?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The hydrosoluble extract from a processing waste, aril of Torreya fargesii Franch. (abbr.as Torreya F), was investigated for its antioxidative properties and resulting protective effect on the oxidation of DHA (docosahexaenoic acid) algal oil. The aril extract of Torreya F contained about 125.2 mg GAE/g dry extract total polyphenols and 18.5 mg RT/g dry extract total flavonoids. The scavenging ability on the DPPH (1,1-diphenyl −2-picrylhydrazyl), hydroxyl and superoxide anion radicals of aril extract of Torreya F was stronger than that of commercial ascorbyl palmitate. With the addition of Torreya F aril extract, the increasing peroxide value and acid value of DHA algal oil was inhibited both in the long-term storage test and the accelerating Schaal test.
RESUMEN
En el presente estudio se investigó el extracto hidrosoluble obtenido de un desecho de procesamiento, el arilo de Torreya fargesii Franch. (abreviado como Torreya F), con el objetivo de determinar sus propiedades antioxidantes y el efecto protector resultante ante la oxidación del aceite de alga DHA (ácido docosahexaenoico). Se comprobó que el extracto del arilo de Torreya F contiene alrededor de 125.2 mg GAE/g del extracto deshidratado de polifenoles totales y 18.5 mg RT/g del extracto deshidratado de flavonoides totales. La capacidad de captación de DPPH (1,1-difenil – 2-picrilhidrazil), hidroxilo y radicales aniones superóxidos del extracto de arilo de Torreya F fue mayor que la del palmitato ascorbilo comercial. A partir de la adición del extracto de arilo de Torreya F se inhibió el incremento de los valores peróxido y ácido del aceite de alga DHA, tanto en la prueba de almacenamiento de largo plazo como en la prueba de Schaal acelerada.
Introduction
Torreya fargesii Franch. is a species of Torreya, Taxaceae, which discontinuously distributes in the subtropical mountainous areas with an altitude around 800–2700 m in Middle and Southwest China, such as the Qinling Mountains, the Wushan Mountains and the Daba Mountains. It is different with the Torreya grandis (abbr. as Torreya G), which widely spreads in the hilly ground of Southeast China (Cheng, Li, Yu, Dai, & Fu, Citation2007). The seeds of these torreya are with well flavor and mainly consumed as expensive edible nuts in Chinese Market. The aril (as shown in of torreya seed is a waste in the husking process; it accounts for the seed’s weight for 15–20%. Huge amount of aril was produced during the harvest time of torreya nuts every year. The casual discard or unsuitable treatment of the aril would cause serious environmental risks. The further utilization with these waste arils became an emerging task in Southwest China. Previous researches already separated out several chemicals, such as the protein, kernel oil, limonene, polyphenols and flavonoids, from the Torreya G (Chen et al., Citation2006; Ni et al., Citation2015; Yu, Ni, Wu, & Sang, Citation2016; Yu, Zeng, Qin, He, & Chen, Citation2017). The lipids and terpenoids were usually extracted by petroleum ether or hexane; the extraction by ethanol usually produced the hydrosoluble polyphenols and flavonoids. Variety kinds of polyphenolic substances, including catechol derivates, phenolic acids, tannin and flavonoids, were found in the torreya extracts (Wang, Citation2008; Yu, Citation2014); the main flavonoids in torreya extract was biflavone, such as kayaflavone, sequoyitol, sciadopitysin, amentoflavone (Kozuka, Tokuda, & Matsumoto, Citation1989; Li et al., Citation2003). The antioxidative efficiency of the aril extract from Torreya G was evaluated with satisfied results by radical scavenging test (He, Chen, Yu, & Ni, Citation2015; Shi, Wang, Wang, & Li, Citation2009; Yu, Guo, Wu, & Ding, Citation2014). However, the corresponding extract from Torreya F or its aril was scarcely reported as so far. revealed the difference of two seeds on morphology; Torreya F has a near round seed; while the seed of Torreya G is like the olive seed. The differences on growing environment and botanic classification of these two torreyas could result in different composition and activity of their arils’ extract. Therefore, the hydrosoluble extract from aril of Torreya F (abbr. as Torreya F extract) still deserved investigation. In this article, the aril extract of Torreya fargesii Franch. was prepared by subcritical extraction method and its antioxidative capacity was evaluated by radical catching tests using DPPH, hydroxyl and superoxide anion radicals as targets.
Figure 1. The whole seeds (the green cortex is the aril) of Torreya fargesii Franch. (left) and Torreya grandis (right).
Figura 1. Semillas enteras (el córtex verde es el arilo) de Torreya fargesii Franch. (izquierda) y de Torreya grandis (derecha).

