ABSTRACT
This study aimed to obtain the drying and water absorption curves of husk rice grain (Oryza sativa L.), cv. Urucuia, as well as the thermodynamic properties. Five temperatures (35°C, 45°C, 55°C, 65°C and 75°C) were used. Two terms, logarithmic, modified Midilli, Page and Verma models were used to model the drying process, and the Peleg model was used to model the soaking process. The modified Midilli and Peleg models satisfactorily accounted for the processes of drying and absorption of water, respectively. The activation energy for the drying and soaking was 51.03 and 37.06 kJ mol−1, respectively. The enthalpy and entropy decreased with increasing temperature, regardless of the process. Their values ranged between −6.60 and 48.47 kJ mol−1 and between −0.1084 and −0.2464 kJ mol−1 K−1 for enthalpy and entropy, respectively. The Gibbs free energy increased with increasing temperature, with values between 69.36 and 86.22 kJ mol−1.
RESUMEN
Este estudio pretende obtener las curvas de secado y absorción de agua de las cáscaras de granos de arroz (Oryza sativa L.), cv. Urucuia, así como sus propiedades termodinámicas. Se utilizaron cinco temperaturas (35°C, 45°C, 55°C, 65°C y 75°C). Se utilizaron dos conceptos, logarítmico y modelos Midilli, Page y Verma modificados para modelar el proceso de secado, además se utilizó el modelo Peleg para modelar el proceso de remojo. Los modelos Midilli and Peleg modificados modelaron satisfactoriamente los procesos de secado y absorción de agua, respectivamente. La energía de activación del secado y el remojo fue de 51,03 y 37,06 kJ mol−1, respectivamente. La entalpía y entropía disminuyeron con el aumento de temperatura, independientemente del proceso. Sus valores se clasificaron entre −6,60 y 48,47 kJ mol−1 además de entre −0,1084 y −0,2464 kJ mol−1 K−1 de entalpía y entropía, respectivamente. La energía libre de Gibbs aumentó con el incremento de la temperatura, con valores entre 69,36 y 86,22 kJ mol−1.
Introduction
Rice is one of the most consumed agricultural products in the world, and in Brazil, along with beans, it is the food base of 84% of the population (Souza, Pereira, Yokoo, Levy, & Sichieri, Citation2013). The average consumption of rice in Brazil varies from 74 to 76 kg/inhabitant/year (Embrapa, Citation2015). In 2014, approximately 12.1 million tons were produced, and approximately 12 million tons were consumed, of which 950,000 tons were imported and 1.2 million tons of rice were exported in the hull (MAPA, Citation2015).
The increasing production of rice has enhanced the study of new technologies and solutions for problems related to the post-harvest of this crop. The drying and hydration of the rice operations are important steps in the production chain, especially in the parboiling of the product (Botelho, Corrêa, Goneli, Martins, & Baptestini, Citation2010).
The process of water absorption in rice grains by soaking at a controlled temperature is essential for the occurrence of partial or complete starch gelatinization (Botelho et al., Citation2010). The drying process reduces the water content of agricultural products, increasing the shelf life and reducing the incidence of losses due to attacks from insects and micro-organisms, as well as the loss of dry matter from the respiration of the product. The correct measurement and prediction of the time needed for water absorption assists in making the decision for the correct storage of the product (Mohsenin, Citation1986).
Many studies have reported the importance of the kinetics of absorption and drying for various agricultural products such as bean and chickpea (Shafaei, Masoumi, & Roshan, Citation2016), yellow peas (Mercier, Villeneuve, Mordor, Moresoli, & Marcos, Citation2015), pumpkin (Hashim, Daniel, & Rahaman, Citation2014) and spearmint (Ayadi, Mabrouk, Zouari, & Bellagi, Citation2014). These studies identified the influence of temperature on the rate and the amount of water that is moved between the product and the environment.
The prediction of the hydration and drying processes is of great importance to the industry, with both for processes contributing to the design of such equipment to determine the production processes. Mathematical models are essential to predict and simulate the behavior of materials under a certain process.
