ABSTRACT
The objective of the article was to compare the types and quantities of phenolic compounds found in seeds of French bean from two green- and two yellow-podded cultivars. The study also comprised determinations of antioxidant properties and reducing capacity of extracts from these seeds, as well as RP-HPLC analysis of their phenolic acids. A relationship was demonstrated between the content of phenolic compounds in the seeds and the colour of their pods. Seeds of the green-podded cultivars were distinguished by a higher total content of phenolics than the yellow-podded varieties. RP-HPLC analysis of the extracts showed the presence of four phenolic acids that were identified as caffeic acid, p-coumaric acid, ferulic acid and sinapic acid. The demonstrated relationship between the seed phenolics and the pod colour can help which bean cultivars most suitable for organic cultivation and better effects on consumer health and those.
Abbreviations: ABTS•+ – 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) enhancer; FRAP – reducing power of extracts; PAL – phenyl-alanine ammonia lyase; ROS – reactive oxygen species; TEAC – Trolox equivalent antioxidant capacity; TPTZ – 2,4,6-tris(2-pyridyl)-s-triazine;
RESUMEN
En el presente estudio se propuso comparar los tipos y las cantidades de compuestos fenólicos presentes en las semillas del frijol procedentes de dos cultivares de vaina verde y dos de vaina amarilla. Además, en el estudio se pudieron determinar las propiedades antioxidantes y la capacidad reductora de los extractos producidos a partir de dichas semillas, realizándose el análisis RP-HPLC de sus ácidos fenólicos. Así, se estableció la relación entre el contenido de compuestos fenólicos en las semillas y el color de su vaina. Las semillas procedentes de cultivares de vaina verde mostraron un contenido total de compuestos fenólicos más elevado que las variedades de vaina amarilla. El análisis RP-HPLC de los extractos reveló la presencia de cuatro ácidos fenólicos: ácido cafeico, ácido p-cumárico, ácido ferúlico y ácido sinápico. La relación comprobada entre los compuestos fenólicos presentes en las semillas y el color de su vaina puede ayudar a determinar qué cultivares de frijol son los más adecuados para cultivos orgánicos y cuáles tienen mejores efectos en la salud de los consumidores.
1. Introduction
Bean seeds are a valuable source of nutrient and non-nutrient ingredients, and their regular consumption is recommended because of the beneficial influence on the human body. Their antioxidant activity is notable among a variety of functional properties of bean seeds. Consumption of beans has been linked to reduced risks of diabetes and obesity, coronary heart disease, colon cancer and gastrointestinal disorders (Oomach, Cardador-Martinez, & Loarca Piǹa, Citation2005; Reynoso-Comacho, Ramos-Gomez, & Loarca-Piǹa, Citation2006). As a rule, the antioxidant capacity of bean seeds arises from their content of phenolic compounds. These are natural antioxidants, able to protect against reactive oxygen species, which are responsible for reactions underlying many serious diseases (Amarowicz & Weidner, Citation2009; Reynoso-Comacho et al., Citation2006). Nyau, Prakash, Rodrigues, and Farrant (Citation2016) have demonstrated that red beans are the richest in total polyphenols, followed by grey mottled, brown and white beans, and that there is a strong positive correlation between antioxidant activity and total polyphenol content.