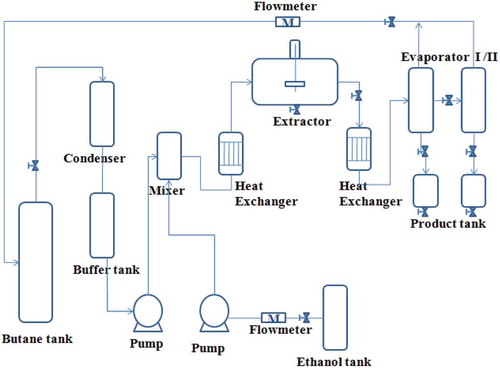
Various extraction processes with different methods have been developed to obtain extracts from natural materials. The subcritical extraction was an effective method in the preparing of hydrosoluble antioxidant from natural plants (Ibanez et al., Citation2003). The subcritical extractor can deal with hundred grams to hundred tons of raw materials, which covered the demand from lab to factory. The subcritical extraction mainly depended on the ultra-permeability of butane; in this study, we added ethanol/water to obtain the hydrosoluble polyphenols and flavonoids. The pressure of subcritical extraction (about 1.0 Mpa) was far below the pressure demand of super-critical extraction method (usually under 15–25 Mpa). The cost of subcritical extraction was only tenth of the super-critical extraction method due to the cutting down of electric power.
The DHA algal oil (abbr. as DHA oil) is a fermented product from Schizochytrium, which contains about 40% DHA and other 10–20% polyunsaturated fatty acid (PUFA). The using of DHA oil as edible blend oil, microencapsulate and softgel held large amount of market shares in global business. The DHA in blend oil was more sensitive to oxidation in air (Kamal-Eldin & Yanishlieva, Citation2002) than in the form of softgel or microencapsulate; the oxidation would deteriorate the flavor and quality of products (Fournier et al., Citation2006). The oxidation of DHA was mainly caused by the chain reaction initiated from oxygen radicals. The ascorbyl palmitate (AP) and tocopherol are the commercial antioxidants for DHA oil on industry. The rosemary extract, oregano, salidroside and other plants extracts (Bhale, Xu, Prinyawiwatkul, King, & Godber, Citation2007; Jayasinghe, Gotoh, & Wada, Citation2013; Navarro-Garcia et al., Citation2017; Wang, Wang, & Chen, Citation2010) were also evaluated for their antioxidative efficiency on DHA oil in the laboratorial research. The antioxidative activity on DHA oil of these natural extracts was attributed to their quenching ability on oxygen radicals. In this article, the aril extract of Torreya F aril was also investigated of its antioxidative effect on DHA oil by the Schaal test and long-term storage test. Because the edible oil is packaged by fill of nitrogen in glass bottle so the oxidation of DHA oil in the whole shelf life is trivial. After the opening of cap with customers, the penetrating of oxygen will initiate the oxidation of the DHA oil. Therefore, we used the daily uncapping method to reflect the antioxidative capacity of Torreya F extract more practically in this study.
Experimental
Materials and reagents
Torreya F nut was collected in October 2016 from Daba Mountain, Chongqing Province. The aril was artificially deprived from the fresh torreya seed, dried in oven under vacuum at 50°C for 48 h. The dried aril (moisture about 1–2%) was milled and sieved through 60 meshes (sieve diameter 0.25–0.30 mm) to obtain the aril powder of Torreya F. The DHA oil was a commercial product of Huison (Xiamen, China) fermented from Schizochytrium. The ascorbyl palmitate (AP), tocopherol (MT 90, Kemin), DPPH, Folin Ciocalteu reagent, enzymes and gallic acid (TraceCERT®) were purchased from Sigma. Other reagents were all analytical grade and purchased from the local producer.
Subcritical extraction
The extraction of aril extract was achieved on a lab-scale subcritical extractor (Henan Subcritical Co. Ltd., China), whose flow chart was shown in . The extractor can handle about 1000 g aril powder for each operation. The solvent was mixed by butane, water and ethanol with a molar ratio of 4:1:4. The volume ratio of aril powder and solvent was set to 1:25. The operating temperature was 45°C with 1 h immersion time under stirring; the de-solvent temperature was 60°C under vacuum. The above conditions were modified from a recommended standard operating procedure (SOP) on extracting hydrosoluble component presented by the supplier. The further optimization of extraction condition was not considered because the purpose of this study was to explore the treatment of aril waste and clarify the antioxidative ability of Torreya F extract.
