ABSTRACT
Microbial transglutaminase (MTGase) is a kind of enzyme preparation which is used in the processing of emulsified meat products for improving the quality. In this paper, the effect of MTGase content on water-holding capacity, texture and protein structure was studied by using Raman spectroscopy. The results showed that emulsion stability and cooking yield were significantly (P < 0.05) improved with increased MTGase content. But when the contents were 0.67% and 1%, the emulsion stability and cooking yield had no significant difference (P > 0.05). The hardness, springiness, cohesiveness and chewiness also were significantly (P < 0.05) improved with increased MTGase content. The result of Raman spectroscopy found that the wave peak of amide I shifted from 1658 to 1660, 1661 and 1661 cm−1 when added various amounts of MTGase, β-sheet and β-turn contents were significantly (P < 0.05) increased with decreased α-helix content, and random coil was not a significant difference (P > 0.05); the wave peak of 3100–3500 cm−1 shifted from 3226 to 3228, 3229 and 3229 cm−1 that indicated OH stretching motions were stronger. Overall, when added appropriately, MTGase to frankfurters could change the protein conformation, improve the texture and water-holding capacity.
RESUMEN
La transglutaminasa microbial (MTGase) es un tipo de preparado encimático utilizado en el procesamiento de productos cárnicos emulsionados para mejorar su calidad. En este estudio se ha investigado el efecto del contenido de MTGase en la capacidad de retención de agua, la textura y la estructura proteínica mediante la utilización de espectroscopia Raman. Los resultados mostraron que la estabilidad de emulsión y el rendimiento de cocinado mejoraron significativamente (P < 0,05) con el aumento del contenido de MTGase. Sin embargo, cuando los contenidos fueron 0,67% y 1%, la estabilidad de emulsión y el rendimiento de cocinado no obtuvieron diferencias significativas (P > 0,05). La dureza, la ligereza, la cohesión y la masticabilidad también fueron mejorados significativamente (P < 0,05) con el aumentó en el contenido de MTGase. Los resultados de la espectroscopia Raman indicaron que la ola pico de amida I cambió de 1658 cm−1 a 1660 cm−1, 1661 cm−y y 1661 cm−1 cuando fueron añadidas diferentes cantidades de Migase. Los contenidos de lámina β y giro β aumentaron significativamente (P < 0,05) cuando el contenido de hélice α disminuyó, además el movimiento aleatorio no fue significativamente diferente (P > 0,05); la ola pico de 3100-3500 cm−1 cambió de 3226 cm−1 a 3228 cm−1, 3229 cm−1 y 3229 cm−1, lo cual indicó que los movimientos de elasticidad de OH eran más fuertes. En general, la adición apropiada de MTGase a las salchichas Frankfurt podría cambiar la conformación proteínica, mejorar la textura y la capacidad de retención de agua.
1. Introduction
Frankfurters are a type of frequently consumed meat products, which have enjoyed widely consumer acceptance in certain sections of the global population (Delgado-Pando, Cofrades, Ruiz-Capillas, Solas, & Jiménez-Colmenero, Citation2010). But traditional frankfurters have high salt level (Desmond, Citation2006). Excessive dietary salt intake can cause cardiovascular disease and other diseases (Askin & Kilic, Citation2009; Yalçın & Şeker, Citation2016). Emulsion formation and stability of emulsified meats are markedly affected by salt content. One of salt’s main functions in emulsified meats is the solubilization of the myofibrillar proteins as it is these proteins that determine the binding and textural characteristics of the product (Pietrasik & Li-Chang, Citation2002). Thus, reduction of salt in frankfurters has therefore become an important research field (Kang et al., Citation2014; Ma et al., Citation2012).
The microbial transglutaminase (MTGase; proteineglutamine g-glutamyltransferase, EC 2.3.2.13) is an efficient and promising alternative, which can catalyze the formation of ε-(g-glutamyl) lysyl cross-links between glutamine and lysine residues. It has been used to catalyze the intra- and intermolecular transverse cross-linking of meat proteins to enhance their interactions and functionality (Chanarat & Benjakul, Citation2013; Han, Zhang, Fei, Xu, & Zhou, Citation2009; Trespalacios & Pla, Citation2007). MTGase has been used to improve textural properties and water-holding capacity of meat protein gel, which is one of the most important functional properties of proteins in low-salt meat systems (Canto et al., Citation2014; Cardoso, Mendes, Vaz-Pires, & Nunes, Citation2010; Han et al., Citation2009).