Determining the best model can be built based on drying curves and hydration. These curves are important to set the drying/soaking time for the industry, according to the water content that is intended to be achieved. Moreover, one can determine the power that is required for drying and absorption, which is represented by thermodynamic properties. Different studies have reported on thermodynamic properties such as the enthalpy, entropy, Gibbs free energy and enthalpy–entropy theory of compensation in different products such as chia (Velásquez et al., Citation2015), potato flakes (Lago, Liendo-Cárdenas, & Noreña, Citation2013) and pineapple (Bispo, Bonafe, Santana, & Santos, Citation2015).
Given the above and the importance of theoretical knowledge of the drying and water absorption processes of agricultural products, the objective of this study was to determine the drying and water absorption curves of husk rice grain, as well as to determine the thermodynamic properties of these processes.
Materials and methods
The study was conducted in the Physical Properties and Quality of Agricultural Products Laboratory belonging to the National Center Storage Training (CENTREINAR), located at the Federal University of Viçosa, Viçosa, MG.
The husk rice grains that were used were the Urucuia variety from the Experimental Farm EPAMIG in the southern region of Minas Gerais.
The grain drying curves
The grains were manually collected with an initial water content of approximately 0.28 g kg−1, which was determined using an oven with forced air circulation at a temperature of 105 ± 1°C for 24 h in three repetitions (Brasil, Citation2009). Grains were dried in an oven at five different temperatures (35°C, 45°C, 55°C, 65°C and 75°C), and the equilibrium was indicated when the variation in three successive weightings was not higher than 0.01 g. To determine the moisture ratios (MRs) during drying in different air conditions, Equation 1 was used as follows:
where MR is the moisture ratio, dimensionless; is the product moisture content at time t, decimal dry basis;
is the product of the equilibrium water content, decimal dry basis; and
is the initial water content of the product, decimal dry basis.
The experimental drying data were adjusted to the five mathematical models (two terms, logarithmic, modified Midilli, Page and Verma) that are cited in the literature and that are used to represent the drying process of agricultural products ().
Table 1. Mathematical models used to predict drying process of husk rice.
Tabla 1. Modelos matemáticos utilizados para pronosticar el proceso de secado de las cáscaras de arroz
The absorption curves
Beans were manually collected with a water content of 0.17 g kg−1, which was determined using an oven with forced air circulation at a temperature of 105 ± 1° C for 24 h in three repetitions (Brasil, Citation2009).
In the absorption process, 100 g of husk rice were soaked with distilled water in a Becker flask at a ratio of four volumes of water to one volume of the product at temperatures of 35°C, 45°C, 55°C, 65°C or 75°C in a water bath for a period of 10 h, with three repetitions per temperature. During this period, samples were periodically weighted at every hour on a digital scale with precision set to 0.01 g. Then, the samples were removed from soaking and left on a paper towel for three minutes so that the surface water was removed. The grains were weighed and then returned to immersion.
For the absorption experimental data, the model Peleg (Peleg, Citation1988) was fitted (Equation 7) as
where C1 is the Peleg constant rate (100 h kgms)kg−1a and C2 is the Peleg constant capacity, (100 kgms)kg−1a.
Thermodynamic properties
To calculate the activation energy of the drying and soaking processes, the Arrhenius equation was used. The Arrhenius equation demonstrates the relationship between the activation energy and the rate at which the reaction occurs. For the drying process, the values of the drying constant rate were used (k) in the Arrhenius equation. For the absorption process, the C1 values were used in the Arrhenius equation because this parameter is related to the mass transfer rate (Botelho et al., Citation2010). This feature can be seen in Equation 8:
where A0 is the pre-exponential factor, h−1; Ea is the activation energy, J mol−1; R is the universal gas constant, 8314 J mol−1 K−1; and T is the temperature, K.
