ABSTRACT
The contents and enantiomeric distributions of three chiral compounds, linalool, phenylethanol and acetoin, were investigated in three different palm wines (i.e. Elaeis guineensis, Borassus flabellifer, and Nypa fruticans). While N. fruticans and B. flabellifer wines were predominated with the (S)-enantiomers of linalool, phenylethanol and acetoin, respectively, E. guineensis wine contained acetoin primarily as (R)-enantiomers in addition to the (S)-forms of linalool and phenylethanol. Interestingly, results revealed a high level of acetoin in all wines with concentrations ranging from 2437 to 6611 µg/L and an average ratio of S/R of 4:96–100:0. Moreover, noticeable differences occurred in the enantiomeric ratios and concentrations of enantiomers of the chiral compounds during storage. In all the wines, concentration of the (S)-form decreased during storage, whereas those of the (R)-form increased.
RESUMEN
En este estudio se investigaron los contenidos y las distribuciones enantioméricas de tres compuestos quirales (linalol, feniletanol y acetoína) en tres vinos de palma distintos (Elaeis guineensis, Borassus flabellifer y Nypa fruticans). Mientras que los (S)-enantiómeros de linalol, feniletanol y acetoína predominan en los vinos N. fruticans y B. flabellifer, el vino E. guineensis contiene principalmente acetoína como (R)-enantiómeros, además de las formas (S) de linalol y feniletanol. Los resultados dan cuenta de la presencia de un elevado nivel de acetoína en todos los vinos, comprobándose que sus concentraciones oscilan en un rango de 2437 a 6611 µg/L y una ratio promedio de S/R de 4:96 a 100:0. Por otra parte, se constató que durante su almacenamiento se presentaron diferencias notables en las ratios enantioméricas y las concentraciones de enantiómeros de los compuestos quirales. En todos los vinos disminuyó la concentración de la forma (S), aumentando la de la forma (R).
Introduction
Palm wine is consumed throughout the tropics and appears as a whitish liquid produced by natural fermentation of the sap of Elaeis guineensis and Raphia hooker (Mbuagbaw & Noorduyn, Citation2012). Palm sap contains abundant sucrose and polar side chain amino acids especially asparagine and glutamine. The sap is oyster-white and translucent, with a nearly neutral pH (Aimi, AbuBakar, & Dzulkifly, Citation2013). However, it is highly susceptible to spontaneous fermentation by natural microbial floral. Fermentation brings about rapid decreases in sugar level as it is converted to alcohol and other products (Obire, Citation2005). The sap becomes milky-white (i.e. palm wine) due to the increased microbial suspension resulting from the prolific growth of the fermenting organisms (Amoa-Awua, Sampson, & Tano-Debrak, Citation2007). Sources of the fermenting organisms are the gourds, tapping implements and air.
The composition, flavour and quality of palm wine have been reported to vary with the time, duration and place of tapping. For instance, the flavour of palm wine thereof is not only dependent on the type, but majorly determined by microbial reactions. Several numbers of volatile compounds have been identified in different types of palm wines (Aimi et al., Citation2013; Jirovetz, Buchbaucer, Fleischhacker, & Ngassoum, Citation2001). In recent years, studies have been focused on the chemical basis of palm wine aroma (Lasekan, Buettner, & Christlebauer, Citation2007, Citation2009; Lasekan & Otto, Citation2009). Recent study has shown that acetoin, linalool and 2-phenyl ethanol (all chiral compounds) are important components of palm wine, and they contributed to the moody, citrusy aroma of palm wine (Lasekan, Citation2013). The enantiomeric compositions of chiral compounds have been well documented in sweet white wine (Takatoshi, Niclass, Frerot, & Dubourdieu, Citation2006), red wine (Lytra, Tempere, de Revel, & Barbe, Citation2012), fruit beverages (Castillo, Caja, & Herraiz, Citation2003) and fruit juices (Weber, Maas, & Mosandl, Citation1995). The enantiomeric compositions of chiral compounds of palm wine varieties are yet to receive attention from researchers. Therefore, a better knowledge of the enantiomer differentiation of the chiral compounds in palm wine would help in the improvement of the flavouring and inhibition of off-flavours through technological processes. Enantiomer differentiation allows a more accurate evaluation of flavours and fragrances (Armstrong, Chang, & Li, Citation1990). In addition, each enantiomer of many chiral compounds is known to evoke different neural responses in consumers (Barba, Flores, & Herraiz, Citation2010).