Plant phenolics are secondary metabolites synthesised either from the shikimate/phenylpropanoid pathway or from the “polyketide” acetate/malonate pathway. These compounds perform a very broad range of physiological roles in plants (Reynoso-Comacho et al., Citation2006). Phenolic compound in plant tissues act as antioxidants (Nascimento & Fett-Neto, Citation2010). This group of compounds protects cells against the negative effects of ROS as well as against lipid peroxidation, protein denaturation and DNA damage (Allakhverdiev et al., Citation2008; Kranner, Minibayeva, Backett, & Seal, Citation2010). Additionally, plants need phenolics for pigmentation, growth, reproduction, resistance to pathogens and for many other functions. It is also thought that phenolic compounds play a defensive role when environmental stresses such as intensive light, low and high temperatures, drought, salinity, pathogen infection, herbivore activity and nutrient deficiency, can lead to an increased production of free radicals and other oxidative species in plants. The defensive role of phenolic compounds present in plants mostly depends on their antioxidant properties and involves stabilisation of unpaired electrons, scavenging of free radicals from lipid peroxidation and the ability to chelate transition metal ions, which results in the inhibition of reactive oxygen species production (Król, Amarowicz, & Weidner, Citation2014; Lattanzio, Citation2013)
Notably, when the quantity of pre-existing antifungal phenolics is insufficient to stop the development of an infection, plants often respond to this situation by raising the level of antifungal phenolics at the infection site. It is then possible to observe an increase in the activity of key PAL (phenyl-alanine ammonia lyase as well as chalcone synthase) enzymes (Bhattacharya, Sood, & Citowsky, Citation2010). Once substrates for key oxidation reactions have been delivered as described above, plant cells initiate the production of fungitoxic quinones. These are highly toxic compounds responsible for an upsurge of active oxygen species, which makes the plant cell an unfavourable medium for further pathogen development. In addition to this, another mechanism has been described through which substances secreted by pathogens induce the synthesis and accumulation of other types of phenolic compounds in infected plants. In many cases, for example, there is an increase in the production of lignin and formation of polyphenolic parenchymal cells, e.g. in the secondary bark of infected plant shoots (Lattanzio, Citation2013). The consequences of more intensive accumulation of lignin (a polymer of aromatic alcohols: p-coumaryl, coniferyl and sinapyl) in cell walls include: improved mechanical resistance of cell walls to the penetration by hyphae; decreased sensitivity to pathogen enzymes degrading the cell wall; and limited permeation of the pathogen-derived enzymes and toxins into cells. The correlation between the concentration and composition of phenolic compounds in plant infected by some pathogens and the degree of resistance of plants has been, and still remains, the fundamental observation suggesting that phenolic compounds may be an important element of the plant defence mechanism (Bhattacharya et al., Citation2010; Lattanzio, Citation2013).
Because of the important role of phenolic compounds in plant resistance to diseases, pests and unfavourable environmental conditions and their importance to human health, the aim of our study was to compare the identity and quantities of phenolic compounds in seeds of different bean cultivars, and to determine the antioxidant properties and reducing capacity of extracts obtained from these seeds. This is the first attempt to compare the content of phenolic compounds in the seeds of green- and yellow-podded beans.
2. Materials and methods
2.1. Plant material
The research material consisted of seeds of four French bean (Phaseolus vulgaris L.) cultivars: two dwarf cultivars (“Lotos” and “Exalto”) and two climbing ones (“Algavre” and “Goldfield”). The cultivars “Lotos” and “Goldfield” are yellow-podded beans, while “Exalto” and “Algrave” are green podded. The seeds were supplied by the company Agro-Plant (Magnuszew, Poland). Bean seeds of all the tested cultivars were collected in 2015. Photographs of the seeds of the analysed bean cultivars are shown in .
2.2. Extraction of phenolic compounds
Phenolic compounds were extracted from plant material (fresh, not dried seeds) in three times into 80% (v/v) acetone and then three times into 80% (v/v) methanol for 15 min at 80°C (Amarowicz, Naczk, Zadernowski, & Shahidi, Citation2000). The extraction was carried out in a shaking water bath (JWE 357, JWElectronic) and the solutions from all extractions were combined. After evaporating the organic solvent in a rotary evaporator (Rotavapor R-3, Büchi) at 45°C, the remaining aqueous solution was lyophilised (Alpha 1–2, Christ). Finally, the crude extracts were stored at −20°C in the dark until further analysis.
2.3. Determination of total phenols
The content of total phenolic compounds in the extracts was determined using the Folin-Ciocalteu’s reagent and (+)-catechin served as a standard (Naczk & Shahidi, Citation1989).
Briefly, 0.25 mL of crude extract (10 mg/ml) were made up to 4 mL with distilled water, mixed thoroughly with 0.25 mL of Folin–Ciocalteu reagent for 5 min, followed by the addition of 0.5 cm3 of sodium carbonate. The mixture was allowed to stand for a further 30 min in the dark, and absorbance was measured at 725 nm. The total phenolic content was calculated from the calibration curve, and the results were expressed as mg of (+)-catechin equivalent per gram of extract, dry and fresh weight of seeds.
2.4. Determination of antioxidant activity of the extracts
Trolox equivalent antioxidant capacity (TEAC)
The antiradical activity was analysed using the method described by Re et al. (Citation1999). Briefly, 0.1–0.5 mg of raw extract was dissolved in 0.1 mL methanol and then 2 mL ABTS•+·radical (2,2ʹ-azinobis(3-ethylbenzthiazoline-6-sulphonic acid) diammonium salt) was added. The mixture was left in the dark at 32°C for 20 min. After incubation, the absorption was quantified at 734 nm. A calibration curve was prepared using Trolox as a standard. The coloured ABTS+·solution becomes colourless when a sample is added to it. The rate of colourless in reaction medium is dependent on extract radical scavenging activity.