Determination of the thermodynamic properties of the mass transfer processes in paddy rice was carried out by a known method (Corrêa, Oliveira, & Santos, Citation2012):
where ∆H is the differential enthalpy, J mol−1; ∆S is the differential entropy, J mol−1 K−1; ∆G is the Gibbs free energy, J mol−1; kB is Boltzmann’s constant, 1.38 × 10–23 J K−1; and hP is Planck’s constant, 6,626 × 10–34 J s−1.
Statistical analyses
To adjust the mathematical models, non-linear regression analysis using the Gauss Newton method was performed using STATISTICA software 8.0®. To analyze the degree of suitability of the models, the standard deviation of the estimate values (SDE), the mean relative error (MRE) and the adjusted coefficient of determination (R2) (explained variance) were used. In addition, the analysis of the residues generated by the models will be taken into consideration. The values of the SDE and MRE are calculated by the following equations:
where SDE is the standard deviation of the estimate, decimal dry basis; Y is the experimental value; Ŷ is the value calculated by the model; GLR are the degrees of freedom of the model; MRE is the mean relative error, %; and n is the number of observed data.
Results and discussion
Drying curves
shows the values of R2, MRE), SDE and the residue analysis of the mathematical models adjusted to the drying data.
Table 2. Statistical parameters of drying models for husk rice grain.
Tabla 2. Parámetros estadísticos de los modelos de secado de las cáscaras de granos de arroz.
The objective of mathematical modeling is to determine the models that present the best possible fit to the experimental data, evaluating the error of the estimate and the distribution of the residuals. In the case of an error associated with the estimation, many authors argue that a model has satisfactory data adjustment if the value of the MRE is less than 10% (Botelho et al., Citation2010; Corrêa, Botelho, Botelho, & Goneli, Citation2014). A maximum of 0.2 % (d.b.) of SDE was considered to be acceptable for model adequacy. Thus, of all the models evaluated, the modified Midilli model showed the smallest magnitudes of SDE and MRE and is the only one that met the criteria used for the description of husk rice drying ().
Usually, the determination coefficient (R2) shows higher values for those models with a better fit, as was observed for the modified Midilli model. The obtained values are above 99.5%. However, this index should only be used as an aid and not as a criterion for deciding between nonlinear models (Botelho et al., Citation2010).
The distribution of the residues, which are the differences between the values that are observed experimentally and those that are estimated by the model, are represented as a function of the estimated levels; this representation allows one to evaluate how the model estimates the values of the variable under study (Corrêa et al., Citation2014). In order to confirm the suitability of the modified Midilli model, the residue distribution was evaluated. This distribution of this model to describe the drying of husk rice is shown in .
Figure 1. Behavior of the residual distribution of modified Midilli model, in order to describe drying of husk rice (cv. Urucuia).
Figura 1. Comportamiento de la distribución residual del modelo Midilli modificado para describir el secado de las cáscaras de arroz (cv. Urucuia).
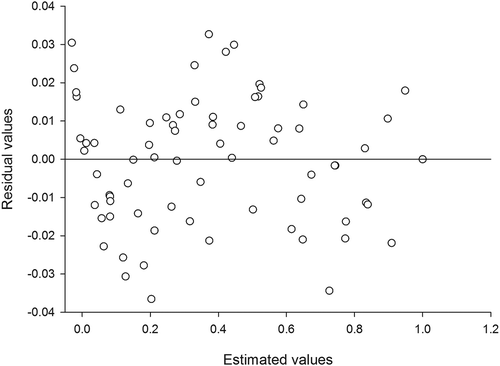
The trend presented by the residues of the modified Midilli model to describe husk rice drying was random. Although it is a subjective assessment, the residual distribution is used in order to determine if a model is considered acceptable when its residual values are in a horizontal zone close to zero, forming random distributions. If residual distributions form geometric figures, then it indicates that there is a great possibility of data tracks in which the model underestimates or overestimates the real conditions, or if the values tend to accumulate at one point off the axis, then the residual distribution is considered to be biased. Thus, the model is considered inadequate to represent the phenomenon in question. In addition to presenting the smallest error magnitudes, the modified Midilli model was also random in its estimates (), and it is therefore recommended to represent the drying of husk rice (cv. Urucuia) in the range of temperatures that were used in this study.