In order to fulfil the lack of study in the area of enantiomeric distribution of chiral compounds in palm wine, this study was carried out to establish the enantiomeric composition and distribution of three key compounds in three palm wine varieties.
Materials and methods
Raw materials
Three bottles (4.5 L) of each wine obtained from three different palm trees (E. guineensis, Borassus flabellifer and Nypa fruticans) were freshly obtained from the production farm in a sterilized container encrusted in ice. The samples were bottled, pasteurized and dispensed into 45 mL glass-tubes. A set was stored (3 months) at ambient temperature (28 ± 2°C), and the others were stored at −18°C prior to analysis. Accordingly, there was no extended storage of samples at elevated temperatures prior to analysis. The alcohol contents of the palm wines were 3.5% (E. guineensis), 3.3% (B. flabellifer) and 3.0% (N. fruticans). Dilute alcohol solution was prepared as described by Lytra, Cameleyre, Tempere, and Barbe (Citation2015) with high-purity ethanol and micro filtered water, to produce an ethanol content of 12% (v/v) and 5 g/L tartaric acid (pH adjusted to 3.5 with sodium hydroxide.
Chemicals
The following reference compounds were obtained from the suppliers given in parentheses: ethyl acetate, 98%; methyl butanoate, 99%; 2-methylbutanal, 98%; ethyl-2-methylbutanoate, 99%; ethylpentanoate, 97%; 2-heptanone, 98%; 3-methylbutanol, 99%; ethylhexanoate, 97%; acetoin, 97%; ethyl lactate, 98%; 3-hydroxy-2-butanone, 98%; (Z)-1,5-octadien-3-one, 97%; 1-hexanol, 99%; ethyl octanoate, 98%; hexyl-3-methylbutanoate, 98%; methional, 98%; 3-isobutyl-2-methoxypyrazine, 70%; 3-methylbutyl acetate, 80%; linalool, 98%; 2-methyl propionic acid, 97%; butanoic acid, 97%; 3-methyl butanoic acid, 80%; (E,E)-2,4-nonadienal, 97%; 3-methylthiol-1,1-propanal, 98%; 3-mercapto-2-methylpentanone, 98%; β-damascenone, 97%; 2-methoxyphenol, 98%; 4-hydroxy-2,5-dimethyl-3(2H)-furanone, 99%; homofuraneol, 98%; ethyl cinnamate, 98%; sotolone, 98%; and phenylacetic acid, 97% (Aldrich, Steinheim, Germany); while, 2,3-butandione, 99%; 2-acetylpyridine, 99%; and pentanoic acid, 97% were purchased from Fluka, Neu-Ulm, Germany. Others such as acetic acid, 99%; 2-ethyl-3,5-dimethylpyrazine, 98%; 2-phenyl ethanol, 98%; and vanillin were from Acros Organics, Morris Plain, NJ, USA & Merck Darmstadt, Germany, respectively. For chiral analysis, the following reference compounds were used: (R, S)-linalool and (R, S)-phenylethanol were from Fluka, Buchs, Switzerland. Absolute ethanol (analytical grade, 99.97%, Scharlau Chemie S.A., Barcelona Spain) was distilled before use. Micro filtered water was obtained from a Milli-Q Plus water system (resistivity = 18 Ω cm, Millipore, Saint-Quentin-en-Yvelines, France)
Synthesis
(R)-acetoin was synthesized by monobenzylation of commercially available (2R, 3R)-butanediol (99% purity) (Sigma-Aldrich, Steinheim, Germany), with sodium hydride and benzyl bromide as described by Crout, Littlechild, and Morrey (Citation1986). Oxidation of the product with pyridinium dichromate was followed by debenzylation of the resulting ketone (H2/Pd-C). Careful workup yielded >98% (R)-acetoin in chemical purity as determined by (Chiral) Gas chromatography (GC). Due to its high racemization rate, it was stored at −70°C until use.
