CGH took essential effects on immunomodulatory activities.
RESUMEN
En este estudio, se obtuvo hidrolisato glutelina de Coix (CGH) de molécula pequeña (≤ 3 kDa) mediante el método de hidrólisis enzimática y después se evaluó la actividad sinmunomoduladora in vitro y in vivo. El índice de estimulación (SI) de los esplenocitos de ratón aumentó de forma dependiente a la dosis después ser tratados con 3,125-25 μg/mL de CGH. La fosfatasa ácida (ACP) en los macrófagos peritoneales de ratón mejoró significativamente después de que se estimulara con CGH en algunas concentraciones. El CGH estimuló la secreción de NO a partir de Raw264.7 de forma dependiente de la concentración e inhibió de forma significativa la activación excesiva de células inducidas con LPS en cierta medida. Después de una administración sucesiva de CGH durante 21 días, el índice esplénico de ICR aumentó significativamente a 3,73 mg/g para el grupo al cual se había administrado 800 mg/kg de CGH. El ensayo de malondialdehído (MDA) mostró que los 200 mg/kg de CGH hicieron aumentar la concentración de MDA del suero de ratón, mientras que la variación fue obsoleta en los otros grupos de CGH. Todos los resultados demostraron que CGH obtuvo efectos esenciales en las actividades inmunomoduladoras.
1. Introduction
The seed of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf), a traditional Chinese herb widely planted in Hebei, Fujian Province, and other places of China, is considered a healthy food supplement. In Chinese medicine, Coix seed is sweet and bland in flavor, slightly cold in nature, and function to dry dampness, strengthen spleen, arrest diarrhea, clear heat, and drain pus. Coix seed traditionally has long been used for treatments of warts, chapped skin, rheumatism, neuralgia, and inflammatory diseases (Li, Citation1998) in China. Modern medicine research has shown that Coix seed has definite antitumor effect on several types of tumor cell and enhances antitumor immunity (Kuo, Chen, & Chiang, Citation2012; Pan et al., Citation2012). It has abundant nutritional components including proteins, lipids, carbohydrates, inorganic salts, and a small quantity of vitamins (Nagao, Otsuka, Kohda, Sato, & Yamasaki, Citation1985). Extensive research had revealed that Coix seed and its extracts possessed a wide spectrum of bioactivities. For example, Kanglaite, one widely applied injection of Coix seed oil, exhibits satisfactory anticancer and immunomodulatory properties (Huang, Qin, & Lu, Citation2014). Injectable Semen coicis, which was extracted from Coix seed using advanced pharmaceutical technology, could significantly increase the apoptosis rate of hepatocellular carcinoma HepG2 cell and the expression of caspase-8 (Lu, Zhang, Jia, Wu, & Lu, Citation2011). It had reported that Coix seed oil obtained by supercritical CO2 extraction significantly decreased the accumulation of epididymal adipose and low-density lipoprotein cholesterol (LDL-C) concentrations and increased the total antioxidant capacity in hyperlipidemia rats (Yu, Gao, Zeng, & Liu, Citation2011). Some researchers found that bioactivity compounds extracted from Adlay seed manifested inhibitory effects on melanin production and cellular oxygen stress in B16F10 melanoma cells (Huang, Hsieh, Niu, & Chang, Citation2014). The ethyl acetate fraction of an ethanol extract of C. lachryma-jobi, which included gallicacid, chlorogenic acid, caffeic acid, and ferulic acid, decreased adipocyte differentiation and increased glucose uptake in 3T3-L1 cells (Ha et al., Citation2010). Taking Coix seeds tablets was beneficial to increase the percentages of CD16+, CD57+ (mature, most active natural killer [NK] cells) and CD3+, CD56+ (major histocompatibility complex-no restricted cytotoxic T cells) from healthy volunteers (Hidaka, Kaneda, Amino, & Miyai, Citation1992). The inflammation of lipopolysaccharide (LPS)-induced in RAW 264.7 macrophages was effectively inhibited by Coix seed hull extracts (Huang, Chung, Kuo, Lin, & Chiang, Citation2009). In clinic, injectable Coix seed oil has great advantages in improving immune system function of an organism manifested as Lung-Qi deficiency and immunologic inadequacy (Wang, Ma, & Wang, Citation1999).
Peptides released from food protein by enzymatic hydrolysis had been reported to manifest various bioactivities such as immune regulation (Gauthier, Pouliot, & Saint-Sauveur, Citation2006), antiinflammatory (Mukhopadhya et al., Citation2014), antihypertensive (Hernández-Ledesma, del Mar Contreras, & Recio, Citation2011), antioxidant (Kleekayai et al., Citation2015), and antimalarial (Lhouvum, Bhuyar, & Trivedi, Citation2015). Amongst them, peptides with immunomodulatory ability had been focused on by more and more researchers. Casein-derived peptides QEPVL and QEPV could significantly increase mice lymphocyte proliferation rate and cAMP levels in vitro and also inhibited LPS-induced inflammation by regulating nitric oxide release and the production of the many cytokines in vivo (Zhou et al., Citation2014). Novel peptides isolated and characterized from the chicken bursa of Fabricius promoted the development of mice B cell and enhanced the production of specific Avian Influenza Virus antibody and cytokines, T-cell immunophenotyping at reachable concentrations (Liu et al., Citation2013). Shark-derived protein hydrolysate digested by trypsin and type II α-chymotrypsin-enhanced mice gut barrier function by upregulating IgA-producing cells and the protein level of intestinal interleukin-6 and tumor necrosis factor-α and downregulating of uncontrolled-inflammatory reaction induced by Escherichia coli infection via increasing transforming growth factor-β and interleukin-10 (Mallet et al., Citation2014). According to our previous research, Coix seed was rich in protein (14.17%) (Wang, Yuan, Zhang, & Qiao, Citation2012); therefore, it is worth exploring immune peptides or protein from Coix seed.