The modified Midilli model also satisfactorily represented the drying of leek slices (Doymaz, Citation2008), mint leaves (Ozbek & Dadali, Citation2007), parsley leaves (Soysal, Oztekin, & Eren, Citation2006), sliced Golden apples (Menges & Ertekin, Citation2006) and coffee fruits (Corrêa, Oliveira, Botelho, Goneli, & Carvalho, Citation2010). It is important to remember the need for studies on the modeling of drying various agricultural products regardless of whether they are similar in size and shape and /or chemically similar. In the absence of drying husk rice data (cv. Urucuia), other variety data may be used, which can result in significant errors. For example, the drying time encountered in this work for the air at 55°C is 2.0 h, less than that found for drying husk rice (cv. Lido), 19.0 h (Iguaz, San Martín, Maté, Fernández, & Vírseda, Citation2003).
shows the coefficients of the modified Midilli model that were obtained in the modeling process; the coefficients represent the drying process of husk rice.
Table 3. Parameters of the modified Midilli model fitted to experimental data of husk rice drying, cv. Urucuia.
Tabla 3. Parámetros del modelo Midilli modificado utilizado con los datos experimentales del secado de las cáscaras de arroz, cv. Urucuia.
It can be seen from that the drying constant ‘k’ increases in absolute values with increasing temperatures. This fact is expected because higher temperatures lead to a higher drying rate, reaching equilibrium water content faster. Note that the other parameters (n and b) also have a relationship with temperature. Thus, we sought to study the correlation between the parameters of the modified Midilli model and the temperature, with 99.2%, 96.3% and 93.4% correlation coefficients for the parameters k, n and b, respectively.
Due to the high degree of correlation that was presented (>90%), the correlation can be reached with a single model of modified Midilli, which accounts for the temperature range that is used in the work (35–75°C). This equation is expressed as follows:
shows the husk rice drying curves (cv. Urucuia) that were observed and estimated by the modified Midilli model at different temperature conditions.
Figure 2. Husk rice drying curves at temperatures of 35°C, 45°C, 55°C, 65°C and 75°C.
Figura 2. Curvas de secado de las cáscaras de arroz a temperaturas de 35°C, 45°C, 55°C, 65°C y 75°C.
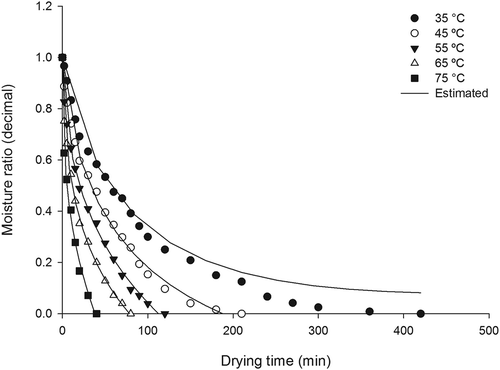
In the study of drying agricultural products, especially grain and seed, it is known that these products have a constant low drying rate period because of the occurrence of a difference between the surface moisture and the moisture inside of the product. This difference essentially occurs because of the different speeds of water molecules as they migrate from inside to the periphery of the product as well as because of the evaporation of surface water molecules. This process can be seen in , wherein the drying occurs mostly in the falling rate period.
Absorption curves
shows the parameters of the Peleg model, the values of R2, MRE, SDE and the analysis of residues of the Peleg model set to the data on the water absorption by the husk rice grain.
Table 4. Statistical parameters of Peleg model for water absorption by husk rice grain.
Tabla 4. Parámetros estadísticos del modelo Peleg de la absorción de agua de las cáscaras de granos de arroz.
According to the values of the MRE and SDE and the residual analysis, as well as the statistical parameters discussed above, it is concluded that the Peleg model was adjusted satisfactorily to the data on the water absorption by the husk rice grain (cv. Urucuia). However, it is noted that for a temperature of 75°C, there is a significant increase in the MRE and SDE, indicating a higher estimation error at this temperature. This fact can be observed in , which presents the curves of the water absorption by the husk rice grains.