Separation of enantiomers from the commercial racemic mixtures (i.e. linalool phenylethanol)
High performance liquid chromatography (HPLC) separation was performed on a Merck L-7100 pump connected to a Merck variable-wavelength UV detector. The preparative column was a Chiralpak AS-H model (250 × 20 mm). The eluent was made up of 10% isopropanol in n-heptane with a flow rate of 20 mL/min at a constant temperature of 25°C. The two compounds were collected after HPLC purification. Each enantiomer of linalool and phenylethanol yielded approximately 47 mg/L.
Chiral identity
The configuration of linalool and phenylethanol was determined by measuring the specific rotation values of the optically active compounds. Optical rotation was determined on a Perkin-Elmer 341 polarimeter with a 1 dm cell. The following specific rotation values were obtained for (R)-linalool: [α]D20 = −15.1° and (S)-linalool: [α]D20 = +17.4°, while the (R/S) – (±)-linalool: [α]D20 = 0° and contains 50.9% of the (R)-form and 49.1% of the (S)-form, respectively. (R)-1-phenylethanol has specific rotation of −55.15° while the (S)-1-phenylethanol has +45°.
Isolation and identification of wines volatile compounds
The isolation of the palm wines’ volatile compounds was performed by extracting 300 mL of each palm wine with diethyl ether (200 mL), followed by distillation in vacuum (Buettner & Schieberle, Citation2001). A similar workup procedure reported earlier (Lasekan & Ng, Citation2015) was carried out on both palm wines to produce 400 µL extract. The flavour compounds were identified by comparison with reference compounds on the basis of the following criteria: retention index (RI) on two stationary phases of different polarities; mass spectra; and odour notes perceived at the sniffing port.
Enrichment and fractionation of compounds
The solvent-assisted flavour extraction (SAFE) distillate (Engel, Bahr, & Schieberle, Citation1999) obtained from (500 mL) of palm wine was concentrated to 100 mL and extracted three times with an aqueous sodium carbonate solution (0.5 M) to remove the acidic volatiles (Lasekan & Ng, Citation2015). The organic phase containing the neutral and basic volatiles fraction (NBF) was dried over anhydrous sodium sulphate and concentrated to 1 mL. The acidic fraction obtained as described previously (Lasekan & Ng, Citation2015) was also concentrated to 1 mL. A portion (1 mL) of the NBF was applied onto a water-cooled glass column (25 × 1 cm i.d., 12°C) filled with a slurry of silica gel 60 in n-pentane, and separated into five fractions by applying the following n-pentane/diethyl ether mixtures: first fraction (100 mL; n-pentane); second fraction (100 mL; 95 + 5 mL); third fraction (100 mL; 90 + 10 mL); fourth fraction (100 mL; 80 + 20 mL); and fifth fraction (100 mL; 50 + 50 mL). The eluate was collected and concentrated to 1 mL, and the concentrate was used for the gas chromatography olfactometry (GC-O) and gas chromatography-mass spectrometry (GC–MS) analyses.
GC-O and GC–MS
The GC-O was performed with an Agilent 6890N instrument equipped with a split–splitless injector, a flame ionization detector (FID) and an ODP2 (Olfactory Detector Port; Gerstel, Mulheim, Germany) (Lasekan & Ng, Citation2015). The following fused silica columns were used: DB-FFAP (30 m × 0.32 mm, film thickness, 0.25 µm; Chrompack, Mulheim, Germany) and DB5 (30 m × 0.32 mm, film thickness 0.25 µm; J & W Scientific, Folsom, CA, USA). An aliquot (2 µL) of the wine extracts was applied by the on-column injection method at 35°C. After 2 min, the temperature of the oven was raised at 40°C min−1 to 50°C and held for 2 min isothermally. It was also raised at 6°C min−1 to 180°C, and it was finally raised at 10°C min−1 to 230°C and held for 10 min. Helium (2.5 mL min−1) was used as the carrier gas. At the end of the capillary, the effluent was split 1:1 (by vol.) into an FID and a sniffing port. The FID and the sniffing port were held at 220°C and 240°C, respectively. Linear RIs were calculated according to Kovats method using a mixture of normal paraffin C6– C28 as external references.
MS analysis was performed with a MS-95S (Finnigan Bremen, Germany) in tandem with the capillaries described above. Mass spectra in the electron impact mode (MS/EI) were generated at ionisation energy of 70 eV; relative electron multiplier voltage (EM) of 400 V, with a resulting voltage of 1553 V, was used. The detector temperature was maintained at 280 C, with the actual temperature in the MS source reaching 180°C (Guen, Prost, & Demaimay, Citation2001).