Determination of total polyphenols and total flavonoids
The content of total polyphenols of the Torreya F extract was determined by the Folin -Ciocalteu’s method using gallic acid as the reference. The standard working curves of total polyphenols was constructed referring the Industrial Standard (GB/T8313-2008, 2008). The absorbance of test solution was measured on a UV-2800 Spectrometer at 760 nm. The detail of measurement was similar with the documents (Singleton, Orthofer, & Lamuela-Raventos, Citation1999). The total polyphenols was calculated from the standard working curves using the absorbance data and expressed as gallic acid equivalent (mg GAE/g dry extract, mg GAE/g DE). The content of total flavonoids was measured with the same method of Liu’s report (Liu et al., Citation2017) .The working curve was established in 60% ethanol using rutin as reference. The absorbance was obtained at 510 nm on the Uv-vis Spectrometer. The linearity was established between 6 and 24 μg/ml (R2, 0.999). The results were expressed as rutin equivalent (mg rutin/g dry extract, mg RT/g DE). Three parallel tests were carried out and their average value was expressed with its standard error.
DPPH radical assay
The DPPH radical scavenging efficiency of Torreya F extract was assessed according to the published document (Blois, Citation1958; Siddeeg, Xu, Jiang, & Xia, Citation2015). 20 μL Torreya F extract (in ethanol with different concentration) was diluted into 2 mL DMSO and then blended with 2 mL 0.1 mM DPPH. The reactant was kept for 30 min in the dark and the absorbance was measured at 565 nm on a spectrometer. BHT and ethanol without Torreya F extract was used as the positive control and blank, respectively.
The scavenging efficiency (E%) of DPPH radicals was calculated using the following equation:
here, A1 is the absorbance of a mixture of reactant solution and A0 is the absorbance of the control reaction without Torreya F extract.
Superoxide anion radical assay
The test method of superoxide anion radical scavenging assay was modified from the reported document (Valentao et al., Citation2001). Superoxide anion radical was generated in a xanthine/xanthine oxidase system. 30 μL Torreya F extract (in ethanol with different concentration) was added into the reactant system consisting of 1 mM xanthine, 1 mM EDTA and 0.3 mM NBT (Nitrotetrazolium Blue chloride, in PBS). Finally, 1 mL of xanthine oxidase (150 mU/mL, in PBS) was added to the mixture and incubated 15 min at 25°C. The absorbance of NBT was measured in a microplate cuvette at 560 nm on spectrometer.
The scavenging efficiency (E%) of superoxide anion radicals was calculated using the following equation:
here, A1 is the absorbance of a mixture of reactant solution and A0 is the absorbance of the control reaction without Torreya F extract.
Hydroxyl radical assay
The hydroxyl radical scavenging activity was performed referring to the method described by Kaur (Kaur & Halliwell, Citation1994). The test mixture contained 120 μL of 30 mM 2-deoxy-2-ribose (in PBS), 50 μL solution of Torreya F extract, 150 μL of 10 mM FeSO4-EDTA and 100 μL H2O2. After incubation at 25°C for 1 h, the solution was stopped by adding 2 mL of thiobarbituric acid (0.5%, w/v). The absorbance was recorded at 536 nm in a micro-cuvette.
The scavenging efficiency (E%) of OH· was calculated by:
here, A1 and A0 are the absorbance of radical reactant with and without Torreya F extract, respectively. A2 is the background absorbance of Torreya F extract.
Antioxidative ability on DHA oil
The antioxidative effect on DHA oil by Torreya F extract was evaluated by the long-term storage test and the accelerating Schaal test. To increase the solubility in DHA oil, the Torreya F extract was first mixed with glycerol monooleate to forming the antioxidative additive (total polyphenols 100 mg GAE/g). The solubility of above additive was higher than 0.5% in DHA oil. Then 250 mg additive (25 mg GAE/g) was added into 50 g DHA oil in a capped flask to carry out a 42 days storage test under room temperature (about 20–25°C). The cap of bottle was opened 5 min every day artificially to simulate the daily using condition. The exposure of sample DHA oil to fresh air could character the oxidative degree more reliability than the sealed shelf-life test. Each 7 days, 2 ml oil sample was fetched to measure the acid value (AV) and peroxide value (PV) according to the AOCS method (AOCS Cd 3d-63; AOCS Cd 8b-90). In the Schaal test, 50 g oil adding with 0.5% extract additives (25 mg GAE/g) was heated in constant temperature oven at (63 ± 1) °C. The PV and AV was tested for every 8 h, the total heating time was set to 96 h. The BHT, tocopherol (MT 90) and AP was employed as comparison to evaluate the antioxidative ability of Torreya F extract. Referring to the commercial dosage, the addition of BHT, AP and tocopherol was 0.5 mg/mL (500 ppm), 1 mg/mL (1000 ppm) and 1 mg/mL (1000 ppm), respectively.