Raman spectroscopy is a direct and noninvasive technique, which can provide information on peptide backbone conformations such as secondary and water structure of meat proteins (Li-Chan, Nakai, & Hirostuka, Citation1994). Due to weak background scattering from water, it is directly applicable to aqueous systems (Li-Chan, Citation1996; Liu, Zhao, Xie, & Xiong, Citation2011). This method has been used to study the structural changes of meat systems (Herrero, Cambero, Ordóñez, Hoz, & Carmona, Citation2008; Schmidt, Scheier, & Hopkins, Citation2013; Wang et al., Citation2013). However, as far as our knowledge, little papers reported that Raman spectroscopy was used to examine the structural changes of meat protein with various amounts of MTGase. Therefore, the objective of the present study was to determine protein structural and physical–chemical differences on low-salt frankfurters with various amounts of MTGase, and thereby to obtain an appropriate concentration of MTGase in frankfurters.
2. Materials and methods
2.1. Raw materials and ingredients
Pork leg lean meat (after 24–48 h of slaughtering, pH 5.75, 72.05% moisture, 20.10% protein, 7.34% fat) and back fat (8.50% moisture, 1.75% protein and 90.12% fat, AOAC Citation2000) were purchased four times in 2 days from a local meat market (Xinxiang, China). All subcutaneous and intramuscular fat and visible connective tissues were removed from the lean meat. The lean meat and back fat were passed through a grinder (JR-120, Shandong, China) fitted with a plate having 6-mm diameter holes. The ground meat (500 g each) was packaged in nylon/polyethylene bags and stored at −20°C until use within 4 weeks. MTGase (1% MTGase, 99% maltodextrin) was purchased from Dongsheng food science and technology Co. Ltd, China. The raw materials and other ingredients are shown in .
Table 1. Formulation (g) of frankfurters with various amounts of MTGase.
Tabla 1. Formulación (g) de las salchichas Frankfurt con diferentes cantidades de MTGase.
2.2. Preparation of frankfurter
Three different frankfurters were prepared with four replications at different occasions. The preparation of frankfurter was as described by Kang et al. (Citation2014) with slight modification. Pork meat was thawed at 4°C overnight prior to use. The thawed meat was processed using a beating machine (MC-7, Shangdong, China) according to the processing as follows: the meat was beaten with salt, MTGase, sodium tripolyphosphate and one-third ice water for 10 min (200 rpm); followed by the pork back fat, spices and two-thirds ice water were mixed (200 rpm) to the batter for 5 min (final temperature less than 10°C). Then, the batter was stuffed by a vacuum stuffer (GZY3500, China) in 24-mm diameter edible collagen sausage casings (Shenguan Holdings [Group] Limited, China). Frankfurters were hand linked at 18-cm intervals, after incubated at 4°C for 4 h and weighed. The heat is processed in smokehouse (T1900 El619, Germany) according to the following processing cycle: drying for 15 min at 50°C and 60% relative humidity (RH), and steam cooking for 25 min at 80°C and 100% RH (the internal temperature of frankfurters at 72°C for 3 min), then showered for 15 min and drying for 10 min at 30°C and 60% RH and finally removed from the smokehouse and chilled at 2°C overnight.
2.3. Emulsion stability
Emulsion stability using the method of Fernández-Martín, López-lópez, Cofrades and Jiménez-Colmenero (Citation2009) with slight modification is as follows: 25 g meat batter was placed in a 50-mL centrifuge tube and centrifuged at 500g for 15 min at 3°C to eliminate any air bubbles. Then each tube was heated in 80°C water bath for 20 min, immediately removed, uncapped and the left inverted on paper tissues to release any exudate at room temperature for 50 min. The water-released component (WR, % of the initial sample weight) was determined from the dry matter content of the total fluid release (TR) after heating at 105°C for 16 h. The TR was expressed as % of the initial sample weight. The fat-released component (FR, % of the initial sample weight) ignored any minor protein or salt components and was taken as the difference between TR and WR. Four determinations (four tubes) were made on each formulation per batch.