Chiral analysis
The wine fractions 3 and 4 () were used for the chiral analysis. Fraction 3 was used for the chiral analyses of acetoin and phenyl ethanol, respectively; while chiral analysis of linalool was conducted with fraction 4. Chiral analyses were performed with an Agilent 6890N gas chromatograph (Agilent Technologies, Deutschland, Gmbh, Waldronn, Germany) by using the following capillaries: Chirasil-β-Dex (25 m × 0.25 mm i.d. fused silica capillary column coated with 0.25 µm film thickness of permethylated β-cyclodextrin; Varian, Middelburg, The Netherlands) for acetoin and BGB-176 (30 m × 0.25 mm, 2,3-dimethyl-6-tert-butyldimethylsilyl-β-cyclodextrin, film thickness, 0.25 µm; BGB Analytik AG, Rothenfluh, Germany) for linalool and phenylethanol, respectively. The wine fractions were injected at 35°C while maintaining the following conditions: the temperature of the oven was initially raised at 40°C min−1 to 50°C, and then raised at 6°C min−1 to 180°C. The flow rate of the helium was 2.5 mL min−1. The compounds were separated into their enantiomers without derivatization, and the order of elution was assigned by employing optically pure reference compounds.
Table 1. Odorants obtained in the SAFE extracts of palm wines (E. guineensis, B. flabellifer and N. fruticans).
Tabla 1. Odorantes obtenidos de las extracciones SAFE de los vinos de palma E. guineensis, B. flabellifer y N. fruticans.
Quantification
Quantification was performed by GC–MS, and triplicate calibration graphs at five concentrations level were constructed by least square linear regression using the results for the standard solution (12% hydro-alcoholic solution) submitted to the same procedure as described above. The concentration ranges employed for the relevant palm wine compounds were: linalool (4.4–68.7 µg/L), ethylbutanoate (3.0–18.1 µg/L), ethylacetate (0.3–19.2 µg/L), 2-phenylethanol (1.5–14.9 µg/L), β-damascenone (1.4–10.5 µg/L), 2,3-butandione (0.9–20.8 µg/L), ethylhexanoate (0.8–12.4 µg/L), geraniol (1.4–17.0 µg/L), 3-methylbutanol (1.9–15.1 µg/L) and acetoin (2.4–20.5 µg/L). The calibration curves were linear with r2 values between 0.974 (linalool) and 0.998 (2-phenylethanol). Student’s t-test was used to evaluate the differences. The statistically significant level was 5% (XLSTAT software, P < 0.05).
Results and discussion
shows the results of odour qualities and the retention indices of the SAFE extracts from three different palm wines (E. guineensis, B. flabellifer and N. fruticans). While a total of 41 odorants were detected in E. guineensis wine, N. fruticans and B. flabellifer wines produced a total of 35 and 37 odorants, respectively. All the compounds identified are well-known constituents of the organic fractions of wine and have previously been described in numerous publications and reviews (Fretz, Kanel, Luisier, & Amado, Citation2005; Guen et al., Citation2001; Lasekan & Abbas, Citation2010; Lasekan et al., Citation2007, Citation2009). Most of the compounds are mainly formed during alcoholic fermentation. In the current study, five fractions were obtained by column chromatography of the NBF fraction and were subsequently analysed by GC-O and GC–MS. Acetic acid, 2-methylpropanoic acid, butanoic acid, 3-methylbutanoic acid, pentanoic acid, 4-hydroxy-2,5-dimethyl-3(2H)-furanone, 3-methylpentanoic acid and phenylacetic acid were detected in the acidic fraction. However, the chiral compounds of interest (i.e. acetoin, linalool and phenylethanol) () were identified and isolated from fractions 3 and 4, respectively.
Figure 1. Enantiomeric separations of racemic mixtures of: (a) phenylethanol, (b) acetoin and (c) linalool.
Figura 1. Separaciones enantioméricas de mezclas racémicas de A) feniletanol, B) acetoína y C) linalol.