Statistical analysis
In the radicals’ scavenging test and DHA oil oxidation test, the average value of three parallel tests was used in the Figure. The standard error was no more than 5% of the average value. The corresponding data with the addition of different antioxidants were analyzed by one-way ANOVA (Analysis of Variance) and multiple mean comparisons using the Tukey–Kramer method by SPSS Statistics 15.0 (SPSS Inc., Chicago, USA). The 0.05 level of significance was used to determine the antioxidative difference between the data of different antioxidants.
Results and discussions
Total polyphenols and total flavonoids
The yield was about 1.2–1.7% (dry extract: dry aril) in the subcritical extraction. The product was ropy solid with yellow to brown color. The solubility of extract was higher than 2% in water, which indicated the hydrosoluble materials were the main component. The content of total polyphenols in the aril extract was 125.2 ± 7.2 mg GAE/g dry extract (DE). For the aril of Torreya G, the content of total polyphenols was reported as 40–50 mg GAE/g DE by supersonic extraction method (Bao, Citation2010); the higher content of total polyphenols (76.67 mg GAE/g DE) was obtained using supercritical extraction method (He et al., Citation2015). The total flavonoids in the aril extract of Torreya F was 18.5 ± 3.1 RT mg/g DE, it was equivalent with the content in Torreya G (about 16 mg/g) by supersonic extraction method (Yu et al., Citation2014). It indicated the subcritical method was effective in the extraction of total polyphenols and total flavonoids from the waste aril. However, the extraction method, solvent and operating conditions could manifestly affect the content of total polyphenols and total flavonoids in the aril extract.
DPPH radical scavenging ability
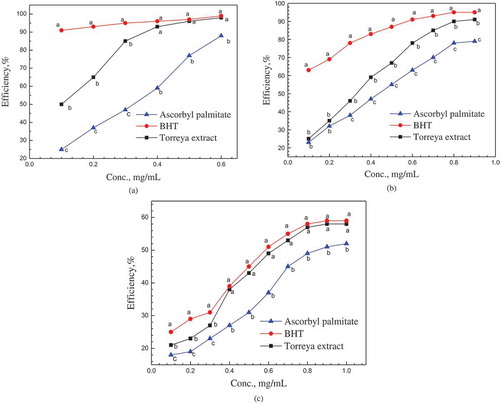
The scavenging of DPPH radical indicated the hydrogen-donation ability of natural antioxidants. In , the Torreya F extract showed stronger scavenging efficiency at each dosage than commercial AP. At the concentration higher than 0.4 mg/mL, the elimination efficiency of Torreya F extract was higher than 90%. The BHT showed the best DPPH radical scavenging efficiency even at low dosage. At concentration higher than 0.5 mg/mL, the scavenging capacity between BHT and Torreya F extract was not significant (P < 0.05). In a previously research on Torreya G, the aril extract obtained by supersonic extraction method showed a lower efficiency than AP in a similar test condition (Bao, Citation2010). The IC 50 values (inhibitory concentration decreasing the absorbance of DPPH solution by 50%) of Torreya F extract was 0.1 mg/mL, converted as 0.0125 GAE mg/mL. The aril extract of Torreya G gave an IC 50 as 1.55 mg/mL (about 1 mg GAE/mL) (Bao, Citation2010) while the pure flavonoids extract from aril of Torreya G presented IC 50 at 0.063 mg/mL in another report (He et al., Citation2015). For the essential oil extract from the aril of Torreya G, the IC 50 on DPPH was less effective (17.13 mg/mL) (Yu et al., Citation2016). A research on polyphenolic-enriched apple skin extract showed the IC 50 about 0.036 GAE mg/mL in the fish oil with 12% DHA (Vasantha, Naciye, & Afsana, Citation2010). However, the amount of phenolic compounds and the antioxidant capacity of plant extract depend on many factors, such as genetic differences, pre-harvest environmental conditions or degree of maturity at harvest.
Figure 3. The scavenging efficiency on DPPH radical, hydroxyl radical and superoxide anion radical of Torreya F extract (a) DPPH radical. (b) hydroxyl radical. (c) superoxide anion radical.
* The standard error ≤5% of each data point.* a,b,c Means with the same letter in the same concentration did not show significant difference (Tukey–Kramer test, α = 0.05).
Figura 3. Eficiencia de captación en el radical DPPH, el radical hidroxilo y el radical anión superóxido del extracto de Torreya F: (a) radical DPPH; (b) radical hidroxilo; y (c) radical anión superóxido.
* El error estándar es de ≤5% de cada dato.* a,b,c Las medias con la misma letra en la misma concentración no demostraron tener diferencias significativas (prueba Tukey-Kramer, α = 0.05).