2.4. Cooking yield
After cooling at 2°C overnight, the frankfurters were weighed and the percentage weight yield was calculated using the following formula:
Cooking yield% = weight of frankfurter after cooking/weight of frankfurter before cooking × 100
2.5. Texture profile analysis
After cooling at 2°C overnight, the frankfurters were left at room temperature for 2 h. The texture profile analysis (TPA) attributes of the frankfurters were determined using a texture analyzer (TA-XT. plus, Stable Micro system Ltd., Surrey, UK) fitted with a cylindrical probe (P/50, 50 mm stainless cylinder). The conditions were as follows: pretext speed 2.0 mm/s, test speed 2.0 mm/s, post-text speed 5.0 mm/s, strain 50%, time 5.0 s and trigger force 5 g for TPA measurement. The frankfurters were cut in two to obtain a 20-mm depth and 20-mm diameter strips. Attributes were calculated as follows: hardness (Hd), the peak force (N) required for first compression; cohesiveness (Ch), the ratio of active work done under the second compression curve to that done under the first compression curve (dimensionless); springiness (Sp), distance (mm) of sample recovery after the first compression and chewiness (Cw), Hd × Ch × Sp (N mm) (Bourne, Citation1978).
2.6. Raman spectroscopic
Raman experiments were determined using a modified procedure of Shao, Zou, Xu, Wu and Zhou (Citation2011). Spectra were smoothed, baselines corrected and normalized against the phenylalanine band at 1003 cm−1 (Herrero, Citation2008; Li-Chan et al., Citation1994) using Labspec version 3.01c (Horiba/Jobin. Yvon, Long-jumeau, France). The secondary structures of the proteins were determined as percentages of α-helix, β-sheet, β-turn and random coil or unordered conformations (Alix, Pedanou, & Berjot, Citation1988). With this aim, the water spectrum was subtracted from the spectra by following the same criteria as that described previously (Alix et al., Citation1988; Herrero, Carmona, Pintado, Jiménez-Colmenero, & Ruíz-Capillas, Citation2011).
2.7. Statistical analysis
The experiment was repeated four times. The data were analyzed using the one-way ANOVA program. The difference between means was considered significant at P < 0.05. Significant differences between means were identified by the least significant difference procedure using the statistical software package SPSS v.18.0 for windows.
3. Results and discussion
3.1. Emulsion stability
Emulsion stability is an important parameter that is critical to the stability of raw meat batter. MTGase level had significant effects (P < 0.05) on the stability of raw meat emulsions (). Emulsion stability of the raw meat batters is increasing with improved MTGase content from 0% to 0.67%. When the MTGase content was 1% (T4), the emulsion stability had not increased significantly (P > 0.05). The samples of T3 and T4 had the lowest TR, WR and FR, and T1 decreased largely. Different TR suggested the differences in water- and fat-holding capacity of gel network. MTGase and heat treatment could induce denaturation of protein structure and the subsequent ordered aggregation of protein forming a well-structured gel on protein interactions and having a good capacity to hold water (Martínez et al., Citation2014; Wang et al., Citation2013). Martínez et al. (Citation2014) reported that gels obtained by adding 6 g/kg MTGase showed higher value of water-holding capacity than gels cooked directly. Wu, He, Hong and Wang (Citation2016) found that the treatment with MTGase, the emulsifying properties of chicken myofibrillar protein isolate/kidney bean protein isolate mixtures increased significantly (P < 0.05) and the emulsifying properties of preheated samples exceeded the non-preheated samples which incubated 240 min.
Table 2. Emulsion stability of raw batters with various amounts of MTGase.
Tabla 2. Estabilidad de emulsión de la masa cruda con diferentes cantidades de MTGase.
3.2. Cooking yield
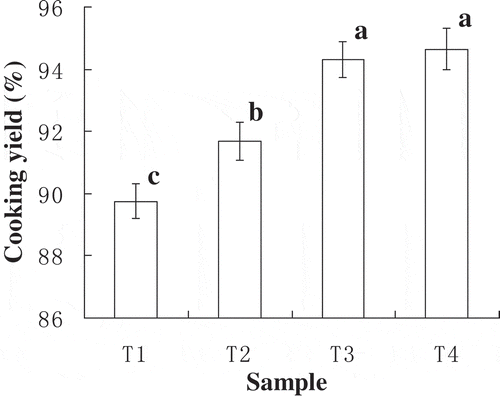
The MTGase content had significant effects (P < 0.05) on cooking yields of frankfurters (). The treatments of T3 and T4 had the highest cooking yield, and T1 had significantly (P < 0.05) lower than other treatments. When improved the enzyme concentration, the number of inter- and intra-chain peptide cross-links increased and formed good gel structure and enhanced the water- and fat-binding capacity of meat batters (Ahhmed, Nasu, & Muguruma, Citation2009). The cooking yield of T3 and T4 was not significantly different (P > 0.05), the reason is the higher the enzyme concentration, the lower the protein–water interaction, and water-holding capacity was decreased (Gaspar & Góes-Favoni, Citation2015). In agreement with our findings, Canto et al. (Citation2014) reported that only added MTGase increased cooking yield of low-sodium-restructured caiman steaks. Pietrasik and Li-Chang (Citation2002) found that MTGase addition reduced cooking loss of gels.