Figure 3. Curves of water absorption by the grains of paddy rice (cv. Urucuia) at temperatures of 35°C, 45°C, 55°C, 65°C and 75°C.
Figura 3. Curvas de absorción de agua de los granos de arroz con cáscara (cv. Urucuia) a temperaturas de 35°C, 45°C, 55°C, 65°C y 75°C.
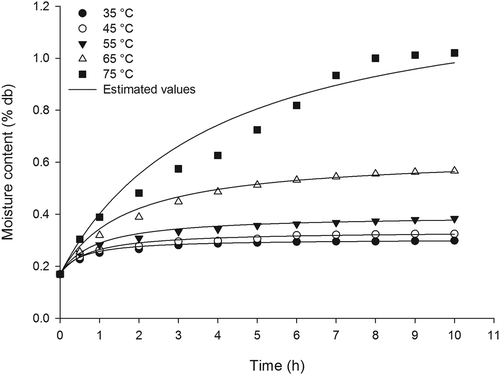
Previous studies have also reported a better fit of the Peleg model at lower temperatures (<70°C) and have attributed this difference to the higher leaching of solids that occurs in such processes at higher temperatures, which is not accounted for by the model (Botelho et al., Citation2010; Shafaei et al., Citation2016).
It is observed that at the beginning of the process, there is a high rate of water absorption, which tends to reduce and stabilize over time (). This fact is pronounced from approximately 3 h for the husk rice grains subjected to soaking at 35°C, 45°C and 55°C. At a temperature of 65ºC, this trend was reached after approximately 6 h. The husk rice grains subjected to 75°C reached equilibrium after approximately 8 h.
The high rate of water absorption at the beginning of the process can be attributed to the inhibition of the capillary outer layers of the pericarp, which hasten the absorption of water (Bello, Tolaba, & Suarez, Citation2004). Another factor that could be attributed to the high initial rate of water absorption is the high matric potential of the various constituents of the grain (Botelho et al., Citation2010).
The constant C1 of the Peleg model is related to the mass transfer rate, and the smaller the values of C1 is, the greater the initial water absorption rates (Turhan, Sayar, & Gunasekaran, Citation2002). This coefficient can also be related to the diffusion coefficient (Shafaei et al., Citation2016). The constants C1 for this experiment decreased with increasing temperature, as shown in . Researchers have also reported that the constant C1 decreases with temperature (Shafaei et al., Citation2016). Higher temperatures result in gelling of the product, resulting in the expansion and softening of the grain; thus, a greater number of pores and cracks are opened, facilitating the migration of water into the product (Shafaei et al., Citation2016).
The constant C2 of the Peleg model is related to the capacity for water absorption, and the lower the value is, the higher the water absorption of the product (Resende & Corrêa, Citation2007). It is noted from that the higher the temperature is, the lower the value of C2, indicating that the product is able to absorb more water. Similar results were observed in previous studies (Botelho et al., Citation2010; Resende & Corrêa, Citation2007). The C2 dependence on temperature can be attributed to an increased product equilibrium water content with increasing hydration temperature (Shafaei et al., Citation2016).
As both constants C1 and C2 showed linear behavior in the absorption temperature (correlation values of 81.2% and 99.3%, respectively), a single model can be described as having occurred with the husk rice drying curves. The Peleg model as a function of temperature is inserted as
Thermodynamic properties
The activation energy for the drying and soaking process was 51.03 and 37.06 kJ mol−1, respectively. Different activation energy values for drying were found, 8.19 and 5.45 kJ mol−1, for raw and pretreated husk rice, respectively (Bazargan, Gebreegziabher, Hui, & McKay, Citation2014). The activation energy for soaking parboiled husk rice was 33.2 kJ mol−1 (Botelho et al., Citation2010). Such differences in the activation energy values are mainly due to the different constitution of the rice cultivars, as well as to the raw material that was used, for example, with or without husk.