Hydroxyl radical scavenging ability
The redox potential of hydroxyl radical is 2.8 V (vs. NHE) (Parsons, Citation2004) which was stronger than that of other common oxygen radicals in aqueous media. It was meaningful to reduce the oxidation of sensitive chemicals by hydroxyl radicals’ scavenger in the antioxidative research. In , the scavenging efficiency on hydroxyl radical by Torreya F extract increased with the increasing of dosage. The Torreya F extract showed stronger scavenging capacity than that of AP but lower than the BHT at concentration higher than 0.3 mg/mL (P < 0.05). The highest scavenging efficiency of Torreya F extract was nearly 90% with 0.9 mg/mL concentration (about 0.1 mg GAE/ml polyphenols, 0.016 mg/mL flavonoids). The BHT with 0.6 mg/mL concentration presented above 90% elimination efficiency at the same condition. It indicated the equivalent capacity on the scavenging of hydroxyl radical between the Torreya F extract and BHT. The IC 50 of Torreya F extract on hydroxyl radical was 0.35 mg/mL (0.043 mg GAE/ml), which was higher than that of on DPPH radical. For the Torreya G, the IC 50 was 1.32 mg/L (about 0.092 GAE mg/mL) in a similar test condition (He et al., Citation2015). For the kernel oil from Torreya G, the IC 50 was about 0.1 mg/mL (Ni & Shi, Citation2014).
Superoxide anion radical scavenging ability
The superoxide anion radical was not sensitive to the quenching from TBHQ, BHT and normal natural antioxidant. The scavenging efficiency of Torreya F extract on superoxide anion radical was lower than that on DPPH and hydroxyl radicals (in ). The IC 50 of Torreya F extract on superoxide anion radical was 0.62 mg/ml, which was higher than that on DPPH and hydroxyl radicals. The aril extract of Torreya F contained about 120 mg GAE/g DE total polyphenols and 18 mg RT/g DE total flavonoids; its elimination capacity on superoxide anion radical was equivalent with BHT and stronger than that of AP at concentration higher than 0.4 mg/mL (P < 0.05). With the increasing of extract concentration, the elimination efficiency on superoxide radicals increased and reached about 59% at the high concentration (0.8 mg/mL).
Oxidation protection on DHA oil
The Schaal test was an accelerating method to evaluate the stability of oil as well as the efficiency of their antioxidative additives. The chain reaction of DHA oxidation was initiated, catalyzed and propagated by the participating of radicals (Yasushi, Sanae, & Kenshiro, Citation1997). Therefore, the addition of Torreya F extract showed manifest inhibition on the oxidative process of DHA oil. In and , the PV and AV of DHA oil after addition of Torreya F extract, commercial AP, commercial tocopherol and BHT were compared. With the heating time increasing, the acid value (AV) and peroxide value (PV) showed a generally rising trend for all sample oils. After heating 24 h, the value of AV was lower in presence of Torreya F extract than the AP and tocopherol. The scavenging difference between AP and tocopherol was not significant within 56 h heating time (P < 0.05). The variation of peroxide value was complicated in long-term test than the AV because of the occurrence of secondary oxidation reaction. After 40 h heating, the PV in presence of Torreya F extract was lower than that with AP and tocopherol at the same heating time (P < 0.05), except the PV of 80 h. The variation of PV and AV indicated the Torreya F extract had better protective effect on the oxidation of DHA oil than the existed commercial AP and tocopherol. The addition of Torreya F extract (0.5 mg/mL, 0.5 g/kg) can keep the PV below 20 meq/kg within 48 h heating. A research on fish oil with 30% PUFA showed the addition of 1 g/kg flavonoids (catechin, morin, qucertin) had the equivalent effect in the same test conditions (Nieto et al., Citation1993). BHT showed strongest protective capacity than the other three antioxidants on the inhibition of increasing PV and AV (P < 0.05). However, the chemical synthesized BHT lagged behind the market development. And it was even prohibited to use in some circumstances, such as formula or food for infant and children.
Figure 4. The variation of acid value of DHA oil with different antioxidants (a) in Schaal test (The significant between each group, P < 0.05). (b) in long-term storage test (The significant between each group, P < 0.05).
* The standard error ≤5% of each data point.* a,b,c,d Means with the same letter in the same time did not show significant difference (Tukey–Kramer test, α = 0.05).
Figura 4. Variación del valor ácido del aceite DHA con los distintos antioxidantes: (a) en la prueba Schaal; (b) en pruebas de almacenamiento de largo plazo. (El valor de significación entre cada grupo, P < 0.05.).
* El error estándar es de ≤5% de cada dato.* a,b,c,d Las medias con la misma letra en los mismos periodos no demostraron tener diferencias significativas (prueba Tukey–Kramer, α = 0.05).