Figure 1. Cooking yield (%) of frankfurters with various amounts of MTGase.a–cDifferent parameter superscripts in the same column indicate significant differences (P < 0.05). T1: Without MTGase; T2: 0.33% MTGase; T3: 0.67% MTGase; T4: 1% MTGase. Each value represents the mean ± SD, n = 4.
Figura 1. Rendimiento del cocinado (%) de las salchichas Frankfurt con diferentes cantidades de MTGase.a-cLos diferentes parámetros en los superíndices de la misma columna indican diferencias significativas (P < 0,05). T1: Sin MTGase; T2: 0,33% de MTGase; T3: 0,67% de MTGase; T4: 1% de MTGase. Cada valor representa el promedio±SD, n = 4.
3.3. TPA
TPA parameters were affected by MTGase content which is presented in . Increasing MTGase levels significantly increased (P < 0.05) the hardness, springiness, adhesiveness and chewiness of frankfurters. The value of hardness was improved from 54.23 to 59.09 N. It was in agreement with Herrero et al. (Citation2008), who reported that the hardness, springiness, adhesiveness and gumminess of pork meat gel were significantly improved (P < 0.05) when MTGase is added. The proteins were aggregated, and protein interactions were enhanced after adding MTGase, then forming a well gel network (Gaspar & Góes-Favoni, Citation2015). Meanwhile, the deamidation promoted by the action of MTGase can increase the proteins’ solubility (Renzetti, Bello, & Arendt, Citation2008). A higher level of salt-soluble protein concentrations enhanced gel network and protein matrix formation (Somboonpanyakula, Barbut, Jantawata, & Chinprahasta, Citation2007) and increased the number of sites in the polypeptide chains capable of interacting during heating, allowing it to form a stable, elastic and rigid protein gel matrix (Verma, Sharma, & Banerjee, Citation2010).
Table 3. Texture profile analysis of frankfurters with various amounts of MTGase.
Tabla 3. Análisis del perfil textural de las salchichas Frankfrut con diferentes cantidades de MTGase.
3.4. Raman spectroscopy analysis
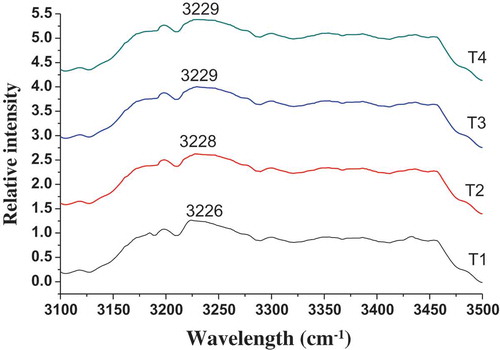
The Raman spectroscopy of frankfurters with different MTGase concentrations on the 1550–1800 and 3100–3500-cm−1 region and quantitative analysis of the selected peaks are shown in and and , respectively. The Raman bands were assigned according to the previous literature (Chen & Han, Citation2011; Li-Chan et al., Citation1994). The changes of frequency and intensity in the Raman bands were mainly indicative of changes in the secondary structure and water structure of the pork meat proteins.
Table 4. Percentages of protein secondary structures α-helix, β-sheet, β-turns, unordered of frankfurters with various amounts of MTGase.
Tabla 4. Porcentajes de las estructura secundarias proteínicas hélice α, lámina β, giro β, desordenadas de las salchichas Frankfurt con diferentes cantidades de MTGase.
Figure 2. Raman spectra of frankfurters with various amounts of MTGase in the region 1550–1800 cm−1.T1: Without MTGase; T2: 0.33% MTGase; T3: 0.67% MTGase; T4: 1% MTGase.