2.5. Determination of the reducing power of the extracts
The reducing power of extracts was measured using the FRAP method (ferric ion reducing antioxidant power) described by Benzie and Strain (Citation1999). A small amount (0.1–0.5 mg of raw extract was dissolved in 0.1 mL water, and 3 mL FRAP reagent was added (acetic acid buffer at pH 3.6 containing TPTZ (2,4,6-tris(2-pyridyl)-s-triazine). After incubation, the absorption was quantified at 593 nm. A calibration curve was prepared using Trolox as a standard. The results were expressed as µmol Fe2+ per g of extract, fresh matter (FM) and dry matter (DM).
2.6. Separation and identification of free, ester-bound and glycoside-bound phenolic acids
The phenolic acids (free acids and those liberated from soluble esters and glycosides) were isolated from the extracts according to the method previously described and modified by Król, Amarowicz, and Weidner (Citation2015). An aqueous suspension of the methanolic extract (400 mg in 10 mL) was adjusted to pH 2 with 6 M HCl, and free phenolic acids were extracted five times into 10 mL diethyl ether using a separating funnel. The ether extract was evaporated to dryness under vacuum at room temperature. The water solution was neutralised and then lyophilised. The residue was dissolved in 10 mL of 2 M NaOH and hydrolysed for 3.5 h in a nitrogen atmosphere at room temperature. After acidification to pH 2 using 6 M HCl, phenolic acids released from soluble esters were extracted from the hydrolysate five times in 15 mL diethyl ether. A 9 mL volume of 6 M HCl was added to the aqueous solution, which was then placed in a nitrogen atmosphere and hydrolysed for 1 h at 100°C. The phenolic acids released from soluble glycosides were separated from the hydrolysate five times into 20 mL diethyl ether. After ether evaporation, the dry residue was dissolved in 2 mL methanol and filtered through a 0.45 μm nylon filter. The sample was injected onto an HPLC column. A Shimadzu HPLC system was employed: LC – 10 ADVP pump; photodiode array detector UV-vis SPD – M10AVP; oven CTO – 10 ASVP; Controller SCL-10AVP. The conditions of the separations were as follows: pre-packed LUNA C18 column (5 μm, 4.6 × 250 mm; Phenomenex); mobile phase water-acetonitrile -acetic acid (88:10:2, v/v/v) (Król et al., Citation2015); flow rate of 1 mL min−1; injection volume of 20 μl; the detector was set at 280 and 320 nm; the oven temperature was 20°C.
2.7. Statistical analysis
All experiments were repeated four times. The results are reported as means ± SD. Statistically significant differences between the mean values were analysed by ANOVA and Duncan’s multiple comparison test (p < 0.05) using the GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
3.1. Content of phenolic compounds
shows the content of total phenolic compounds in all samples as per gram of extract, per gram of fresh matter and dry matter in tissues. The results show that the total content of phenolic compounds in extracts from seeds of the analysed bean cultivars ranged from 2.27 to 4.55 mg g−1 of extract. The highest content of phenolic compounds was found in seeds of the green-podded cultivar “Algarve” (4.55 mg g−1 of extract). Significantly less phenolics were observed in seeds of the other green-podded cultivar, “Exalto” (3.33 mg g−1 of extract). The yellow-podded variety “Goldfield” contained even less phenolics in seed extracts (2.84 mg g−1 of extract). The lowest content of phenolic compounds was detected in the extract from seeds of the other yellow-podded cultivar, “Lotos” (2.27 mg g−1 of extract). Similar tendencies were observed after calculating the total content of phenolic compounds per gram of dry and fresh matter. The content of phenolic compounds in seeds of the analysed bean varieties ranged from 0.23 to 0.46 mg g−1 of FM and from 0.25 to 0.52 mg g−1 of DM. To recapitulate, seeds of the green-podded bean cultivars in our study contained significantly more phenolic compounds than the seeds of the yellow-podded varieties.
Table 1. Content of total phenolics in extracts and seeds.
Tabla 1. Contenido de fenólicos totales en extractos y semillas.