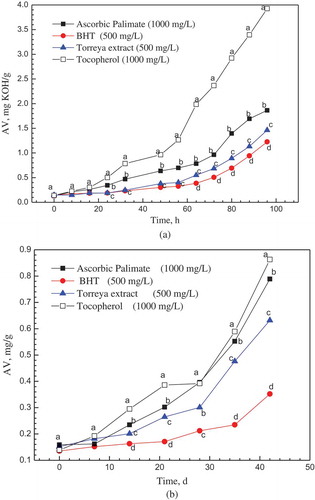
Figure 5. The variation of peroxide value of DHA oil with different antioxidants (a) in Schaal test (The significant between each group, P < 0.05). (b) in long-term storage test (The significant between each group, P < 0.05).
* The standard error ≤5% of each data point.* a,b,c,d Means with the same letter in the same time did not show significant difference (Tukey–Kramer test, α = 0.05).
Figura 5. Variación del valor peróxido del aceite DHA con distintos antioxidantes (b) en la prueba de almacenamiento de largo plazo (El valor de significación entre cada grupo, P < 0.05.).
* El error estándar es de ≤5% de cada dato.* a,b,c,d Las medias con la misma letra en el mismo periodo no demostraron tener diferencias significativas (prueba Tukey–Kramer, α = 0.05).
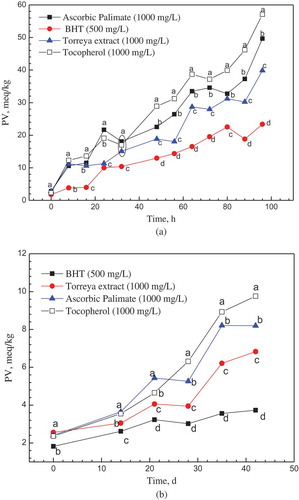
The Schaal test reflected the antioxidative capacity of Torreya F extract under the extremely heating condition. The long-term storage test under room temperature indicated the protective ability of antioxidants more practical. As shown in , the increment of AV after 42 days test decreased from tocopherol, AP, Torreya F extract to BHT. The behavior of AP and tocopherol on AV inhibition was similar with that in Schaal test. The peroxide value directly demonstrated the content of primary oxidative products in the oxidation of oil. In , after 14 days storage, the PVs in different antioxidants groups showed manifest difference (P < 0.05), which indicated the Torreya F extract with better protective capacity than the AP and tocopherol. BHT still showed the best antioxidative ability than the other three natural sourcing antioxidants in the long-term test. The long-term storage test presented the similar results with the accelerating Schaal test, which reflected the reliability of these tests and the stability of these antioxidants in DHA oil.
In 30 days, the addition of Torreya F extract can keep the AV of DHA oil no more than 0.5 mg KOH/g. It was up to standard of the DHA oil (0.5 mg KOH/g) (GRAS UCM264032). The PV of 5.0 meq/kg was the upper limit for DHA oil and its blend oil (GRAS UCM264032). The addition of Torreya F extract can keep the PV of DHA oil samples below 5.0 meq/kg within 30 days storage term. The commercial edible blend oil with DHA oil can be consumed within about 30–45 days (250–500 mL per bottle/10–15 mL per day). In the commonly using condition, the daily exposing time to air of the DHA oil was shorter than one minute. The practical oxidation of commercial DHA oil should be slower than the experimental condition. Therefore, the Torreya F extract qualified as a potential replacement of existing AP and tocopherol.
The hydroxyl radical and superoxide anion radical act as oxidant, catalyst and propagator in the chain reaction of triglyceride oxidation (Vasantha et al., Citation2010). Especially, the DHA oil contains more than 60% of polyunsaturated fatty acid, which made it more sensitive to active oxygen radicals, such as hydroxyl radical and superoxide anion radical. The polyphenols and flavonoids in Torreya F extract can effective scavenging the correlating radicals, terminating the chain reaction and inhibiting the oxidation of DHA oil.
Conclusion
The hydrosoluble extracts of aril of Torreya fargesii Franch. contained 125.2 GAE mg/g DE total polyphenols and 18.5 mg RT/g DE total flavonoids preparing from the subcritical method. The aril extract showed satisfied scavenging capacity on DPPH radical and hydroxyl radical with about 90% efficiency, while the efficiency can reach 50% for superoxide anion radical. The concentration-dependent activity was observed in some concentration range in the radical scavenging tests. The scavenging efficiency of Torreya F extract was better than ascorbyl palmitate and tocopherol for all investigated radicals. Due to its radicals scavenging properties, the aril extract of Torreya F showed good antioxidative effect on DHA oil both in the accelerating Schaal test and the long-term storage test. The protective ability of Torreya F extract was better than the existed ascorbyl palmitate and tocopherol for DHA oil. The potential as an antioxidant presented a new utilized route of waste aril from Torreya F seed. The detail of commercial application of Torreya F extract, as well as its toxicity and safety, deserves further investigation.