Figura 2. Espectro Raman de las salchichas Frankfurt con diferentes cantidades de MTGase en la región 1550–1800 cm−1.T1: Sin MTGase; T2: 0,33% de MTGase; T3: 0,67% de MTGase; T4: 1% de MTGase.
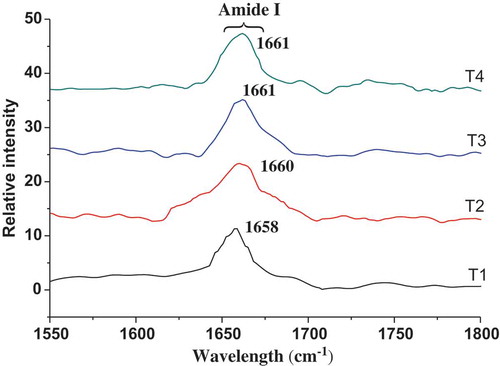
Figure 3. Raman spectra of frankfurters with various amounts of transglutaminase in the region 3100–3500 cm−1.T1: Without MTGase; T2: 0.33% MTGase; T3: 0.67% MTGase; T4: 1% MTGase.
Figura 3. Espectro Raman de las salchichas Frankfurt con diferentes cantidades de transglutaminasa en la región 3100–3500 cm−1.T1: Sin MTGase; T2: 0,33% de MTGase; T3: 0,67% de MTGase; T4: 1% de MTGase.
3.4.1. Changes of secondary structures
The Raman spectra of meat products in 1600–1700 cm−1 region showed that the most prominent band has been assigned to the amide I vibrational mode, which centered near 1665 cm−1 region (Herrero et al., Citation2011; Krimm & Bandekar, Citation1986). This Raman band could provide secondary structural information about protein (Alix et al., Citation1988; Herrero, Citation2008; Tu, Citation1982), which involves mainly C=O stretching and to lesser degrees C–N stretching, Cα–C–N bending and N–H in-plane bending of peptide groups. Raman spectra () showed a slight shift from 1658 cm−1 to higher frequencies of the maximum intensity of this band (1660, 1661 and 1661 cm−1) after MTGase was added to frankfurters, indicating a reduction in the α-helical structure.
The Raman spectra is sensitive to changes in the hydrogen-bonding scheme involving the peptide linkages, the amide I band consists of overlapping band components falling in the 1650–1660, 1665–1680, 1680 and 1660–1665 cm−1 ranges, which are attributable to α-helices, β-sheets, β-turn and random coil structures, respectively (Li-Chan, Citation1996; Ngarize, Herman, Adams, & Howell, Citation2004). Thus, quantitative information about secondary structure of meat protein could be estimated from the amide I spectral profile (Alix et al., Citation1988). According to , there were significant effects (P < 0.05) of MTGase on the values of secondary structural percentages content in frankfurters, mainly decreased α-helices and increased β-sheet, β-turn, which is consistent with the amide I spectral changes (). Because of an increase in hydrogen bonding, more α-helical structures could change into β-sheet structures during protein denaturation with MTGase addition 0.335–0.67% (Perisic, Afsetha, Ofstada, Hassani, & Kohler, Citation2013). When the MTGase addition was increased from 0.67% to 1%, there were no significant differences (P > 0.05) on the values of secondary structural percentages content. After the enzyme concentration reaches a certain point in meat proteins, it lowered the protein–water interaction, enhanced the inter- and intra-chain peptide cross-links and decreased the number of hydrogen bonding (Damodaran, Citation2010). Herrero et al. (Citation2008) reported that it was a significant (P < 0.05) increase in the β-sheet and β-turn accompanied by a concomitant decrease in α-helices when MTGase is added. Some researchers have reported positive correlations between the content of β-sheet and hardness, and β-sheet structure is the base of formed gel (Herrero et al., Citation2008; Shao et al., Citation2011). In the present study, an increased β-sheet content could improve emulsion stability (), cooking yield () and hardness () of frankfurters.