3.2. Trolox equivalent antioxidant capacity (TEAC)
The ABTS•+· scavenging activity is illustrated in . The results confirm that all of the analysed samples were able to scavenge the ABTS•+· free cation radical. The blue-green solution of the free cation radical ABTS•+· becomes colourless under the effect of the antioxidant properties of the tested extracts. The highest antioxidant capacity was determined for extracts from seeds of the green-podded cultivar “Algrave” (56.1 µmol Trolox g−1 of extract). A significantly lower but statistically similar ability to scavenge the cation radical ABTS•+· was found in extracts from seeds of the cultivars “Exalto”, “Goldfield” and “Lotos”: 40.2, 37.5 and 40.3 µmol Trolox g−1 of extract, respectively. Similar tendencies were observed when recalculating the scavenging power per µmol Trolox g−1 of FM and DM. The antioxidant capacity of seeds ranged then from 4.07 to 5.67 µmol Trolox g−1 of FM and from 4.21 to 6.40 µmol Trolox g−1 of DM. It is worth emphasising that an especially high antioxidant capacity was determined in extracts from seeds of the green-podded cultivar “Algarve”. As mentioned above, seeds of this bean cultivar also contained the highest amounts of phenolic compounds among the analysed bean cultivars.
Table 2. Trolox equivalent antioxidant capacity of extracts and seeds.
Tabla 2. Equivalentes Trolox de capacidad antioxidante de extractos y semillas.
3.3. Determination of the reducing power of the extracts
The results of the determination of the reducing power of extracts from seeds of the four bean cultivars are presented in . Under the influence of the reducing power of extract, Fe3+ ions were reduced to Fe2+ ions and the colour of the mixture changed from light orange to blue to dark blue. The results confirm that all the analysed samples were able to reduce Fe3+ ions. The highest reducing power was detected in extracts from seeds of the green-podded cultivar “Exalto” (86.0 µmol Fe2+ g−1 of extract). Significantly lower but similar reducing power was determined for extracts from seeds of the cultivars “Algarve”, “Goldfield” and “Lotos”: 68.8, 63.2 and 65.6 µmol Fe2+ g−1 of extract, respectively. Similar relationships emerged when the results were recalculated as FRAP values relative to the weight of seed dry or fresh matter. The FRAP values of seeds ranged from 6.45 to 8.51 µmol Fe2+ g−1 of FM and from 7.10 to 9.28 µmol Fe2+ g−1 of DM. It is worth adding that the ferric-reducing antioxidant power (FRAP) assay measures the antioxidant effects of any substance in the reaction medium as reducing power.
Table 3. FRAP values of extracts and seeds.
Tabla 3. Valores FRAP de extractos y semillas.
3.4. Content of phenolic acids
A representative chromatogram of RP-HPLC analysis of bean seed extracts is shown in . Four peaks dominated in the chromatograms obtained from the separation of extracts of analysed seeds. The peaks in the chromatograms are labelled with the identified phenolic acids. It was demonstrated that extracts from seeds of the examined bean cultivars contained caffeic acid, p-coumaric acid, ferulic acid and sinapic acid. The retention time of caffeic acid was 12.1 min, p-coumaric acid – 23.5 min, ferulic acid – 31.3 min and sinapic acid – 34.9 min. The acids occurred in free and ester-bound forms. No glycoside-bound phenolic acids were detected in the extracts from the analysed bean cultivars. The content of free phenolic acids in bean extracts and seeds is shown in . Caffeic acid in the free form occurred only in seeds of the green-podded cv. “Exalto,” where it was quantified at 69.6 µg g−1 of extract. The content of free p-coumaric acid in the extracts was several-fold lower than the content of caffeic acid in the seed extracts from cv. “Exalto” p-coumaric acid was present in seeds of all examined cultivars, at 14.9, 19.4, 25.0 and 22.2 µg g−1 of extract from the cultivars “Algarve”, “Exalto”, “Goldfield” and “Lotos”, respectively. Free ferulic acid also appeared in seeds of all of the bean cultivars. Its content in seed extract samples of the cultivars “Algarve”, “Exalto”, “Goldfield” and “Lotos” was 36.3, 47.4, 60.9 and 54.1 µg g−1 of extract, respectively. Similarly, sinapic acid was found in seed extracts of all the cultivars. Expressed in µg g−1 of extract, its content was lowest in extracts of the cultivar “Algarve” (4.5), while being significantly higher in seed extracts of the cultivars “Exalto”, “Goldfield” and “Lotos”: 25.0, 30.1, and 32.3 µg g−1 of extract, respectively.