In our previous study, Coix glutelin hydrolysate (CGH) and Coix prolamin hydrolysate showed ideal angiotensin-converting enzyme (ACE) inhibitory activity in vitro (Yuan et al., Citation2014a, Citation2014b). It is reported that NK cell activity of healthy human was increased significantly after wheat gluten hydrolysate intake (Horiguchi, Horiguchi, & Suzuki, Citation2005). Garcia and Villarroel (Citation2009) found that corn gluten had a positive influence on macrophage phagocytosis in tilapia (Oreochromis niloticus L.), and the number of bacteria that was phagocytosed by macrophages was significantly increased at 4-h postinfection. The nucleotide and amino acid sequence of Coix exhibited high homology with wheat and corn (Ary, Shewry, & Richardson, Citation1988; Vettore et al., Citation1998). Thus, the function of CGH on immune system is worth to be investigated. In this study, we evaluated the immunomodulating effects of CGH in cell and mice level.
2. Materials and methods
2.1. Plant materials and reagents
The seeds of adlay (C. lachryma-jobi L. var. ma-yuen Stapf) were purchased from Tongrentang, Beijing, China.
Dimethyl sulfoxide (DMSO), LPS, Pepsin, Concanavalin A (Con A), Erythrocyte Lysate, Penicillin, Streptomycin, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Co (St. Louis, MO, USA). Roswell Park Memorial Institute (RPMI) 1640, Dulbecco’s modification of Eagle medium (DMEM), and fetal bovine serum (FBS) were the product of Gibco (Grand Island, NY, USA). The assay kits including nitric oxide (NO) assay kit (No. A013-2), acid phosphatase (ACP) assay kit (No. A060-2), and malondialdehyde (MDA) assay kit (No. A003-1) were all procured from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). BCA Protein Assay Kit was obtained from Biomiga (No. PW0104). All other chemicals and reagents were analytical grade.
2.2. Animals and cell line
ICR and BALB/c mice were provided by Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). All animals were housed under the condition of 22 ± 2°C, 50% ± 5% relative humidity, and a 12-h light–dark cycle in Barrier Environment. Commercial pellet diet and tap water were supplied ad libitum. All animal experiments were performed in strict accordance with the PR China legislation on use and care of laboratory animals. Raw264.7 cell line was a gift from Dr. Wang Rufeng of Beijing University of Chinese Medicine.
2.3. Methods
2.3.1. Preparation of small molecular (≤3 kDa) CGH
The preparation of small molecular weight (≤3 kDa) CGH was performed according to the previously described method (Yuan et al., Citation2014b). In brief, Coix seeds were smashed with grinder and then defatted using cool petroleum ether. Afterward, Coix glutelin solution was obtained by the method of sequential extraction (Wallace, Lopes, Paiva, & Larkins, Citation1990) and then dialyzed at 4°C using a 3500 molecular weight cutoff cellulose membrane (Type MD34-3.5, VISKASE® Companies, Inc., Chicago, USA) against 20 volumes of deionized water, for 24 h with water changes every 4 h. The lyophilized Coix glutelin was suspended in distilled water, and the mixture was adjusted to the optimum pH 2.0 for pepsin. The optimized hydrolysis conditions were substrate concentration of 2%, enzyme-to-substrate ratio of 1:10, and hydrolysis duration for 48 h at 37°C. After enzymatic reaction, the slurry was centrifuged at 2250g for 20 min at 4°C and the soluble aqueous fraction was decanted and passed through a 3000 molecular weight cutoff ultrafiltration membrane (Type PES3-1812, Dalian Yidong Membrane Engineering Equipment Co. Ltd., Dalian, China). The CGH (≤3 kDa) was collected and freeze dried for further analysis.
2.3.2. Splenocyte proliferation assay (in vitro)
Spleen lymphocyte proliferation was performed according to the method as Sugiura, Sugiura, Ueya, Ueya, and Mirbod described (Citation2000). BALB/c mice (Female, 6–8 weeks old, and SPF grade) were sacrificed by cervical dislocation. The spleens were collected aseptically, placed on a sterile petri dish containing 3 mL of RPMI-1640, and pressed in the medium on sterile steel sieve. The cell suspension was collected and treated with erythrocyte lysis buffer. The resulting cells suspension was washed twice with the medium and centrifuged (90g, 4°C) for 5 min. Splenocytes were resuspended in RPMI-1640 complete medium (10% FBS, 100 U/mL penicillin, and 10 μg/mL streptomycin). The cell count was performed using the trypan blue exclusion method and the cell concentration was adjusted to 2.4 × 106 cells/mL.
An aliquot of 100-μL splenocyte suspensions was seeded into the well of 96-well flat bottom microtiter plate; thereafter, CGH (final concentration 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL) were added giving a final volume of 150 μL. RPMI-1640 medium and Con A (10 μg/mL) were used for the negative control and positive control, separately. The MTT-based colorimetric assay was performed (Chen et al., Citation2010). Plates were incubated for 68 h at 37°C in a 5% CO2 incubator, then 20 μL MTT (1 mg/mL) was added to each well and incubated for 4 h under the same conditions. At the end of the culture, the supernatant was discarded and DMSO (150 μL/well) was added to each well. The absorbance at 570 nm was measured. The stimulation index (SI) was calculated according to the following formula:
where ODN = optical density value of negative control, ODT = optical density value of the test sample.