3.4.2. Water structure
The Raman bands of 3100–3500 cm−1 region could provide information about the changes of water structure in meat protein (Herrero et al., Citation2008; Maeda & Kitano, Citation1995). It is attributable to OH stretching vibration which has been related to intramolecular vibrations of hydrogen-bonded water. shows the Raman spectrum of frankfurters with various amounts of MTGase in 3100–3500-cm−1 region. Compared with T1 (3226 cm−1), it could be observed as light frequency upshifting of the band (3225 cm−1) in frankfurters with various amounts of MTGase, T2, T3 and T4, were separately located at 3228, 3229 and 3229 cm−1, respectively. The results indicated that hydrogen-bonded water was stronger (Socrates, Citation2001). It was in compliance with the results of emulsion stability () and cooking yield (). Some authors have reported that the addition of MTGase promoted the G–L cross-links and lead to the formation of a strong and stable gel with better water-holding capacity in the gel network (Bönisch, Huss, Weitl, & Kulozik, Citation2007; Yokoyama, Nio, & Kikuchi, Citation2004). When the MTGase is added from 0.67% to 1%, the bands had little changes. Thus, excessive addition of the MTGase did not affect the OH stretching vibration of protein. The changes of water structure also affected the secondary structure of meat protein, the stronger hydrogen bonding promotes secondary structural transitions toward β-sheets (Mukherjee, Chowdhury, & Gai, Citation2007).
4. Conclusions
The additional difference levels of MTGase significantly affected the physical–chemical and protein structure of frankfurters. When the different levels of MTGase are added to frankfurters, the emulsion stability, cooking yield, hardness, springiness and chewiness were increased. When the MTGase content is increased to 0.67%, the emulsion stability and cooking yield increased and has little effects from 0.67% to 1%. But the hardness, springiness and chewiness of frankfurters increased with the increase in the MTGase content. In addition, the frankfurters with MTGase had a higher β-sheet and β-turn content and lower α-helices content. Moreover, the frequency changes in OH stretching vibration band showed that the addition of MTGase to frankfurters generates stronger hydrogen bonds as water–meat protein interactions, which can be related to the enhancement of water binding. In conclusion, added MTGase from 0.33% to 1% could improve the quality of frankfurters and transform protein structure.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahhmed, A.M., Nasu, T., & Muguruma, M. (2009). Impact of transglutaminase on the textural, physicochemical, and structural properties of chicken skeletal, smooth, and cardiac muscles. Meat Science, 83, 759–767. doi:10.1016/j.meatsci.2009.08.018
- Alix, A.J.P., Pedanou, G., & Berjot, M. (1988). Fast determination of the quantitative secondary structure of proteins by using some parameters of the Raman Amide I band. Journal of Molecular Structure, 174, 159–164. doi:10.1016/0022-2860(88)80151-0
- AOAC. (2000). AOAC, Official methods of analysis of AOAC international (17th ed.). Gaithersburg, MD: AOAC International.
- Askin, O.O., & Kilic, B. (2009). Effect of microbial transglutaminase, sodium caseinate and non-fat dry milk on quality of salt-free, low fat Turkey döner kebab. LWT – Food Science and Technology, 42(10), 1590–1596. doi:10.1016/j.lwt.2009.06.005
- Bönisch, M.P., Huss, M., Weitl, K., & Kulozik, U. (2007). Transglutaminase crosslinking of milk proteins and impact on yoghurt gel properties. International Dairy Journal, 17(11), 1360–1371. doi:10.1016/j.idairyj.2007.01.019
- Bourne, M.C. (1978). Texture profile analysis. Food Technology, 32, 62–66.
- Canto, A., Lima, B.C., Suman, S.P., Lazaro, C., Monteiro, M.L., Conte-Junior, C., … Silva, T. (2014). Physico-chemical and sensory attributes of low-sodium restructured caiman steaks containing microbial transglutaminase and salt replacers. Meat Science, 96, 623–632. doi:10.1016/j.meatsci.2013.08.003
- Cardoso, C., Mendes, R., Vaz-Pires, P., & Nunes, M.L. (2010). Effect of salt and MTGase on the production of high quality gels from farmed sea bass. Journal of Food Engineering, 101, 98–105. doi:10.1016/j.jfoodeng.2010.06.017
- Chanarat, S., & Benjakul, S. (2013). Impact of microbial transglutaminase on gelling properties of Indian mackerel fish protein isolates. Food Chemistry, 136(2), 929–937. doi:10.1016/j.foodchem.2012.09.021
- Chen, H.Y., & Han, M.Y. (2011). Raman spectroscopic study of the effects of microbial transglutaminase on heat-induced gelation of pork myofibrillar proteins and its relationship with textural characteristics. Food Research International, 44, 1514–1520. doi:10.1016/j.foodres.2011.03.052
- Damodaran, S. (2010). Aminoácidos, peptídeos e proteínas. In S. Damodaran, K.L. Parkin, & O.R. Fennema (Eds.), Química de alimentos de Fennema (4th ed., pp. 179–262). Porto Alegre: Artmed.