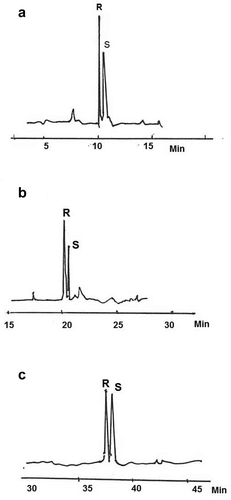
The analysis of the chiral compounds is shown in . Chiral GC analysis of the three palm wine varieties revealed three types of distribution patterns, namely the racemic form, an excess of (R) and an excess of (S). The results showed a high excess of laevorotatory enantiomers over the dextrorotatory enantiomers in all wines. Generally, the results revealed a high level of acetoin in all the wine varieties with concentrations ranging from 2437 to 6611 µg/L and an average ratio of S/R of 4:96–100:0. While the (R)-acetoin predominates in E. guineensis wine, the (S)-configuration was prevalent in the N. fruticans and B. flabellifer wines, respectively. The probable explanation for this observation might be due to the type of yeast strain responsible for the wine fermentation or the enzyme-catalysed processes that occurred during fermentation. While studies have shown that fermentation can induce stereo selective synthesis of some chiral compounds (Romano et al., Citation2000), other studies have reported the role of enzyme-catalysed processes in stereo selective synthesis of chiral compounds as well (Crout et al., Citation1986; Luddeke & Harder, Citation2011). For instance, Romano et al. (Citation2000) reported the prevalence of (R)-acetoin in wines obtained by Saccharomyces cerevisiae as compared to those obtained by other yeast strains. However, in an earlier study, Crout et al. (Citation1986) reported the production of acetoin by Klebsiella aerogenes during the condensation of pyruvate to α-acetolactate in a reaction catalysed by acetolactate synthase, followed by decarboxylation of the (S)-isomer in a reaction catalysed by acetolactate decarboxylase.
Table 2. Enantiomeric concentrations (µg/L) and enantiomeric ratios of some selected chiral compounds in E. guineensis, B. flabellifer and N. fruticans wines.
Tabla 2. Concentraciones enantioméricas (µg/L) y ratios enantioméricas de algunos compuestos quirales seleccionados de los vinos E. guineensis, B. flabellifer y N. fruticans.
Apart from the influence of the type of fermentation yeast and enzyme-catalysed processes that occurred during fermentation, there was a noticeable impact of varietal differences on the S/R ratio in the palm wine samples. Contrary to the results obtained for chiral acetoin in E. guineensiswine, the (S)-forms predominate in all wine varieties (). However, (S)-acetoin exhibited higher concentrations in N. fruticans and B. flabellifer wine varieties, while lower concentrations were found in (S)-linalool. The maximum enantiomeric (S)-form of acetoin was found in N. fruticans wine with an S/R average ratio of 100:0 and a concentration of 3073 µg/L. On the other hand, the least (S)-acetoin was obtained in E. guineensis wine with an enantiomeric ratio S/R of 4:96 (). In the case of linalool, the maximum enantiomeric (S)-form was produced in B. flabellifer wine with an S/R average ratio of 100:0 and a concentration of 10.7 µg/L. For phenylethanol, the maximum (S)-form was obtained in E. guineensis wine with an enantiomeric ratio of 100:0 and a concentration of 588 µg/L. Previous studies have shown that the enantiomeric ratio of chiral compounds can be used as important indicators of varietal differences and quality in wines (Askari, Hener, Schmarr, Rapp, & Mosandl, Citation1991; Chiavaro, Caligiani, & Palla, Citation1998). The enantiomeric ratio of acetoin is considered important in distinguishing between traditionally aged and common vinegars (Chiavaro et al., Citation1998). Similarly, linalool as well as phenylethanol has been used as an important indicator of good quality in wines (Askari et al., Citation1991; Carrau et al., Citation2005). In addition, the chiral compounds are known to exhibit clear odour distinctiveness between enantiomers. For instance, while the (S)-form of linalool has been described as sweet and petigrain-like, and the (R)-form as having a lavender-like note (Brenna, Fuganti, & Serra, Citation2003), the (S)-form of phenylethanol and its (R)-form are noted for their delicate hyacinth, strawberry and honey-like nuances, respectively (Szaleniec, Citation2007). On the other hand, (R)-acetoin is characterized by buttery note, while the (S)-form has been described as almond-like (Crout et al., Citation1986).