2.3.3. Determination of ACP activity in peritoneal macrophages
Peritoneal macrophages were prepared following the reported method (El-Mahmoudy et al., Citation2002) with some modification. That is, female ICR mice (25 ± 2 g, SPF grade) sacrificed by cervical dislocation were completely wet with 75% alcohol for 60–90 s and mounted on the styrofoam block with the abdomen facing up. After 5 mL of cold DMEM (3% FBS, 100 U/mL penicillin, and 10 μg/mL streptomycin) being intraperitoneally injected, the abdomen was gently massaged for 5 min. The medium was then drawn back and centrifuged (90g, 5 min, and 4°C). The peritoneal cells were resuspended in DMEM (10% FBS, 100 U/mL penicillin, and 10 μg/mL streptomycin) and adjusted the cell concentration to 2.4 × 106 cells/mL. After the cell suspension being seeded into the 96-well tissue culture plate with 100 μL in each well, plates were incubated for 4 h at 37°C in a humid atmosphere with 5% CO2. To remove non-adherent cells, plates were washed with the medium for three times. The peritoneal macrophages were then treated with different concentrations of CGH (25, 50, 100, and 200 μg/mL). DMEM and LPS (20 μg/mL) were acted as the negative and positive control, respectively.
After incubated for 48 h, the intracellular ACP activity was measured as some researchers described (Sugiura, Inaba, Iwata, Nishida, & Tanaka, Citation1998) and made a little modification. The culture medium was gently removed and the plate was washed twice with phosphate buffer saline. When an aliquot of 100 μL of normal saline (pH = 5.5) was added into each well, macrophages were lysed by repeated freeze–thaw at ±20°C thrice. Macrophages lysates were gently collected and centrifuged (9000g, 10 min, and 4°C). The total protein concentration was then determined using BCA Protein Assay Kit. ACP activity was assayed according to the kit protocol when the optical density was measured at 520 nm. The ACP activity was evaluated as follows:
where ODB = optical density value of blank (double distilled water), ODS = optical density value of standard (phenol, 0.1 mg/mL), and ODT = optical density value of the test sample (macrophages lysates).
2.3.4. Measurement of NO in the raw264.7 culture supernatant
Raw264.7 cells were cultured in DMEM supplemented with 10% FBS and antibiotics (100 U/mL of penicillin and 100 U/mL of streptomycin) and maintained at 37°C in a humidified incubator containing 5% CO2 until logarithmic growth phase. The cells seeded in the 96-well plate at the density of 2 × 106 cells/mL with the volume of 100 μL were incubated for 24 h. The supernatant was then abandoned, and then cells were treated with CGH and LPS for 48 h. NO production in the supernatants was measured using the NO assay kit and the absorbance at 550 nm was determined. The formula of NO concentration (N value) was as follow:
where ODB = optical density value of blank (double distilled water), ODS = optical density value of standard (sodium nitrite, 20 μmol/L), and ODT = optical density value of the test sample.
2.3.5. Evaluation animal immune response by repeat oral administration
Fifty-five ICRs (male, 20 ± 2 g, and SPF grade) were divided randomly into five groups, namely, the negative control group (normal saline), the positive control group (Vc 100 mg/kg), and CGH groups of low-dose (200 mg/kg), middle-dose (400 mg/kg), and high-dose (800 mg/kg). All the mice were intragastrically administered once daily for a period of 3 weeks with the volume of 0.1 mL/10 g body weight. Symptoms and body weight were recorded daily up to 24 h after the last treatment. The blood was collected from the orbit and used for the determination of serum MDA concentration by the assay kit (Li et al., Citation2013; Zhu, Hu, Wu, & Hu, Citation2014). The MDA concentration (M value) was determined by the formula:
where ODB = optical density value of blank, ODS = optical density value of the standard (1, 1, 3, 3-tetrathoxypropane, 10 nmol/mL), and ODT = optical density value of the test sample.
Afterward, the lungs, spleen, thymus, and the liver were removed and weighted. The organ index was expressed in the relative organ weight described by Lu et al. (Citation1996).
2.3.6. Statistical analysis
Each experiment was repeated at least three times and all the data were presented as mean ± standard deviation. SAS9.2 software was adopted in our experiments for the analysis of variance.
3. Results and discussion
3.1. Effect of CGH on splenocyte proliferation of mice
Splenocyte proliferation assay was carried out to evaluate the general effect of CGH on immune cells. As shown in , the proliferative response of lymphocyte cells was significantly improved on the stimulation of Con A (10 μg/mL). Different dosage of CGH caused a significant increase of the SI compared with the blank control group. It was noteworthy that, as the concentration of CGH ranging from 3.125 to 25 μg/mL, the SI increased in a dose-dependent manner with the maximum SI of 1.13 at 25 μg/mL CGH. However, when the dosage of CGH further increased above 100 μg/mL, the cells reached plate form. These data suggested that the small molecular (≤3 kDa) CGH exerted positive effect on mice splenocyte proliferation at some extent.
Table 1. Effects of CGH on the proliferation of mice splenocytes.
Tabla 1. Efectos de CGH en la proliferación de esplenocitos de ratón.
The spleen is composed of white pulp and red pulp, in which exist various immune cells including T lymphocytes, B lymphocytes, phagocytes etc. (den Haan, Mebius, & Kraal, Citation2012). Spleen plays unique role in immunoregulation by modulating splenocyte migration and proliferation, which is different from other secondary lymphoid organs (Zhao, Liu, Guo, & Zhu, Citation2015). The increase of lymphocyte number usually indicates an effect on immune system by secretion of cytokines and the activation of accessory cells (de Oliveira Silva et al., Citation2011). Ma et al. (Citation2014) found that splenocyte proliferation stimulated by whey protein concentrate hydrolysate (WPCH) in a dose-dependent manner from 7.81 to 250 μg/mL, while the S/P (the ratio of sample optical density to positive control optical density) decreased following further higher concentration of WPCH. The tendency of stimulation effect was similar to our result. In general, CGH could induce the proliferation of murine splenocytes at the applied concentrations, which provided important insights into immunological research on Coix.