- Delgado-Pando, G., Cofrades, S., Ruiz-Capillas, C., Solas, M.T., & Jiménez-Colmenero, F. (2010). Healthier lipid combination oil-in-water emulsions prepared with various protein systems: An approach for development of functional meat products. European Journal of Lipid Science and Technology, 112(7), 791–801. doi:10.1002/ejlt.v112:7
- Desmond, E. (2006). Reducing salt: A challenge for the meat industry. Meat Science, 74, 188–196. doi:10.1016/j.meatsci.2006.04.014
- Fernández-Martín, F., López-lópez, I., Cofrades, S., & Jiménez-Colmenero, F. (2009). Influence of adding sea spaghetti seaweed and replacing the animal fat with olive oil or a konjac gel on pork meat batter gelation. Potential protein/alginate association. Meat Science, 83, 209–217. doi:10.1016/j.meatsci.2009.04.020
- Gaspar, A.L.C., & Góes-Favoni, S.P. (2015). Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chemistry, 171, 315–322. doi:10.1016/j.foodchem.2014.09.019
- Han, M., Zhang, Y., Fei, Y., Xu, X., & Zhou, G. (2009). Effect of microbial transglutaminase on NMR relaxometry and microstructure of pork myofibrillar protein gel. European Food Research and Technology, 228(4), 665–670. doi:10.1007/s00217-008-0976-x
- Herrero, A.M. (2008). Raman spectroscopy a promising technique for quality assessment of meat and fish: A review. Food Chemistry, 107, 1642–1651. doi:10.1016/j.foodchem.2007.10.014
- Herrero, A.M., Cambero, M.I., Ordóñez, J.A., Hoz, L., & Carmona, P. (2008). Raman spectroscopy study of the structural effect of microbial transglutaminase on meat systems and its relationship with textural characteristics. Food Chemistry, 109, 25–32. doi:10.1016/j.foodchem.2007.12.003
- Herrero, A.M., Carmona, P., Pintado, T., Jiménez-Colmenero, F., & Ruíz-Capillas, C. (2011). Olive oil-in-water emulsions stabilized with caseinate: Elucidation of protein–lipid interactions by infrared spectroscopy. Food Hydrocolloids, 25(1), 12–18. doi:10.1016/j.foodhyd.2010.04.014
- Kang, Z.-L., Wang, P., Xu, X.-L., Zhu, C.-Z., Li, K., & Zhou, G.-H. (2014). Effect of beating processing, as a means of reducing salt content in frankfurters: A physico-chemical and Raman spectroscopic study. Meat Science, 98, 171–177. doi:10.1016/j.meatsci.2014.05.025
- Krimm, S., & Bandekar, J. (1986). Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Advances in Protein Chemistry, 38, 181–365.
- Li-Chan, E., Nakai, S., & Hirostuka, M. (1994). Raman spectroscopy as a probe of protein structure in food systems. In RL. Yada & JL. Smith (Eds.), Protein structure-function relationships in foods (pp. 163–197). London: Blackie Academics & professional.