Table 4. Content of free phenolic acids in the extracts and seeds.
Tabla 4. Contenido de ácidos fenólicos libres en extractos y semillas.
Figure 2. HPLC chromatograms of the separation of phenolic acids isolated from bean seeds with marked peaks for caffeic acid (1), p-coumaric acid (2), ferulic acid (3) and sinapic acid (4). A – free phenolic acids, B – ester-bound phenolicacids, C – glycoside-bound phenolic acids.
Figura 2. Cromatogramas HPLC de la separación de los ácidos fenólicos aislados de las semillas de frijol, con picos marcados para el ácido cafeico (1), ácido p-cumárico (2), ácido ferúlico (4). A- ácidos fenólicos libres; B- ácidos fenólicos unidos al éster; C – ácidos fenólicos unidos al glucósido.
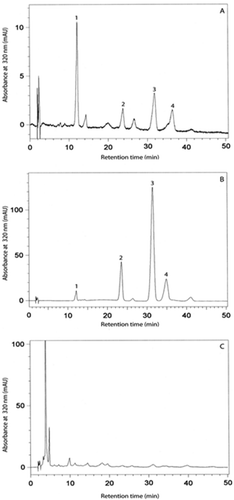
The content of esterified phenolic acids in extracts from seeds of the analysed bean cultivars is presented in . The content of esterified phenolic acids in seeds of all the cultivars was significantly higher than that of free phenolic acids ( and ). The content of esterified caffeic acid expressed in µg g−1 of extract was much higher in the cultivars “Lotos” and “Algarve” (131 and 116 µg g−1 of extract, respectively) than in the cultivars “Exalto” and “Goldfield” (90 and 88 µg g−1 of extract, respectively). The content of p-coumaric acid was high and similar in cv. “Exalto” and “Lotos”: 863 and 826 µg g−1 of extract, respectively. The content of p-coumaric acid in the other cultivars, “Algarve” and “Goldfield”, was 618 and 697 µg g−1 of extract, respectively. The content of ester-bound ferulic acid was high in extracts from seeds of the cultivars “Exalto” and “Lotos”: 2104 and 2014 µg g−1 of extract, respectively. Lower amounts of ester-bound ferulic acid were detected in extracts from seeds of the other two cultivars, “Algarve” and “Goldfield”: 1505 and 1699 µg g−1 of extract, respectively. With respect to ester-bound sinapic acid, its content was high in extracts from seeds of the cultivars “Exalto” and “Lotos”: 479 and 418 µg g−1 of extract, respectively, while being significantly lower in seed extracts obtained from cv. “Algarve” and “Goldfield”: 303 and 283 µg g−1 of extract, respectively. It is worth noting that phenolic acids appeared in both free and ester-bound forms. No glycoside-bound phenolic acids were detected. Ferulic acid, present in all of the analysed seeds, proved to be the dominant phenolic acid. There were intermediate concentrations of by p-coumaric and sinapic acids in the extracts. Of the phenolic acids, ester-bound caffeic acid occurred in the lowest amounts in the analysed bean seed extracts and, as already mentioned, free caffeic acid appeared in considerable amounts only in seeds of cv. “Exalto”.
Table 5. Content of esterified phenolic acids in the extracts and seeds.
Tabla 5. Contenido de ácidos fenólicos esterificados en extractos y semillas.
4. Discussion
Beans grown for their dry seeds contain from 21.4 to 25.5% of protein, having high nutritional value. Bean seeds are also a rich source of mineral salts, phosphorus, magnesium and iron as well as group B vitamins. Cultivated green-podded string beans are distinguished by a higher vitamin content and therefore have a higher biological value than yellow-podded varieties (Crépon et al., Citation2010).