3.2. Effect of CGH on ACP activity in mice peritoneal macrophages
Our data () showed that small molecule CGH had a positive effect on ACP activity in mouse peritoneal macrophages. ACP activities of the peritoneal macrophage stimulated by different concentrations of CGH were all significantly higher than that of negative control, among which the maximum ACP activity reached 73.46 King Unit/g prot (25 μg/mL CGH). However, the effect of CGH on ACP activity was not in a dose-dependent manner. Additionally, it was worth mention that ACP activity of the positive group (20 μg/mL LPS) was significantly higher than the blank control and also significantly superior to four CGH groups (P < 0.05).
Figure 1. Effect of CGH (≤3 kDa) on ACP activity in the peritoneal macrophages.
*Different letters above the columnar diagram represented significant difference (P < 0.05).
Figura 1. Efecto de CGH (≤ 3 kDa) en la actividad ACP en los macrófagos peritoneales.
*Las distintas letras encima del diagrama de columnas representan diferencias significativas (P < 0,05).
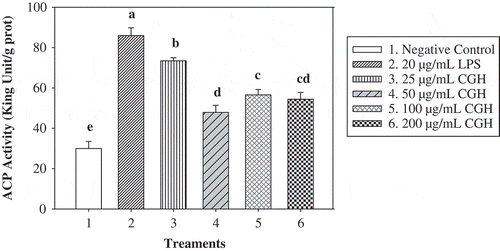
It is well known that macrophages are involved at all stages of the immune response and play essential roles in phagocytosis, antigen processing and presentation, secretion of cytokines, and antibody-dependent cell-mediated cytotoxicity (Moradali, Mostafavi, Ghods, & Hedjaroude, Citation2007). The activated macrophages are considered as the pivotal immunocytes of host defense which inhibit or kill invading pathogen or cancer cells in immune response (Jeong, Jeong, Yang, & Song, Citation2006). ACP, mainly localized in the lysosome of macrophages, is regarded as a marker for the ability of macrophages and nonspecific immune (Broeg, Citation2003). Macrophages incubated with acetylated LDL increased ACP activity as compared to their controls, which was similar to that stimulated with LPS (Kondomerkos, Kalamidas, Michalis, & Kanavaros, Citation2004). Our research clearly demonstrated that CGH could enhance the activity of ACP in peritoneal macrophages, which contributed to macrophages to play elite roles on phagocytosis and digestion.
3.3. Effects of CGH (≤3 kDa) on NO secretion of the RAW264.7
NO has been implicated as an important inflammatory factor in the process of macrophage-mediated inflammation. The influence of CGH on NO production in RAW264.7 was detected. Compared to the blank control, the stimulation of LPS (20 μg/mL) drastically increased the NO production for RAW264.7 cells. As shown in , the effect of CGH on NO concentration in the culture supernatant manifested a positive dosage-dependent pattern with the highest data of 18.46 μg/mL (100-μg/mL CGH group). Another interesting phenomenon was that 100 μg/mL CGH significantly inhibited LPS-induced NO production of RAW264.7 cells.
Figure 2. Effect of CGH on the production of NO in RAW264.7 cells.
*Different letters above the columnar diagram represented significant difference (P < 0.05).
Figura 2. Efecto de CGH en la producción de NO en las células RAW264.7.
*Las distintas letras encima del diagrama de columnas representan diferencias significativas (P < 0,05).
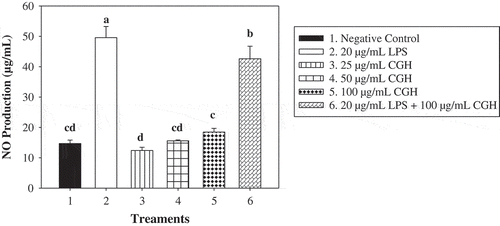
Substantial evidence has showed that NO, an essential inflammatory molecule, is closely related to innate immune function and is used to evaluate the therapeutic effect of various immune modulators (Pan et al., Citation2010). The interaction of NO secreted by the activated macrophages between cytokines could regulate immune function to prevent occurrence and development of autoimmune diseases and inflammation (Commins, Borish, & Steinke, Citation2010; Medzhitov & Janeway, Citation2000). Some reports demonstrated that the methanol (MeOH) extract of Coix seed and the ethyl acetate (AcOEt) fraction of the unhulled adlay had marked inhibitory effect on NO production by activated Raw264.7 cells via suppressing the production of inducible NO synthase (Choi et al., Citation2015; Seo et al., Citation2000). Other research revealed that some components other than the nonproteins and defatted components in Coix seeds could contribute to activate Murine peritoneal macrophages infected by Toxoplasma gondii through induction of NO for the biostatic activity (Soh et al., Citation1996).
3.4. Effects of CGH (≤3 kDa) repeat administration on mice organ index
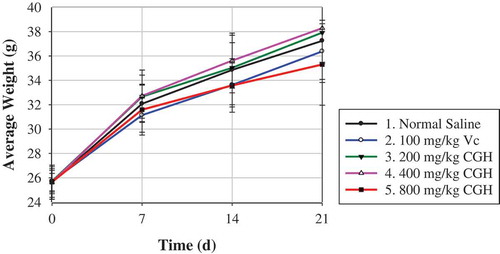
The body weight was recorded once per week after intragastric administration. As shown, mice body weight of 200-mg/kg CGH and 400-mg/kg CGH groups were slightly higher than that of the blank, while that of 100-mg/kg Vc and 800-mg/kg CGH groups were lower than the control since 7 days after administration, with insignificant difference (P > 0.05). At 14 day and 21 day after administration, mice average body weight treated with 400 mg/kg CGH was significantly higher than 800 mg/kg CGH (P < 0.05). As revealed that the spleen index of all three CGH groups increased with a dose-dependent manner, while only high-dose group showed statistical significant increasing (P < 0.05). There was no remarkable difference between normal control group and other groups in the thymus index, despite the thymus index of normal mice was briefly higher. For the liver index, there was no significant difference among all groups, except that 800-mg/kg group elevated notably the liver index comparing with Vc group (P < 0.05).