Acknowledgments
The financial support was provided by the National Natural Science Foundation of China [Grant Number 31470568], the Spring Light Program of the Ministry of Education of China [Grant Number Z2017], the Foundation of the Key Laboratory for Protection and Utilization of Wulingshan Region’s Unique Plant Resources of Chongqing, China (2017), the Science and Technology Project of Fuling District, Chongqing, Chin [Grant Number FLKJ,2017ABA].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bao, J. F. (2010). Function and composition of extract of aril of Torreya Grandis (Master’s thesis). Retrieved from http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD2010&filename=2010063909.nh&v=MzExMTJUM3FUcldNMUZyQ1VSTDJmYitabkZ5emhWTDdQVjEyNkhyTytIZGpNcHBFYlBJUjhlWDFMdXhZUzdEaDE=
- Bhale, S. D., Xu, Z., Prinyawiwatkul, W., King, J. M., & Godber, J. S. (2007). Oregano and rosemary extracts inhibit oxidation of long-chain n-3 fatty acids in menhaden oil. Journal of Food Science, 72, 504–508. doi:10.1111/j.1750-3841.2007.00569.x
- Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181, 1199–1200. doi:10.1038/1811199a0
- Chen, B. Q., Cui, X. Y., Zhao, X., Zhang, Y. H., Piao, H. S., Kim, J. H., … Yun, Y. P. (2006). Antioxidative and acute antiinflammatory effects of Torreya grandis. Fitoterapia, 77, 262–267. doi:10.1016/j.fitote.2006.03.019
- Cheng, X. J., Li, Z. J., Yu, W. W., Dai, W. S., & Fu, Q. G. (2007). Distribution and ecological characteristics of Torreya grandis in China (in Chinese). Journal of Zhejiang Forestry College, 24, 383–388.
- Fournier, V., Juaneda, P., Destaillats, F., Dionisi, F., Lambelet, P., Sebedio, J. L., & Berdeaux, O. (2006). Analysis of eicosapentaenoic and docosahexaenoic acid geometrical isomers formed during fish oil deodorization. Journal of Chromatography A, 1129, 21–28. doi:10.1016/j.chroma.2006.06.089
- GRAS Notification for DHA Algal Oil Derived from Schizochytrium sp. (2003). FDA. UCM 264032. Retrieved from https://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm264032.pdf
- He, W. Y., Chen, Y. X., Yu, Y. J., & Ni, S. (2015). The antioxidant activity evaluation of the extract from Torreya grandis cv.Merrillii Aril (in Chinese). Chinese Wild Plant Resources, 34, 1–4.
- Ibanez, E., Kubatova, A., Senorans, F. J., Cavero, S., Reglero, G., & Hawthorne, S. B. (2003). Subcritical water extraction of antioxidant compounds from rosemary plants. Journal of Agricultural and Food Chemistry, 51, 375–382. doi:10.1021/jf025878j
- Jayasinghe, C., Gotoh, N., & Wada, S. (2013). Pro-oxidant/antioxidant behaviours of ascorbic acid, tocopherol, and plant extracts in n-3 highly unsaturated fatty acid rich oil-in-water emulsions. Food Chemistry, 141, 3077–3084. doi:10.1016/j.foodchem.2013.05.143
- Kamal-Eldin, A., & Yanishlieva, N. V. (2002). N-3 fatty acids for human nutrition: Stability considerations. European Journal of Lipid Science Technology, 104, 825–836. doi:10.1002/1438-9312(200212)104:12<825::AID-EJLT825>3.0.CO;2-N
- Kaur, H., & Halliwell, B. (1994). Detection of hydroxyl radicals by aromatic hydroxylation oxygen radicals. Methods in Enzymology, 233, 67–82. doi:10.1016/S0076-6879(94)33009-3
- Kozuka, M., Tokuda, H., & Matsumoto, T. (1989). Hinokiflavone and kayaflavone as antiviral agents. Kokai Tokkyo Koho, 22, 133–135.