Generally, the three chiral compounds currently being studied are produced by the yeast metabolism during alcoholic fermentation (Loscos, Hernandez-Orte, Cacho, & Ferreira, Citation2007). However, the origins of these compounds are quite different and complex. In addition, each of their enantiomers also exhibited clearly different pathways. For instance, Luddeke and Harder (Citation2011) studied the reaction of linalool dehydratase-isomerase (LDI) on the si-face of the prochiral β-myrcene, and they observed a high enantiospecific hydration reaction to (S)-linalool with an enantiomeric excess (ee) value of 95.4%. Similarly, Hummel (Citation1990) demonstrated that the (R)-form of phenylethanol was predominantly produced by the dehydrogenase catalysing the NADPH-dependent reduction of acetophenone.
The effect of three-month storage on the concentration and the enantiomeric ratio of the chiral compounds are shown in . Noticeable differences were observed in enantiomeric ratio S/R and concentration of the chiral compounds. In all the wines, concentration of the (S)-form decreased during storage, whereas those of the (R)-form increased when compared with the fresh wines (). Similar trend was reported by Lytra et al. (Citation2015) for white wines. In this study, results have shown that the maximum (R)-form concentrations were found in the chiral acetoin of all wines. For example, in E. guineensis wine, the maximum (R)-form of 5289 µg/L was obtained in acetoin with an enantiomeric ratio of 20:80. Similar trend was obtained in N. fruticans wine and B. flabellifer wine, respectively. Also, the maximum (S)-form concentration followed the same trend. The probable explanation for this might be due to the high initial concentrations of acetoin in the three wines. Moreover, the significant differences in the enantiomeric ratios of the chiral compounds after 3 months of storage might be due to racemization. Racemization of some natural flavour compounds such as 4-hydroxyl-2,5-dimethyl-3(2H)-furanone (HDMF, Furaneol®) and sotolone has been attributed to keto-enol-tautomerism (Pons, Lavigne, Landais, Darriet, & Dubourdieu, Citation2008; Raab et al., Citation2003). Under mild acidic medium of palm wine (pH 3–3.5), racemization occurs probably via a keto-enol tautomerism, catalysed by the presence of an acid (Raab et al., Citation2003). This phenomenon results in the decrease in the enantiomeric ratio. Under extreme acidic conditions (pH <3), the racemization will increase significantly and the ee value would decrease significantly.
Table 3. Enantiomeric concentrations (µg/L) and enantiomeric ratios of some selected chiral compounds in stored (3 months) palm wines (E. guineensis, B. flabellifer and N. fruticans).
Tabla 3. Concentraciones enantioméricas (µg/L) y ratios enantioméricas de algunos compuestos quirales seleccionados y almacenados durante 3 meses de los vinos de palma E. guineensis, B. flabellifer y N. fruticans.
Conclusion
The analysis by GC–MS/GC-O of the SAFE extracts from three palm wine varieties allowed the identification of 41 odorants. Of this, 37 odorants were identified in B. flabellifer wine, while E. guineensis and N. fruticans wines produced 41 and 35 odorants, respectively. Chiral GC analysis of the chiral compounds of interest (i.e. acetoin, linalool and phenylethanol) revealed different distributions and concentrations of their enantiomers. With the exception of E. guineensiswine, the other wines contained both (S)-and (R)-forms of the chiral compounds in various ratios. Generally, results revealed a high level of acetoin in all the wines with concentrations ranging from 2437 to 6611 µg/L and an average ratio of S/R of 4:96–100:0. Moreover, noticeable differences were observed in enantiomeric ratios and concentrations of the enantiomers of the compounds during storage. In all the wines, concentration of the (S)-form decreased during storage, whereas those of the (R)-form increased.
Acknowledgements
The author is grateful for the extensive financial support of the Fundamental Research grant scheme (No. 5524558) at the Univ. Putra Malaysia, Malaysia.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Aimi, N.R., AbuBakar, F., & Dzulkifly, M.H. (2013). Determination of volatile compounds in fresh and fermented Nipa sap (Nypa fruticans) using static headspace gas chromatography-mass spectrometry (GC-MS). International Food Research Journal, 20, 369–376.