Table 2. Effects of repeat administration CGH on mice organ indexes.
Tabla 2. Efectos de repetir la administración de CGH en los índices de órganos de ratón.
Figure 3. Effect of CGH (≤3 kDa) on mice body weight after repeat administration for 21 days.
Figura 3. Efecto de CGH (≤ 3 kDa) en el peso corporal de ratón después de repetir la administración durante 21 días.
According to Traditional Chinese Medicine theory, the physiological functions of spleen-Qi include governing transformation and transportation, controlling blood, and sending up the clear (Cheng, Tsai, Chen, & Hung, Citation2015). The herb for tonifying spleen can also enhance organ immunity at some extent. Spleen, the largest secondary lymphoid organ, takes a critical effect on immune modulations, including the clearance of effete or damaged cells from the bloodstream and host resistance to infection (Dailey, Citation2002). Certain research had indicated that active component of Coix seeds have an positive effect on strengthening spleen such as increasing spleen index, which can enhance immunity (Pan et al., Citation2012). In this study, the similar conclusion that CGH increased the spleen index and splenocyte proliferation of mice was obtained. The liver plays an important role in regulating metabolism, secretion of bile, and detoxification. It contains so many immunologically active components that can also be engaged in immune activities as an immune organ according to the existing reports (Nemeth, Baird, & O’Farrelly, Citation2009; Tian, Zhang, & Lian, Citation2014). Our data showed that the liver index of mice was slightly increased at 3 weeks after oral administration of CGH, while the relative weight of thymus showed no significant variation. Thus, it was concluded that CGH had different influence on various immune organ, and the impact on the spleen was more remarkable.
3.5. Effects of CGH repeat administration on mice serum MDA concentration
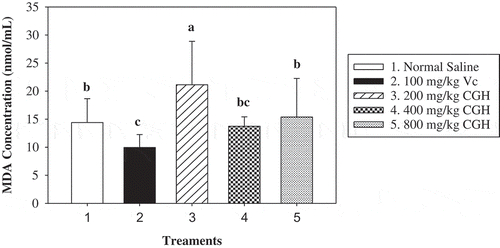
MDA concentration in each group was measured (). The MDA concentration reached 14.40 nmol/mL after long administration of normal saline, while 9.95 nmol/mL for the positive control group (100 mg/kg Vc). The data showed that different doses of CGH caused different MDA content variation. The 400-mg/kg CGH group manifested lower MDA content than the blank control. The MDA concentration of 21.14 nmol/mL (200 mg/kg CGH) was extremely significant higher than 14.40 nmol/mL (normal saline), 13.74 nmol/mL (400 mg/kg CGH), and 15.38 nmol/mL (800 mg/kg CGH), respectively. So it was derived that different CGH dosages had different even opposite influence on serum MDA concentration in mice.
Figure 4. Mice sera MDA concentration (nmol/mL) after repeat administration for 21 days.
*Different letters in the columnar diagram represented significant difference (P < 0.05).
Figura 4. Concentración de MDA en los sueros de ratón (nmol/mL) después de repetir la administración durante 21 días.
*Las distintas letras del diagrama de columnas representan diferencias significativas (P < 0,05).
As we all know, lipid peroxidation could be represented by MDA, which contributes a lotto processes of host defense, inflammation, and tissue damage (Augustin, Wiswedel, Noack, Reinheckel, & Reichelt, Citation1997; Doménech et al., Citation2004; Eze, Anene, & Chukwu, Citation2008). The therapeutic dosage of Coix seed in human body is approximately 9–30 g/kg at present; thus, the dosage of this herb can be properly adjusted higher for the sake of enhancing immunity based on our data.
4. Conclusion
In our experiment, small molecular CGH promoted mice splenocyte proliferation in a concentration-dependent manner in certain concentration range. It was observed that peritoneal macrophages of mice were obviously activated by CGH so as to increase its ACP activity. CGH alone stimulated Raw264.7 to secrete NO in a dose-dependent manner, meanwhile CGH have a negative synergic effect on LPS-induced cell activation at certain condition. Through the repeat oral administration, we found that spleen index of mice was significantly increased at the dosage of 800 mg/kg CGH compared with the blank control, even though no significant variation was detected in thymus index and liver index. The concentration of CGH also had influence on mice average body weight. Additionally, CGH had diverse effects on serum MDA concentration of mice depending on the different concentration. In summary, small molecular (≤3 kDa) gluten enzymatic hydrolysate (bioactive peptides) from Coix showed the immune regulating activity at some extent, while the concrete mechanism deserved further explosion.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NO. 81102750) and the Basic Scientific Research Funding Project of Beijing University of Chinese Medicine (NO. 2015-JYB-JSMS026). Thanks to all the people who joined and helped in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ary, M.B., Shewry, P.R., & Richardson, M. (1988). The amino acid sequence of a cereal Bowman‐Birk type trypsin inhibitor from seeds of Jobs’ tears (Coix lachryma‐jobi L.). FEBS Letters, 229(1), 111–118. doi:10.1016/0014-5793(88)80808-1
- Augustin, W., Wiswedel, I., Noack, H., Reinheckel, T., & Reichelt, O. (1997). Role of endogenous and exogenous antioxidants in the defence against functional damage and lipid peroxidation in rat liver mitochondria. In F.N. Gellerich & S. Zierz (Eds.), Detection of mitochondrial diseases (pp. 199–205). Dordrechet: Springer-Science+Business Media.