In seeds, phenolic compounds, which play an important role in plant resistance to environmental stresses and in human diet, are mostly found in the seed cover (Cardador-Martinez, Loarca-Piǹa, & Oomah, Citation2002). In general, the content of total phenolics in extracts and seeds of the bean cultivars we examined was similar to that reported by other authors from studies performed on other cultivars. However, certain differences have emerged. For example, the total phenolic content in extracts from white bean varieties ranged between 2.79 and 5.34 mg g of extract (Orak, Karamać, Orak, & Amarowicz, Citation2016). In our studies it was found that green-podded bean varieties and one yellow-podded variety had similar phenolic content as in white seed varieties. Only one green variety of “Lotos” had a lower phenolic content. Generally, earlier comparative studies conducted on different legumes and different bean varieties demonstrated that the total phenolic content in white beans was lower than in some other legume seeds and coloured beans (Djordjevic, Śiler-Marinkovic, & Dimitrijevic-Brankovic, Citation2011; Nyau et al., Citation2016; Orak et al., Citation2016; Xu, Yusan, & Chang, Citation2007). Marathe, Rajalakshmi, Jamdar, and Sharma (Citation2011) categorised legumes into three groups depending on their phenolic content. White beans were classified as low content phenolic legumes together with chickpea (cream, green), pea (white, green) and lentil.
The antioxidant activity of bean seed extracts was evaluated using two polar systems – the ability to scavenge radicals and the ability to reduce Fe3+, using the ABTS•+· and FRAP assays, respectively. Ours paper is the first study in which the content of phenolic compounds has been compared between two green-podded and two yellow-podded string bean cultivars. We have demonstrated that seeds of the green-podded beans are distinguished by a generally higher content of phenolics than yellow-podded bean seeds. Extracts from seeds of one of the green-podded bean varieties were also characterised by the highest antioxidant capacity, while extracts from seeds of the other green-podded cultivar had the highest reducing power. The yield of French beans and the availability of material for processing depends on many factors, such as a cultivar, sowing date, harvest method and, above all, environmental conditions (Łabuda & Brodaczewska, Citation2011). It should be highlighted that French bean is a thermophilous plant with a short growing period. Hence, cold stress seems to be the major factor influencing yields. Studies on the role of phenolic compounds in cold tolerance have demonstrated that the antioxidant potential can be activated and can function effectively even under severe stress conditions (Turk et al., Citation2014), when the activity of basic antioxidant enzymes is depressed (Posmyk, Janas, & Szafrańska, Citation2002; Szafrańska, Posmyk, & Janas, Citation2002). Analyses of the activity of PAL, the key enzyme in the metabolism of phenols, have shown that some phenolic compounds can be both synthesised de novo and released from cell walls. The effect of phenolic compounds on the growth and development of plants is complex and multidirectional. Phenolic components can participate in the metabolism of auxins, they can alter the permeability of membranes, affect respiratory processes or alter synthesis of certain proteins. However, the demonstrated inverse proportionality between the degree of accumulation of phenols and the level of accumulation of lipid peroxidation products (TBARS – triobarbituric acid reactive substances) can be suggestive of the principal role of phenolic compounds as oxidants responding to cold stress. Owing to these chemical substances, oxidative damage to cellular structure is minimised, which allows for a more rapid and effective regeneration of seedlings once the stress conditions have disappeared (Janas, Cvikrova, Pałągiewicz, Szafrańska, & Posmyk, Citation2002; Posmyk et al., Citation2002; Szafrańska et al., Citation2002). Further results are presented here for the first time from a comparative analysis of phenolic acids present in seeds of green-podded and yellow-podded beans. Problems in analysing phenolic acids arise from their structural complexities, as these compounds may exist in multiple forms, e.g. free, esterified, glycosylated or polymerised forms (Robbins, Citation2003). In addition, phenolic acids are not uniformly distributed in plants at the tissue, cellular and sub-cellular levels and may coexist as complexes with proteins, carbohydrates, lipids or other plant components. Hence, the polarity of phenolic acids varies significantly, and it is difficult to develop a uniform analysis procedure for an assay of all phenolic acids.
The results of our investigations on the seeds of two green-podded and two yellow-podded French bean cultivars have demonstrated the presence of four phenolic acids: caffeic acid, p-coumaric acid, ferulic acid and sinapic acid, in all samples.