- Li-Chan, E.C.Y. (1996). The applications of Raman spectroscopy in food science. Trends in Food Science & Technology, 7, 361–370. doi:10.1016/S0924-2244(96)10037-6
- Liu, R., Zhao, S.-M., Xie, B.-J., & Xiong, S.-B. (2011). Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocolloids, 25, 898–906. doi:10.1016/j.foodhyd.2010.08.016
- Ma, F., Chen, C.G., Sun, G.J., Wang, W., Fang, H.M., & Han, Z. (2012). Effects of high pressure and CaCl2 on properties of salt-soluble meat protein gels containing locust bean gum. Innovative Food Science & Emerging Technologies, 14, 31–37. doi:10.1016/j.ifset.2011.12.001
- Maeda, Y., & Kitano, H. (1995). The structure of water in polymer systems asrevealed by Raman spectroscopy. Spectrochimica Acta, 51, 2433–2446. doi:10.1016/0584-8539(95)01446-2
- Martínez, M.A., Robledo, V., Velazquez, G., Ramírez, J.A., Vázquez, M., & Uresti, R.M. (2014). Effect of precooking temperature and microbial transglutaminase on the gelling properties of blue crab (Callinectes sapidus) proteins. Food Hydrocolloids, 35, 264–269. doi:10.1016/j.foodhyd.2013.06.001
- Mukherjee, S., Chowdhury, P., & Gai, F. (2007). Infrared study of the effect of hydration on the amide I band and aggregation properties of helical peptides. The Journal of Physical Chemistry B, 111, 4596–4602. doi:10.1021/jp0689060
- Ngarize, S., Herman, H., Adams, A., & Howell, N.K. (2004). Comparison of changes in the secondary structure of unheated, heated, and high-pressure-treated β-lactoglobulin and ovalbumin proteins using fourier transform raman spectroscopy and self-deconvolution. Journal of Agricultural and Food Chemistry, 52, 6470–6477. doi:10.1021/jf030649y
- Perisic, N., Afsetha, N.K., Ofstada, R., Hassani, S., & Kohler, A. (2013). Characterising protein, salt and water interactions with combined vibrational spectroscopic techniques. Food Chemistry, 138, 679–686. doi:10.1016/j.foodchem.2012.10.117
- Pietrasik, Z., & Li-Chang, E.C.Y. (2002). Binding and textural properties of beef gels as affected by protein, κ-carrageenan and microbial transglutaminase addition. Food Research International, 35, 91–98. doi:10.1016/S0963-9969(01)00123-5
- Renzetti, S., Bello, F.D., & Arendt, E.K. (2008). Microstructure, fundamental rheology and baking characteristics of batters and breads from different glutenfree flours treated with a microbial transglutaminase. Journal of Cereal Science, 48(1), 33–45. doi:10.1016/j.jcs.2007.07.011
- Schmidt, H., Scheier, R., & Hopkins, D.L. (2013). Preliminary investigation on the relationship of Raman spectra of sheep meat with shear force and cooking loss. Meat Science, 93, 138–143. doi:10.1016/j.meatsci.2012.08.019
- Shao, J.-H., Zou, Y.-F., Xu, X.-L., Wu, J.-Q., & Zhou, G.-H. (2011). Evaluation of structural changes in raw and heated meat batters prepared with different lipids using Raman spectroscopy. Food Research International, 44, 2955–2961. doi:10.1016/j.foodres.2011.07.003
- Socrates, G. (2001). Infrared and Raman characteristic group frequencies. Chichester: Wiley.
- Somboonpanyakula, P., Barbut, S., Jantawata, P., & Chinprahasta, N. (2007). Textural and sensory quality of poultry meat batter containing malva nut gum, salt and phosphate. LWT – Food Science and Technology, 40, 498–505. doi:10.1016/j.lwt.2005.12.008
- Trespalacios, P., & Pla, R. (2007). Simultaneous application of transglutaminase and high pressure to improve functional properties of chicken meat gels. Food Chemistry, 100(1), 264–272. doi:10.1016/j.foodchem.2005.09.058
- Tu, A. (1982). Raman spectroscopy in biology. New York, NY: John Wiley.
- Verma, A.K., Sharma, B.D., & Banerjee, R. (2010). Effect of sodium chloride replacement and apple pulp inclusion on the physico-chemical, textural and sensory properties of low fat chicken nuggets. LWT – Food Science and Technology, 43, 715–719. doi:10.1016/j.lwt.2009.12.006
- Wang, R., Peng, Z., Hui, T., Wang, F., Yao, Y., Zhang, Y., & Zhou, G. (2013). Potential use of crude extracts from Alaska Pollock muscle as meat tenderizer. CyTA-Journal of Food, 11(1), 50–59. doi:10.1080/19476337.2012.684357
- Wu, M., He, Q., Hong, Y., & Wang, S. (2016). Preheating of kidney bean proteins enhances cross-linking and functional properties with chicken myofibrillar proteins induced by transglutaminase. LWT – Food Science and Technology, 65, 816–822. doi:10.1016/j.lwt.2015.09.019
- Yalçın, M.Y., & Şeker, M. (2016). Effect of salt and moisture content reduction on physical and microbiological properties of salted, pressed and freeze dried turkey meat. LWT – Food Science and Technology, 68, 153–159. doi:10.1016/j.lwt.2015.12.032
- Yokoyama, K., Nio, N., & Kikuchi, Y. (2004). Properties and applications of microbial transglutaminase. Applied Microbiology and Biotechnology, 64(4), 447–454. doi:10.1007/s00253-003-1539-5