- Broeg, K. (2003). Acid phosphatase activity in liver macrophage aggregates as a marker for pollution-induced immunomodulation of the non-specific immune response in fish. Helgoland Marine Research, 57(3–4), 166–175. doi:10.1007/s10152-003-0154-2
- Chen, J.-R., Yang, Z.-Q., Hu, T.-J., Yan, Z.-T., Niu, T.-X., Wang, L., … Wang, M. (2010). Immunomodulatory activity in vitro and in vivo of polysaccharide from Potentilla anserina. Fitoterapia, 81(8), 1117–1124. doi:10.1016/j.fitote.2010.07.009
- Cheng, Y.-C., Tsai, M.-Y., Chen, C.-J., & Hung, Y.-C. (2015). Combination therapy of traditional Chinese medicine and western medicine to treat refractory polymyositis: A case report. The Journal of Alternative and Complementary Medicine, 21(5), 304–306. doi:10.1089/acm.2014.0145
- Choi, G., Han, A.R., Lee, J.H., Park, J.Y., Kang, U., Hong, J., … Seo, E.K. (2015). A comparative study on hulled adlay and unhulled adlay through evaluation of their LPS‐induced anti‐inflammatory effects, and isolation of pure compounds. Chemistry & Biodiversity, 12(3), 380–387. doi:10.1002/cbdv.201400242
- Commins, S.P., Borish, L., & Steinke, J.W. (2010). Immunologic messenger molecules: Cytokines, interferons, and chemokines. Journal of Allergy and Clinical Immunology, 125(2), S53–S72. doi:10.1016/j.jaci.2009.07.008
- Dailey, M.O. (2002). The immune functions of the spleen. In A.J. Bowdler (Ed.), The complete spleen (pp. 51–69). Totowa, NJ: Humana Press.
- de Oliveira Silva, F., das Neves Santos, P., de Melo, C.M.L., Teixeira, E.H., de Sousa Cavada, B., Pereira, V.A.R., … Almeida, A.C. (2011). Immunostimulatory activity of ConBr: A focus on splenocyte proliferation and proliferative cytokine secretion. Cell and Tissue Research, 346(2), 237–244. doi:10.1007/s00441-011-1239-x
- den Haan, J.M., Mebius, R.E., & Kraal, G. (2012). Stromal cells of the mouse spleen. Frontiers in Immunology, 3(201.10), 3389. eCollection 2012. 10.3389/fimmu.2012.00201
- Doménech, P., Carbonell, L., Cárceles, M.P., Falcon, M., Luna, A., & Osuna, E. (2004). Application of postmortem lipid peroxidation in heart tissue to the diagnosis of myocardial damage. International Journal of Legal Medicine, 118(1), 19–23. doi:10.1007/s00414-003-0410-7
- El-Mahmoudy, A., Matsuyama, H., Borgan, M.A., Shimizu, Y., El-Sayed, M.G., Minamoto, N., & Takewaki, T. (2002). Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophages. International Immunopharmacology, 2(11), 1603–1611. doi:10.1016/s1567-5769(02)00139-x
- Eze, J., Anene, B., & Chukwu, C. (2008). Determination of serum and organ malondialdehyde (MDA) concentration, a lipid peroxidation index, in Trypanosoma brucei-infected rats. Comparative Clinical Pathology, 17(2), 67–72. doi:10.1007/s00580-008-0722-6
- Garcia, J., & Villarroel, M. (2009). Effect of feed type and feeding frequency on macrophage functions in tilapia (Oreochromis niloticus L.). Fish & Shellfish Immunology, 27(2), 325–329. doi:10.1016/j.fsi.2009.05.018
- Gauthier, S.F., Pouliot, Y., & Saint-Sauveur, D. (2006). Immunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. International Dairy Journal, 16(11), 1315–1323. doi:10.1016/j.idairyj.2006.06.014
- Ha, D.T., Nam Trung, T., Bich Thu, N., Van On, T., Hai Nam, N., Van Men, C., … Bae, K. (2010). Adlay seed extract (Coix lachryma-jobi L.) decreased adipocyte differentiation and increased glucose uptake in 3T3-L1 cells. Journal of Medicinal Food, 13(6), 1331–1339. doi:10.1089/jmf.2010.1155
- Hernández-Ledesma, B., del Mar Contreras, M., & Recio, I. (2011). Antihypertensive peptides: Production, bioavailability and incorporation into foods. Advances in Colloid and Interface Science, 165(1), 23–35. doi:10.1016/j.cis.2010.11.001
- Hidaka, Y., Kaneda, T., Amino, N., & Miyai, K. (1992). Chinese medicine, coix seeds increase peripheral cytotoxic T and NK cells. Biotherapy, 5(3), 201–203. doi:10.1007/bf02171052
- Horiguchi, N., Horiguchi, H., & Suzuki, Y. (2005). Effect of wheat gluten hydrolysate on the immune system in healthy human subjects. Bioscience, Biotechnology, and Biochemistry, 69(12), 2445–2449. doi:10.1271/bbb.69.2445
- Huang, D.-W., Chung, C.-P., Kuo, Y.-H., Lin, Y.-L., & Chiang, W. (2009). Identification of compounds in adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) seed hull extracts that inhibit lipopolysaccharide-induced inflammation in RAW 264.7 macrophages. Journal of Agricultural and Food Chemistry, 57(22), 10651–10657. doi:10.1021/jf9028514
- Huang, H.-C., Hsieh, W.-Y., Niu, Y.-L., & Chang, T.-M. (2014). Inhibitory effects of adlay extract on melanin production and cellular Oxygen stress in B16F10 melanoma cells. International Journal of Molecular Sciences, 15(9), 16665–16679. doi:10.3390/ijms150916665
- Huang, X., Qin, J., & Lu, S. (2014). Kanglaite stimulates anticancer immune responses and inhibits HepG2 cell transplantation-induced tumor growth. Molecular Medicine Reports, 10(4), 2153–2159. doi:10.3892/mmr.2014.2479
- Jeong, S.-C., Jeong, Y.-T., Yang, B.-K., & Song, C.-H. (2006). Chemical characteristics and immuno-stimulating properties of biopolymers extracted from Acanthopanax sessiliflorus. Journal of Biochemistry and Molecular Biology, 39(1), 84–90. doi:10.5483/BMBRep.2006.39.1.084
- Kleekayai, T., Harnedy, P.A., O’Keeffe, M.B., Poyarkov, A.A., CunhaNeves, A., Suntornsuk, W., & FitzGerald, R.J. (2015). Extraction of antioxidant and ACE inhibitory peptides from Thai traditional fermented shrimp pastes. Food Chemistry, 176, 441–447. doi:10.1016/j.foodchem.2014.12.026
- Kondomerkos, D.J., Kalamidas, S.A., Michalis, L.K., & Kanavaros, P. (2004). The effects of phosphoinositide/calcium-or cyclic AMP-mediated signal transduction pathway inhibitors on the activation of rat peritoneal macrophages by acetylated low-density lipoprotein. In Vivo, 18(5), 653–660.