These acids appeared in both the free and ester-bound forms. Caffeic acid in the free form appeared only in seeds of the green-podded cultivar “Exalto”, while in seeds of the other cultivars, it was present solely in the esterified form. Similar results were achieved by Luthria and Pastor-Corrales (Citation2006), who studied dry beans from ten market classes and 15 varieties commonly consumed in the United States. The authors identified the same phenolic acids that were present in all the bean classes and varieties. Caffeic acid was determined in quantifiable amounts only in two varieties, namely ‘T-39ʹ and ‘Eclipse of the Black’ bean class. Analogous to our study, ferulic acid was the most predominant phenolic acid, and intermediate levels of p-coumaric and sinapic acids were detected in all bean varieties (Luthria & Pastor-Corrales, Citation2006; Sosulski & Dąbrowski, Citation1984). With respect to the total quantity of phenolic acids isolated in the current study, there are certain differences relative to earlier reports (Luthria & Pastor-Corrales, Citation2006; Orak et al., Citation2016). These differences in total phenolic acids can be attributed to various factors such as the variety tested, the assay procedure used, and the plant growing and storage conditions. (Dixon & Paiva, Citation1995; Hakkinen & Torronen, Citation2000; Ninfali & Bacchiocca, Citation2003). Król et al. (Citation2015) demonstrate that plants within one species developed different tolerance to the same environmental factors. These authors also reported that analysis of the content of phenolics and their ability to scavenging free radicles could be one of the ways for detecting the degree of a plant’s tolerance to abiotic stressor. It seems that this analysis can also be used to select varieties of beans with a higher tolerance to adverse conditions. The selection of varieties seems to be justified to increase the profitability of cultivation of beans.
5. Conclusions
The present study, for the first time, compared the antioxidant potential and content of phenolics in seeds of green-podded and yellow-podded French bean cultivars. The results demonstrated that seeds of green-podded varieties (“Algarve” and “Exalto”) were generally characterised by a higher total content of phenolic compounds than seeds of yellow-podded cultivars (“Goldfield” and “Lotos”). The highest antioxidant capacity was determined in extracts from seeds of one of the green-podded cultivars (“Algarve”), while the highest reducing power of ferrous ions was found in extracts from seeds of the other green-podded variety, “Exalto”. The presence of three phenolic acids: p-coumaric acid, ferulic acid and sinapic acid, was detected in all samples. These acids appeared in both free and ester-bound forms. Caffeic acid in the free form appeared only in seeds of the green-podded variety “Exalto”, while in seeds of the other cultivars it was present as an esterified phenolic acid. Ferulic acid was the predominant phenolic acid, and intermediate levels of p-coumaric and sinapic acids were detected in all of the bean varieties. The relationship, demonstrated in this research, between the content of phenolic compounds and the colour of the bean pods could be helpful in identifying French bean cultivars that would be most beneficial for human health and best for organic farming. It is a well-known fact that seed production in organic farming encounters serious problems due to the shortage of effective “green” plant protection measures.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Allakhverdiev, S. I., Kreslavski, V. D., Klimov, V. V., Los, D. A., Carpentier, R., & Mohanty, P. (2008). Heat stress: An overview of molecular responses in photosynthesis. Photosynthesis Research, 98, 541–550.
- Amarowicz, R., Naczk, M., Zadernowski, R., & Shahidi, F. (2000). Antioxidant activity of condensed tannins of beach pea, canola hulls, evening primrose, and faba beans. Journal of Food and Lipids, 7, 199–211.
- Amarowicz, R., & Weidner, S. (2009). Biological activity of grapevine phenolic compounds. In K. A. Roubelakis-Angelakis (Eds.), Grapevine molecular physiology & biotechnology (2nd ed., pp. 389–405). Heraklion: Springer Science + Business Media B.V.
- Benzie, F. F., & Strain, J. J. (1999). Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology, 299, 15–23.
- Bhattacharya, A., Sood, P., & Citowsky, V. (2010). The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Molecular Plant Pathology, 11, 705–719.
- Cardador-Martinez, A., Loarca-Piǹa, G., & Oomah, B. D. (2002). Antioxidant activity in common brans (Phaseolus vulgaris L.). Journal of Agricultural Food and Chemistry, 50, 6975–6980.
- Crépon, K., Marget, P., Peyronnet, C., Carrouée, B., Arese, P., & Duc, G. (2010). Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crops Research, 115, 329–339.
- Dixon, R. A., & Paiva, N. L. (1995). Stress-induced phenylpropanoid metabolism. Plant Cell, 7, 1085–1097.
- Djordjevic, T. M., Śiler-Marinkovic, S. S., & Dimitrijevic-Brankovic, S. I. (2011). Antioxidant activity and total phenolic content in some cereals and legumes. International Journal of Food Properties, 14, 175–184.
- Hakkinen, S., & Torronen, R. (2000). Content of flavonols and selected phenolic acids in strawberries Vaccinium species: Influence of cultivar, cultivation site and technique. Food Reviews International, 33, 517–524.