- Li, S. H., Zhang, H. J., Niu, X. M., Ping, Y., Sun, H. D., & Fong, H. H. S. (2003). Chemical constituents from Amentotaxus yunnanensis and Torreya yunnanensis. Journal of Natural Products, 66, 1002–1005. doi:10.1021/np030117b
- Liu, Y., Luo, X. L., Lan, Z. Q., Tang, J. R., Zhao, P., & Kan, H. (2017). Ultrasonic-assisted extraction and antioxidant capacities of flavonoids from Camellia fascicularis leaves. CyTA – Journal of Food, 16, 105–112. doi:10.1080/19476337.2017.1343867
- Navarro-Garcia, G., Gamez-Meza, N., Medina-Juarez, L. A., Ortega-Garcia, J., Cota-Quinones, E., & Ramirez-Suarez, J. C. (2017). Natural antioxidants in the stability of ray liver oil. Ciencia Rural, 47, 47–48. doi:10.1590/0103-8478cr20160240
- Ni, L., & Shi, W. Y. (2014). Composition and free radical scavenging activity of kernel oil from Torreya grandis, Carya Cathayensis and Myrica Rubra. Iranian Journal of Pharmaceutical Research, 13, 221–226.
- Ni, Q. X., Gao, Q. X., Yu, W. W., Liu, X. Q., Xu, G. Z., & Zhang, Y. Z. (2015). Supercritical carbon dioxide extraction of oils from two Torreya grandis varieties seeds and their physicochemical and antioxidant properties. LWT-Food Science and Technology, 60, 1226–1234. doi:10.1016/j.lwt.2014.09.007
- Nieto, S., Garrido, A., Sanhueza, J., Loyola, L. A., Morales, G., Leighton, F., & Valenzuela, A. (1993). Flavonoids as stabilizers of fish oil: An alternative to syntheticantioxidants. Journal of American Oil Chemistry Society, 70, 773–779. doi:10.1007/BF02542599
- Parsons, S. (2004). Advanced oxidation processes for water and wastewater treatment. UK: IWA Publishing.
- Shi, H. M., Wang, H. D., Wang, M. Y., & Li, X. B. (2009). Antioxidant activity and chemical composition of Torreya grandis cv. Merrillii Seed. Natural Product Communications, 4, 1565–1570.
- Siddeeg, A., Xu, Y. S., Jiang, Q. X., & Xia, W. S. (2015). In vitro antioxidant activity of protein fractions extracted from seinat (Cucumis melo var. tibish) seeds. CyTA – Journal of Food, 13, 472–481. doi:10.1080/19476337.2014.1003199
- Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent in oxidants and antioxidants. Methods in Enzymology, 299, 152–178.
- Valentao, P., Fernandes, E., Carvalho, F., Andrade, P. B., Seabra, R. M., & Bastos, M. L. (2001). Antioxidant activity of Centaurium erythraea infusion evidenced by its superoxide radical scavenging and xanthine oxidase inhibitory activity. Journal of Agricultural and Food Chemistry, 49, 3476–3479. doi:10.1021/jf001145s
- Vasantha, R. H. P., Naciye, E., & Afsana, Y. (2010). Antioxidant protection of eicosapentaenoic acid and fish oxidation by polyphenolic-enriched apple skin extract. Journal of Agricultural and Food Chemistry, 58, 1233–1239. doi:10.1021/jf903162k
- Wang, H. D. (2008). The chemical composition, antioxidative activity and soluble protein of Torreya seed (Postdoc Dissertation). Retrieved from http://d.wanfangdata.com.cn/Thesis/Y1400944.
- Wang, X. H., Wang, W. T., & Chen, G. (2010). Effects of 4 kinds of antioxidants on the stability of DHA and EPA (in Chinese). China Pharmacy, 21, 2717–2719.
- Yasushi, E., Sanae, H., & Kenshiro, F. (1997). Kinetics for the autoxidation of triacylglycerols containing eicosapentaenoic acid. Bioscience Biotechnology and Biochemistry, 61, 1036–1037. doi:10.1271/bbb.61.1036
- Yu, M., Zeng, M. M., Qin, F., He, Z. Y., & Chen, J. (2017). Physicochemical and functional properties of protein extracts from Torreya grandis seeds. Food Chemistry, 227, 453–460. doi:10.1016/j.foodchem.2017.01.114
- Yu, Y. (2014). Study on the extraction,analysis and antioxidant ability of the main ingredients in the Torreya Grandis (Master’s thesis). Retrieved from http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201601&filename=1015403351.nh&v=MDcwNDdTN0RoMVQzcVRyV00xRnJDVVJMMmVadVpxRnlya1ZMcktWRjI2RzdlNEhkTEpycEViUElSOGVYMUx1eFk=
- Yu, Y., Guo, L., Wu, M. H., & Ding, Z. E. (2014). Study on the extraction and antioxidant activity of total flavonoids of Torreya grandis Aril (in Chinese). Food Industry, 35, 23–26.
- Yu, Y. J., Ni, S., Wu, F., & Sang, W. G. (2016). Chemical composition and antioxidant activity of essential oil from Torreya grandis cv. merrillii Arils. Journal of Essential Oil Bearing Plant, 19, 1170–1180. doi:10.1080/0972060X.2014.989183