- Kuo, -C.-C., Chen, -H.-H., & Chiang, W. (2012). Adlay (yì yĭ;“soft-shelled job’s’ tears”; the seeds of Coix lachryma-jobi L. var. ma-yuen Stapf) is a Potential Cancer Chemopreventive Agent toward Multistage Carcinogenesis Processes. Journal of Traditional and Complementary Medicine, 2(4), 267–275. doi:10.1016/S2225-4110(16)30112-2
- Lhouvum, K., Bhuyar, K.S., & Trivedi, V. (2015). Molecular modeling and correlation of PFI1625c-peptide models of bioactive peptides with antimalarial properties. Medicinal Chemistry Research, 24(4), 1527–1533. doi:10.1007/s00044-014-1232-5
- Li, H.-X., Xiao, Y., Cao, -L.-L., Yan, X., Li, C., Shi, H.-Y., … Ye, Y.-H. (2013). Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots. Plos One, 8(9), e73380. doi:10.1371/journal.pone.0073380
- Li, S.-Z. (1998). Pen-t’sao kangmu (Systematic Pharmacopoeia). Beijing: China Press of Traditional Chinese Medicine.
- Liu, X.-D., Zhou, B., Cao, R.-B., Feng, X.-L., Li, X.-F., & Chen, P.-Y. (2013). Comparison of immunomodulatory functions of three peptides from the chicken bursa of Fabricius. Regulatory Peptides, 186, 57–61. doi:10.1016/j.regpep.2013.07.007
- Lu, C., Schoknecht, P., Ellis, K., Shypailo, R., Su, D., & Pond, W. (1996). Differential compensatory organ growthin young pigs after short-term rehabilitation from protein deficiency. Nutrition Research, 16(4), 627–637. doi:10.1016/0271-5317(96)00040-1
- Lu, Y., Zhang, B.-Y., Jia, Z.-X., Wu, W.-J., & Lu, Z.-Q. (2011). Hepatocellular carcinoma HepG2 cell apoptosis and caspase-8 and Bcl-2 expression induced by injectable seed extract of Coix lacryma-jobi. Hepatobiliary & Pancreatic Diseases International, 10(3), 303–307. doi:10.1016/s1499-3872(11)60050-7
- Ma, -J.-J., Mao, X.-Y., Wang, Q., Yang, S., Zhang, D., Chen, S.-W., & Li, Y.-H. (2014). Effect of spray drying and freeze drying on the immunomodulatory activity, bitter taste and hygroscopicity of hydrolysate derived from whey protein concentrate. LWT-Food Science and Technology, 56(2), 296–302. doi:10.1016/j.lwt.2013.12.019
- Mallet, J.-F., Duarte, J., Vinderola, G., Anguenot, R., Beaulieu, M., & Matar, C. (2014). The immunopotentiating effects of shark-derived protein hydrolysate. Nutrition, 30(6), 706–712. doi:10.1016/j.nut.2013.10.025
- Medzhitov, R., & Janeway, C. (2000). Innate immune recognition: Mechanisms and pathways. Immunological Reviews, 173(1), 89–97. doi:10.1034/j.1600-065X.2000.917309.x
- Moradali, M.-F., Mostafavi, H., Ghods, S., & Hedjaroude, G.-A. (2007). Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). International Immunopharmacology, 7(6), 701–724. doi:10.1016/j.intimp.2007.01.008
- Mukhopadhya, A., Noronha, N., Bahar, B., Ryan, M.T., Murray, B.A., Kelly, P.M., … Sweeney, T. (2014). Anti‐inflammatory effects of a casein hydrolysate and its peptide‐enriched fractions on TNFα‐challenged Caco‐2 cells and LPS‐challenged porcine colonic explants. Food Science & Nutrition, 2(6), 712–723. doi:10.1002/fsn3.153
- Nagao, T., Otsuka, H., Kohda, H., Sato, T., & Yamasaki, K. (1985). Benzoxazinones from Coix lachryma-jobi var. ma-yuen. Phytochemistry, 24(12), 2959–2962. doi:10.1016/0031-9422(85)80035-2
- Nemeth, E., Baird, A.W., & O’Farrelly, C. (2009). Microanatomy of the liver immune system. Paper Presented at the Seminars in Immunopathology, 31, 333–343. doi:10.1007/s00281-009-0173-4
- Pan, D., Bera, A., Das, S., Bandyopadhyay, S., Rana, T., Bandyopadhyay, S., … Bhattacharya, D. (2010). Use of zinc chloride as alternative stimulant for in vitro study of nitric oxide production pathway in avian splenocyte culture. Molecular Biology Reports, 37(5), 2223–2226. doi:10.1007/s11033-009-9708-y
- Pan, P., Wu, Y., Guo, Z.-Y., Wang, R., Wang, Y.-J., & Yuan, Y.-F. (2012). Antitumor activity and immunomodulatory effects of the intraperitoneal administration of Kanglaite in vivo in Lewis lung carcinoma. Journal of Ethnopharmacology, 143(2), 680–685. doi:10.1016/j.jep.2012.07.025