The smaller the activation energy is, the more easily a particular process occurs, in other words, the lower the energy that is required for the physical processing. Thus, immersion in water for husk rice grains occurs more easily than does the drying of that product.
shows the values that were obtained from the thermodynamic properties relating to the drying processes and water absorption by the husk rice grains.
Table 5. Thermodynamic properties (∆H: enthalpy; ∆S: entropy; ∆G: Gibbs free energy) of drying and water absorption processes of husk rice grain (cv. Urucuia).
Tabla 5. Propiedades termodinámicas (∆H: entalpía; ∆S: entropía; ∆G: energía libre de Gibbs) de los procesos de secado y absorción de agua de las cáscaras de granos de arroz (cv. Urucuia).
The differential enthalpy decreased with increasing temperature, irrespective of the reported procedure (). For drying, the differential enthalpy values were positive as this is a process with heat absorption, that is, endothermic (Shafaei et al., Citation2016). As for the absorption, differential enthalpy values were negative, indicating that during soaking, the water content of the product changed according to an exothermic transformation and was energetically favorable (Reusch, Citation2007). Similar results are reported in previous studies (Jideani & Mpotokwana, Citation2009; Shafaei et al., Citation2016).
Lower values of differential enthalpy indicate that a lower energy is required for the process to occur. This study showed, as expected, lower differential enthalpy values at higher drying temperatures, indicating that less energy is required for drying at higher temperatures. Similarly, at higher temperatures, the absorption requires less energy for water to migrate into the product.
Differential entropy is a thermodynamic quantity that is associated with the degree of disorder, as it is a state function where the values increase during a natural process in an isolated system. Analyzing the behavior of the differential entropy, it is concluded that this thermodynamic property showed similar behavior to the differential enthalpy in which the values decreased with increasing temperature (). This trend indicates that there is an increase in the system order, as it is entropically unfavorable (Jideani & Mpotokwana, Citation2009). This fact can be explained by the theory of activated complex in which a substance in an activation condition may acquire negative entropy if the degrees of freedom of translation or rotation are lost during the formation of the activated complex (Dannenberg & Kessler, Citation1988).
The Gibbs free energy increased with increasing temperature, and their values were positive, indicating that the drying and absorption in the present study conditions were not spontaneous. The positive value of the Gibbs free energy is characteristic of an endergonic reaction that requires the addition of energy from the environment in which the product is involved for the reaction to occur (Reusch, Citation2007). This same result was reported previously (Jideani & Mpotokwana, Citation2009; Shafaei et al., Citation2016).
Conclusions
The modified Midilli model was the one that was best fitted to the experimental data of drying husk rice, variety Urucuia, with rising drying constant values with increasing temperature. As for the soaking process, the Peleg model satisfactorily describes the water absorption curves by the husk rice grains. The constants C1 and C2 decreased with increasing temperature, indicating a higher initial absorption rate and higher product water absorption capacity, respectively.
The activation energy for the drying and soaking was 51.03 and 37.06 kJ mol−1, respectively. In the processes of drying and soaking husk rice grains, both differential enthalpy and entropy decreased with increasing temperature. For drying and soaking, the differential enthalpy values are positive and negative, respectively, indicating endothermic and exothermic processes. The Gibbs free energy increased with increasing temperature and with positive values, indicating that drying and water absorption do not occur spontaneously in the working conditions; thus, these processes require the addition of energy from an external source.