- Janas, K. M., Cvikrova, M., Pałągiewicz, A., Szafrańska, K., & Posmyk, M. M. (2002). Constitutive elevated accumulation of phenylpropanoids in soybean roots at low temperature. Plant Science, 163, 369–373.
- Kranner, I., Minibayeva, F. V., Backett, R. P., & Seal, C. E. (2010). What is stress? Concepts, definitions and applications in seed science. New Phytologist, 188, 655–673.
- Król, A., Amarowicz, R., & Weidner, S. (2014). Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiologiae Plantarum, 36, 1491–1499.
- Król, A., Amarowicz, R., & Weidner, S. (2015). The effect of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitis vinifera L.) leaves. Journal of Plant Physiology, 189, 97–104.
- Łabuda, H., & Brodaczewska, A. (2011). Sowing date as a factor determining French bean yield for processing. Folia Horticulturae, 23, 73–81.
- Lattanzio, V. (2013). Phenolic compounds: Introduction. In K. G. Ramawat & J. M. Mérilon (Eds.), Natural products (pp. 1543–1580). Berlin: Springer – Verlag.
- Luthria, D. L., & Pastor-Corrales, M. A. (2006). Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. Journal of Food Composition and Analysis, 19, 205–211.
- Marathe, S. A., Rajalakshmi, V., Jamdar, S. N., & Sharma, A. (2011). Comparative study on antioxidant activity of different varieties of commonly consumed legumes in India. Food and Chemical Toxicology, 49, 2005–2012.
- Naczk, M., & Shahidi, F. (1989). The effect of methanol - ammonia - water treatment on the content of phenolic acids of canola. Food Chemistry, 31, 159–164.
- Nascimento, N. C., & Fett-Neto, F. (2010). Plant secondary metabolism and challenges in modifying its operation: An overview. Methods in Molecular Biology, 643, 1–13.
- Ninfali, P., & Bacchiocca, M. (2003). Polyphenols and antioxidant capacity of vegetables under fresh and frozen conditions. Journal of Agricultural Food and Chemistry, 51, 2222–2226.
- Nyau, V., Prakash, S., Rodrigues, J., & Farrant, J. (2016). Screening different Zambian market classes of common beans (Phaseolus vulgaris) for antioxidant properties of total phenolic profiles. Journal of Food Nutrition Research, 4, 230–236.
- Oomach, B. D., Cardador-Martinez, A., & Loarca Piǹa, G. (2005). Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L.). Journal of the Science of Food and Agriculture, 85, 935–942.
- Orak, H. H., Karamać, M., Orak, A., & Amarowicz, R. (2016). Antioxidant potential and phenolic compounds of some widely consumed Turkish white bean (Phaseolus vulgaris L.) varieties. Polish Journal of Food and Nutrition Science, 66, 253–260.
- Posmyk, M. M., Janas, K. M., & Szafrańska, K. (2002). Osmoconditioning reduces physiological and biochemical damage induced by chilling in soybean seed. Physiologia Plantarum, 111, 473–483.
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice - Evans, C. A. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26, 1231–1237.
- Reynoso-Comacho, R., Ramos-Gomez, M., & Loarca-Piǹa, G. (2006). Bioactive components in common beans (Phaseolus vulgaris L.). In R. G. Guevera-González & I. Torres-Pacheco (Eds.), Advances in agricultural and food biotechnology research (2nd ed., pp. 217–236). Trivandrum: Fort P.O.
- Robbins, R. J. (2003). Phenolic acids in foods: An overview of analytical methodology. Journal of Agricultural Food and Chemistry, 51, 2866–2887.
- Sosulski, F. W., & Dąbrowski, K. J. (1984). Composition of free and hydrolysable phenolic acids in the flours and hulls of ten legume species. Journal of Food Composition and Analysis, 32, 131–133.
- Szafrańska, K., Posmyk, M., & Janas, K. M. (2002). Activity of phenylalanine ammonia lyase and soluble phenols content in two cultivars of soybean differing in sensitivity to low-temperature stress. Zeszyty Problemów I Postępów W Nauk Rolniczych, 481, 211–215.
- Turk, H., Erdal, S., Genisel, M., Aticio, O., Demir, Y., & Yanmis, D. (2014). The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedling. Plant Growth Regulation, 74, 139–152.
- Xu, B. J., Yusan, S. H., & Chang, S. K. C. (2007). Comparative analyses of phenolic composition antioxidant capacity, and colour of cool season legumes and other selected food legumes. Journal of Food Science, 72, S167–S177.