- Seo, W.-G., Pae, H.-O., Chai, K.-Y., Yun, Y.-G., Kwon, T.-H., & Chung, H.-T. (2000). Inhibitory effects of Methanol extract of Seeds of Job’s Tears (Coix Lachryma-Jobi L Var. Ma-Yuen) On Nitric Oxide and Superoxide Production in Raw 264.7 Macrophages. Immunopharmacology and Immunotoxicology, 22(3), 545–554. doi:10.3109/08923970009026011
- Soh, C.-T., Kim, S.-H., Kim, K.-Y., Park, H., Chung, H.-T., Kim, T.-U., … Han, Y.-B. (1996). Biostatic activity of Coix lacryma seed extract on Toxoplasma gondii in macrophages. The Korean Journal of Parasitology, 34(3), 197–206. doi:10.3347/kjp.1996.34.3.197
- Sugiura, H., Inaba, R., Iwata, H., Nishida, H., & Tanaka, T. (1998). Modifying effects of Maharishi Amrit Kalash 4 and 5 on phagocytic and digestive functions of macrophages in male ICR mice. Environmental Health and Preventive Medicine, 3(1), 50–54. doi:10.1007/bf02931239
- Sugiura, H., Sugiura, H., Ueya, E., Ueya, S., & Mirbod, S.M. (2000). Enhanced macrophage functions and cytokine production of lymphocytes after ingestion of bon narine in female BALB/c mice. Life Sciences, 68(5), 505–515. doi:10.1016/s0024-3205(00)00946-2
- Tian, Z., Zhang, C., & Lian, Z.-X. (2014). The liver and immune tolerance. In M.E. Gershwin, J.M. Vierling, & M.P. MannsLiver (Eds.), Liver immunology (pp. 79–94). New York, NY: Springer International Publishing.
- Vettore, A.L., Yunes, J.A., Neto, G.C., Da Silva, M.J., Arruda, P., & Leite, A. (1998). The molecular and functional characterization of an Opaque2 homologue gene from Coix and a new classification of plant bZIP proteins. Plant Molecular Biology, 36(2), 249–263. doi:10.1023/A:1005995806897
- Wallace, J.C., Lopes, M.A., Paiva, E., & Larkins, B.A. (1990). New methods for extraction and quantitation of zeins reveal a high content of γ-zein in modified opaque-2 maize. Plant Physiology, 92(1), 191–196. doi:10.1104/pp.92.1.191
- Wang, L.-Z., Yuan, J.-N., Zhang, X.-H., & Qiao, Y.-J. (2012). Protein Composition Analysis ofCoix Lacryma-Jobi L. var. ma-yuen (Roman.). Stapf Seeds Progress in Modern Biomedicine, 23, 006. (In Chinese)
- Wang, X.-F., Ma, C.-J., & Wang, X.-Q. (1999). Clinical observation on effect of Kanglaite in treating pulmonary carcinoma patients with Lung-Qi Deficiency and immunologic inadequacy after pneumonectomy) in treating pulmonary carcinoma patients with Lung-Qi Deficiency and immunologic inadequacy after pneumonectomy. Chinese Journal of Integrated Traditional and Western Medicine, 5(2), 105–107.
- Yu, F., Gao, J., Zeng, Y., & Liu, C.X. (2011). Effects of adlay seed oil on blood lipids and antioxidant capacity in hyperlipidemic rats. Journal of the Science of Food and Agriculture, 91(10), 1843–1848. doi:10.1002/jsfa.4393
- Yuan, J.-N., Chen, X.-M., Cui, S., Liang, Y.-X., Wang, Z.-L., Zhang, X.-H., & Wang, L.-Z. (2014a). Optimization on hydrolysis conditions of coix prolamin and angiotensin-converting Enzyme inhibitory activity of hydrolysates. World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica, 16(4), 806–810. In Chinese. 10.11842/wst.2014.04.022
- Yuan, J.-N., Liang, Y.-X., Cui, S., Zhang, X.-H., Wang, L.-Z., & Qiao, Y.-J. (2014b). Angiotensin I converting enzyme inhibitory and antioxidant activity of adlay (Coix lachrymal-jobi L.var. ma-yuen Stapf) glutelin hydrolysate. Italian Journal of Food Science, 26(3), 282.
- Zhao, L., Liu, L., Guo, B., & Zhu, B. (2015). Regulation of adaptive immune responses by guiding cell movements in the spleen. Frontiers in Microbiology, 6. doi:10.3389/fmicb.2015.00645
- Zhou, J.-H, Ma, -L.-L., Xu, -H.-H., Gao, Y, Jin, Y.-K, Zhao, L, & Zhang, S.-H. (2014). Immunomodulating effects of casein-derived peptides QEPVL and QEPV on lymphocytes in vitro and in vivo. Food & Function, 5(9), 2061–2069. doi:10.1039/c3fo60657k
- Zhu, C.-P., Hu, W., Wu, H., & Hu, X. (2014). No evident dose-response relationship between cellular ROS level and its cytotoxicity-a paradoxical issue in ROS-based cancer therapy. Scientific Reports, 4. doi:10.1038/srep05029