Acknowledgements
To CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico, the Instituto Federal do Sudeste de Minas Gerais and FAPEMIG - Fundação de Amparo à Pesquisa do Estado de Minas Gerais, for the essential support and financial aid.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ayadi, M., Mabrouk, S.B., Zouari, I., & Bellagi, A. (2014). Kinetic study of the convective drying of spearmint. Journal of the Saudi Society of Agricultural Sciences, 13, 1–7. doi:10.1016/j.jssas.2013.04.004
- Bazargan, A., Gebreegziabher, T., Hui, C.W., & McKay, G. (2014). The effect of alkali treatment on rice husk moisture content and drying kinetics. Biomass and Bioenergy, 70, 468–475. doi:10.1016/j.biombioe.2014.08.018
- Bello, M., Tolaba, M.P., & Suarez, C. (2004). Factors affecting water uptake of rice grain during soaking. Lebensmittel-Wissenschaft und –Technology, 37, 811–816. doi:10.1016/j.lwt.2004.02.014
- Bispo, J.A.C., Bonafe, C.F.S., Santana, K.M.O.V., & Santos, E.C.A. (2015). A comparison of drying kinetics based on the degree of hydration and moisture ratio. LWT - Food Science and Technology, 60, 192–198. doi:10.1016/j.lwt.2014.07.014
- Botelho, F.M., Corrêa, P.C., Goneli, A.L.D., Martins, M.A., & Baptestini, F.M. (2010). Análise da hidratação do arroz na parboilização. Ciência e Tecnologia de Alimentos, 30, 713–718. doi:10.1590/S0101-20612010000300023
- Brasil. (2009). Regras para análise de sementes (p. 399). Brasília: Ministério da Agricultura e Reforma Agrária.
- Corrêa, P.C., Botelho, F.M., Botelho, S.C.C., & Goneli, A.L.D. (2014). Isotermas de sorção de água de frutos de Coffea canephora. Revista Brasileira de Engenharia Agrícola e Ambiental, 18, 1047–1052. doi:10.1590/1807-1929/agriambi.v18n10p1047-1052
- Corrêa, P.C., Oliveira, G.H.H., Botelho, F.M., Goneli, A.L.D., & Carvalho, F.M. (2010). Modelagem matemática e determinação das propriedades termodinâmicas do café (Coffea arabica L.) durante o processo de secagem. Revista Ceres, 57, 595–601. doi:10.1590/S0034-737X2010000500005
- Corrêa, P.C., Oliveira, G.H.H., & Santos, E.S. (2012). Thermodynamic properties of agricultural products processes. In I. Arana (Ed.), Physical properties of foods: Novel measurement techniques and applications (pp. 131–141). Boca Raton: CRC Press.
- Dannenberg, F., & Kessler, H. (1988). Reaction kinetics of the denaturation of whey proteins in milk. Journal of Food Science, 53, 258–263. doi:10.1111/jfds.1988.53.issue-1
- Diamente, L.M., & Munro, P.A. (1993). Mathematical modelling of the thin layer solar drying of sweet potato slices. Solar Energy, 51, 271–276. doi:10.1016/0038-092X(93)90122-5
- Doymaz, I. (2008). Influence of blanching and slice thickness on drying characteristics of leek slices. Chemical Engineering Process, 47, 41–47. doi:10.1016/j.cep.2007.09.002
- Embrapa. (2015). Consumo, Mercado e Comercialização do Arroz no Brasil. Retrieved from http://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Arroz/ArrozIrrigadoBrasil/cap18.htm
- Ghazanfari, A., Emami, S., Tabil, L.G., & Panigrahi, S. (2006). Thin-layer drying of flax fiber: II. Modeling drying process using semi-theoretical and empirical models. Drying Technology, 24, 1637–1642. doi:10.1080/07373930601031463
- Hashim, N., Daniel, O., & Rahaman, E. (2014). A preliminary study: Kinetic model of drying process of pumpkins (Cucurbita Moschata) in a convective hot Air dryer. Agriculture and Agricultural Science Procedia, 2, 345–352. doi:10.1016/j.aaspro.2014.11.048
- Henderson, S.M. (1974). Progress in developing the thin-layer drying equation. Transactions of the ASAE, 17, 1167–1168. doi:10.13031/2013.37052