- Amoa-Awua, W.K., Sampson, E., & Tano-Debrak, K. (2007). Growth of yeasts, lactic and acetic acid bacteria in palm wine during tapping and fermentation from felled oil palm in Ghana. Journal of Applied Microbiology, 102, 599–606. doi:10.1111/j.1365-2672.2006.03074.x
- Armstrong, D.W., Chang, C.D., & Li, W.Y. (1990). Relevance of enantiomeric separations in food and beverage analysis. Journal of Agricultural and Food Chemistry, 38, 1674–1677. doi:10.1021/jf00098a010
- Askari, C., Hener, U., Schmarr, H.G., Rapp, A., & Mosandl, A. (1991). Stereodifferentiation of some chiral monoterpenes using multidimensional gas chromatography. Fresenius Journal of Analytical Chemistry, 340, 768–772. doi:10.1007/BF00321500
- Barba, C., Flores, G., & Herraiz, M. (2010). Stereodifferentiation of some chiral aroma compounds in wine using solid phase micro extraction and multidimensional gas chromatography. Food Chemistry, 123, 846–857. doi:10.1016/j.foodchem.2010.05.021
- Brenna, E., Fuganti, C., & Serra, S. (2003). Enantioselective perception of chiral odorants. Tetrahedron: Asymmetry, 14, 1–42. doi:10.1016/S0957-4166(02)00713-9
- Buettner, A., & Schieberle, P. (2001). Evaluation of aroma difference between hand-squeezed juices from Valencia late and Navel oranges by quantitation of key odorants and reconstitution experiments. Journal of Agricultural and Food Chemistry, 49, 2387–2394. doi:10.1021/jf0013631
- Carrau, F.M., Medina, K., Boido, E., Farina, L., Gaggero, C., Dellacassa, E., … Henschke, P.A. (2005). De novo synthesis of monoterpene by Saccharomyces cerevisiae wine yeasts. FEMS Microbiology Letter, 243, 107–115. doi:10.1016/j.femsle.2004.11.050
- Castillo, M.L.R., Caja, M.M., & Herraiz, M. (2003). Use of the enantiomeric composition for the assessment of the authenticity of fruit beverages. Journal of Agricultural and Food Chemistry, 51, 1284–1288. doi:10.1021/jf025711q
- Chiavaro, E., Caligiani, A., & Palla, G. (1998). Chira indicators of ageing in balsamic vinegars of Modena. Italian Journal of Food Science, 10, 329-337.
- Crout, D.H.G., Littlechild, J., & Morrey, S.M. (1986). Acetoin metabolism: Stereochemistry of the acetoin produced by the pyruvate decarboxylase of wheat germ and by the α-acetolactate decarboxylase of Klebsiella aerogenes. Journal of Chemical Society Perkin Transaction, 1, 105–107. doi:10.1039/P19860000105
- Engel, W., Bahr, W., & Schieberle, P. (1999). Solvent assisted flavour evaporation – A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. European Food Research Technology, 209, 237–241. doi:10.1007/s002170050486
- Fretz, C., Kanel, S., Luisier, J.-L., & Amado, R. (2005). Analysis of volatile components of petite Arvine wine. European Food Research Technology, 221, 504–510. doi:10.1007/s002170051163y
- Guen, S.L., Prost, C., & Demaimay, M. (2001). Evaluation of the representativeness of the odour of cooked mussel extracts and the relationship between sensory descriptors and potent odorants. Journal of Agricultural and Food Chemistry, 49, 1321–1327. doi:10.1021/jf000781n
- Hummel, W. (1990). Enzyme-catalysed synthesis of optically pure R (+)-phenylethanol. Biotechnology Letter, 12(403–408). doi:10.1007/BF01024393
- Jirovetz, L., Buchbaucer, G., Fleischhacker, W., & Ngassoum, M.B. (2001). Analysis of the aroma compounds of two different palm wine species (“Matango and Raffia”) from Cameroon using SPME-GC-FID, SPME-GC-MS and olfactometry. Ernahrung Nutrition, 25, 67–71.
- Lasekan, O. (2013). A comparative analysis of the influence of human salivary enzymes on odorant concentration in three palm wines. Molecules Journal, 18, 11809–11823. doi:10.3390/molecules181011809
- Lasekan, O., & Abbas, K.A. (2010). Flavour chemistry of palm toddy and palm juice: A review. Trends in Food Science & Technology, 21, 494–501. doi:10.1016/j.tifs.2010.07.007
- Lasekan, O., Buettner, A., & Christlebauer, M. (2007). Investigation of important odorants of Palm wine (Elaeis guineensis). Food Chemistry, 105, 15–23. doi:10.1016/j.foodchem.2006.12.052
- Lasekan, O., Buettner, A., & Christlebauer, M. (2009). Investigation of the retronasal perception of palm wine aroma by application of sensory analysis and exhaled odorant measurement (EXOM). African Journal of Food Agriculture, Nutrition and Development, 9, 793–813.