- Iguaz, A., San Martín, M.B., Maté, J.I., Fernández, T., & Vírseda, P. (2003). Modelling effective moisture difusivity of rough rice (Lido cultivar) at low drying temperatures. Journal of Food Engineering, 59, 253–258. doi:10.1016/S0260-8774(02)00465-X
- Jideani, V.A., & Mpotokwana, S.M. (2009). Modeling of water absorption of Botswana bambara varieties using Peleg’s equation. Journal of Food Engineering, 92, 182–188. doi:10.1016/j.jfoodeng.2008.10.040
- Lago, C.C., Liendo-Cárdenas, M., & Noreña, C.P.Z. (2013). Thermodynamic sorption properties of potato and sweet potato flakes. Food and Bioproducts Processing, 91, 389–395. doi:10.1016/j.fbp.2013.02.005
- MAPA. (2015). Estatísticas e Dados Básicos de Economia Agrícola. Retrieved from http://www.agricultura.gov.br/arq_editor/Pasta%20de%20Fevereiro%20-%202015.pdf
- Menges, H.O., & Ertekin, C. (2006). Mathematical modeling of thin layer drying of Golden apples. Journal of Food Engineering, 77, 119–125. doi:10.1016/j.jfoodeng.2005.06.049
- Mercier, S., Villeneuve, S., Mordor, M., Moresoli, C., & Marcos, B. (2015). Modeling of the water absorption during the steeping of yellow peas. Food and Bioproducts Processing, 94, 20–28. doi:10.1016/j.fbp.2014.12.004
- Mohsenin, N.N. (1986). Physical properties of plant and animal materials (p. 841). New York, NY: Gordon and Breach.
- Ozbek, B., & Dadali, G. (2007). Thin-layer drying characteristics and modelling of mint leaves undergoing microwave treatment. Journal of Food Engineering, 83, 541–549. doi:10.1016/j.jfoodeng.2007.04.004
- Peleg, M. (1988). An empirical model for the description of moisture sorption curves. Journal of Food Science, 53, 1216–1219. doi:10.1111/jfds.1988.53.issue-4
- Resende, O., & Corrêa, P.C. (2007). Modelagem matemática do processo de hidratação de sementes de feijão. Acta Scientiarum. Agronomy, 29, 373–378. doi:10.4025/actasciagron.v29i3.387
- Reusch, W. (2007). The nature of energy. Virtual Textbook of Organic Chemistry. Michigan: MSU.
- Shafaei, S.M., Masoumi, A.A., & Roshan, H. (2016). Analysis of water absorption of bean and chickpea during soaking using Peleg model. Journal of the Saudi Society of Agricultural Sciences, 15, 135–144. doi:10.1016/j.jssas.2014.08.003
- Souza, A.M., Pereira, R.A., Yokoo, E.M., Levy, R.B., & Sichieri, R. (2013). Most consumed foods in Brazil: National Dietary Survey 2008–2009. Revista Saúde Pública, 47, 190–199. doi:10.1590/S0034-89102013000700005
- Soysal, Y., Oztekin, S., & Eren, O. (2006). Microwave drying of parsley: Modelling, kinetics, and energy aspects. Biosystems Engineering, 93, 403–413. doi:10.1016/j.biosystemseng.2006.01.017
- Turhan, M., Sayar, S., & Gunasekaran, S. (2002). Application of Peleg model to study water absorption in chickpea during soaking. Journal of Food Engineering, 53, 153–159. doi:10.1016/S0260-8774(01)00152-2
- Velásquez, G.S.K., Figueira, A.C., Rodriguez, H.M.E., Roman, G.A., Carrillo, N.H., & Perez, A.C. (2015). Sorption isotherms, thermodynamic properties and glass transition temperature of mucilage extracted from chia seeds (Salvia hispanica L.). Carbohydrate Polymers, 121, 411–419. doi:10.1016/j.carbpol.2014.11.068
- Verma, L.R., Bucklin, R.A., Endan, J.B., & Wratten, F.T. (1985). Effects of drying air parameters on rice dryingmodels. Transactions of the ASAE, 28, 296–301. doi:10.13031/2013.32245
- Yagcioglu, A., Degirmencioglu, A., & Cagatay, F. (1999). Drying characteristic of laurel leaves under different conditions. In Proceedings of 7th International congress on agricultural mechanization and energy (pp. 565–569). Turkey: Adana.