- Lasekan, O., & Ng, S.S. (2015). Key volatile aroma compounds of three black velvet tamarind (Dialium) fruit species. Food Chemistry, 168, 561–565. doi:10.1016/j.foodchem.2014.07.112
- Lasekan, O., & Otto, S. (2009). In vivo analysis of palm wine (Elaeis guineensis) volatile organic compounds (VOCs) by proton transfer reaction-mass spectrometry. International Journal of Mass Spectrometry, 282, 45–49. doi:10.1016/j.ijms.2009.02.005
- Loscos, N., Hernandez-Orte, P., Cacho, J., & Ferreira, V. (2007). Release and formation of varietal aroma compounds during alcoholic fermentation from non-floral grape odourless flavour precursors fraction. Journal of Agricultural and Food Chemistry, 55, 6674–6684. doi:10.1021/jf0702343
- Luddeke, F., & Harder, J. (2011). Enantiospecific (S)- (+)-l linalool formation from β-myrcene by linalool dehydratase-isomerase. Zeitschrift Fur Naturforchung, 66 c, 409–412.
- Lytra, G., Cameleyre, M., Tempere, S., & Barbe, J.C. (2015). Distribution and organoleptic impact of ethyl-3-hydroxybutanoate enantiomers in wine. Journal of Agricultural. Food Chemistry, 63, 10484–10491. doi:10.1021/acs.jafc.5b04332
- Lytra, G., Tempere, S., de Revel, G., & Barbe, J.-C. (2012). Distribution and organoleptic impact of ethyl 2-hydroxy-4-methylpentanoate enantiomers in wine. Journal of Agricultural Food Chemistry, 60, 1503–1509. doi:10.1021/jf204378u
- Mbuagbaw, L., & Noorduyn, S.G. (2012). The palm wine trade: Occupational and health hazards. The International Journal of Occupational Environmental Medicine, 3, 157–164.
- Obire, O. (2005). Activity of Zymomonas species in palm sap obtained from three areas in Edo State, Nigeria. Journal of Applied Science and Environment Management, 9, 25–30.
- Pons, A., Lavigne, V., Landais, Y., Darriet, P., & Dubourdieu, D. (2008). Distribution and organoleptic impact of sotolon enantiomers in dry white wines. Journal of Agricultural and Food Chemistry, 56, 1606–1610. doi:10.1021/jf072337r
- Raab, T., Hauck, T., Knecht, A., Schmitt, U., Holzgrabe, U., & Schwab, W. (2003). Tautomerism of 4-hydroxy-2,5-dimethyl-3(2H)-furanone: Evidence for its enantioselective biosynthesis. Chirality, 15, 573–578. doi:10.1002/(ISSN)1520-636X
- Romano, P., Palla, G., Caligiani, A., Brandolini, V., Maiett, A., & Salzano, G. (2000). Evaluation of stereoisomers of 2,3-butanediol and acetoin to differentiate Saccharomyces cerevisiae and Kloeckera apiculata wine strains. Biotech Letter, 22, 1947–1951. doi:10.1023/A:1026741625019
- Szaleniec, M. (2007). An enzyme that can switch scents from gasoline to hyacinth: Research in progress chemistry. Academia Journal, 15, 34–35.
- Takatoshi, T., Niclass, Y., Frerot, E., & Dubourdieu, D. (2006). Stereoisomeric distribution of 3-mercaptohexan-1-ol and 3-mercaptohexyl acetate in dry and sweet white wines made from Vitis vinifera (Var. Sauvignon blanc and Semillon). Journal of Agricultural and Food Chemistry, 54, 7251–7255. doi:10.1021/jf061566v
- Weber, B., Maas, B., & Mosandl, A. (1995). Stereoisomeric flavour compounds. 72 stereoisomeric distributions of some chiral sulphur-containing traces components of yellow passion fruits. Journal of Agricultural and Food Chemistry, 43, 2438–2441. doi:10.1021/jf00057a023
