ABSTRACT
Species of the genus Prosopis were a major staple food in Aridoamerica before the arrival of Europeans. In the present work, chemical and nutritional properties of Prosopis laevigata pods were described. The composition in weight of pods of P. laevigata was 44% mesocarp, 35% endocarp, and 21% exocarp. Sugars, including sucrose, glucose, fructose, and xylose, were important components of pods, reaching a total sugars content of 447 g/kg in mesocarp flour. Considering the FAO-recommended amino-acid scoring patterns for humans older than 3 years, high values of Lys and sulfur-containing amino acids were found in flours. Thermal treatment of flours increases significantly the phenolic compounds content and free-radical scavenging capacity, an effect associated with the generation of Maillard reaction products. Flours of mesquite pods are a good source of phenolic compounds, with significantly higher free-radical scavenging capacity than soybean and common bean.
RESUMEN
Especies del genero Prosopis fueron alimento importante en Aridoamérica antes de la llegada de los Europeos. En el presente trabajo se describen las propiedades químicas y nutrimentales de vainas de Prosopis laevigata. La composición en peso de las vainas de P. laevigata fue de 21% exocarpio, 44% mesocarpio y 35% endocarpio. Azúcares, incluyendo sacarosa, glucosa, fructosa y xilosa, fueron importantes componentes de la vaina, alcanzando un contenido tota de azúcares de 447 g/kg en harina de mesocarpio. Considerando los valores de aminoácidos recomendados por la FAO para mayores de 3 años, altos valores de Lys y aminoácidos azufrados fueron encontrados en las harinas. El tratamiento térmico de las harinas incrementa significativamente el contenido de compuestos fenólicos y la capacidad captadora de radicales libres, un efecto asociado a la generación de productos de la reacción de Maillard. Las harinas de vaina de mezquite son una buena fuente de compuestos fenólicos, con una capacidad captadora de radicales libres más alta que las semillas de soya y frijol.
1. Introduction
Legumes have been an essential part of the human diet for centuries, with a major role in global food security, environmental challenges and healthy diets (FAO, Citation2016). The genus Prosopis is a group of nitrogen-fixing trees belonging to the Fabaceae family, which involves 44 species distributed mainly in arid and semiarid regions of Asia, Africa, and America (Felker, Takeoka, & Dao, Citation2013). Prosopis species were a major staple food for indigenous peoples in arid regions of America before the arrival of Europeans. The mature fruit of the genus Prosopis is an indehiscent pod conformed by an exocarp, a developed mesocarp, and a woody endocarp which protects the seed (Felker et al., Citation2013). Pods and pods fractions of Prosopis species have been described in their nutritional profile: P. africana (Igwe, Ojiako, Anugweje, Nwaogu, & Ujowundu, Citation2012), P. alba (Cattaneo et al., Citation2016; Felker, Grados, Cruz, & Prokopiuk, Citation2003; Sciammaro, Ferrero, & Puppo, Citation2016), P. chilensis (Astudillo, Schmeda-Hirschmann, Herrera, & Cortes, Citation2000), P. glandulosa (Harden & Zolfaghari, Citation1988), P juliflora (Marangoni & Alli, Citation1988), P. laevigata (Barba de la Rosa, Frias-Hernandez, Olalde-Portugal, & Gonzalez-Castañeda, Citation2006; Gallegos-Infante, Rocha-Guzman, Gonzalez-Laredo, & Garcia-Casas, Citation2013), P. nigra (Felker et al., Citation2003), P. ruscifolia (Bernardi, Sanchez, Freyre, & Osella, Citation2010), and P. tamarugo (Astudillo et al., Citation2000); active compounds content and in vitro biological activity: P. alba (Cardozo et al., Citation2010; Cattaneo et al., Citation2016; Perez et al., Citation2014; Sciammaro et al., Citation2016), P. chilensis (Astudillo et al., Citation2000; Briones–Labarca, Muñoz, & Maureira, Citation2011; Schmeda-Hirschmann et al., Citation2015), P. laevigata (Gallegos-Infante et al., Citation2013), P. nigra (Cardozo et al., Citation2010; Perez et al., Citation2014), P. ruscifolia (Bernardi et al., Citation2010), and P. tamarugo (Astudillo et al., Citation2000); and in vivo biological activity: P. glandulosa (George, Lochner, & Huisamen, Citation2011; Huisamen, George, Dietrich, & Genade, Citation2013). Nutritionally, pods of Prosopis species are gluten free (Bernardi et al., Citation2010; Felker et al., Citation2013) and rich in good quality protein (Barba de la Rosa et al., Citation2006) or limited in sulfur amino acids (Felker & Bandurski, Citation1977; Felker et al., Citation2013) and threonine (Felker & Bandurski, Citation1977; Igwe et al., Citation2012). The mesocarp of pods is rich in sugars (31%–50%), sucrose principally (Felker et al., Citation2013; Sciammaro et al., Citation2016). Trypsin inhibitors and phytohemagglutinins are present in seeds, which are denatured and inactivated upon heating (Barba de la Rosa et al., Citation2006; Gallegos-Infante et al., Citation2013). Pods of Prosopis species have been reported as a source of bioactive compounds (Felker et al., Citation2013; Schmeda-Hirschmann et al., Citation2015). Mesocarp (Schmeda-Hirschmann et al., Citation2015), seed (Bernardi et al., Citation2010; Briones–Labarca et al., Citation2011; Cattaneo et al., Citation2016; Sciammaro et al., Citation2016) and whole pod flours are a good source of polyphenolic compounds (Cardozo et al., Citation2010; Cattaneo et al., Citation2016; Gallegos-Infante et al., Citation2013; Perez et al., Citation2014; Sciammaro et al., Citation2016) with antioxidant (Bernardi et al., Citation2010; Briones–Labarca et al., Citation2011; Cardozo et al., Citation2010; Cattaneo et al., Citation2016; Perez et al., Citation2014; Schmeda-Hirschmann et al., Citation2015; Sciammaro et al., Citation2016), anti-inflammatory (Cattaneo et al., Citation2016; Perez et al., Citation2014), antihypertensive (Gallegos-Infante et al., Citation2013; Huisamen et al., Citation2013), and hypoglycemic (George et al., Citation2011) activities. Best biochemically described Prosopis species are the South American species P. alba, P. chilensis, and the North American P. glandulosa. The specie P. laevigata distributed in Aridoamerica (Palacios, Citation2006) is particularly poor described. Therefore, the objective of this work was to describe the chemical and nutritional properties of raw and thermal-treated flours of P. laevigata pods and their residual brans, including proximate composition, sugar and phenolic compounds content, amino-acid profile, free-radical scavenging capacity, and Maillard reaction products (MRPs).
2. Materials and methods
2.1. Plant material
Dry mature pods of 12 P. laevigata trees with the same phenotypic traits and growing conditions were collected during August 2016, at the experimental field of the Universidad Politécnica de Francisco I. Madero in the semiarid region of the Mezquital Valley in Hidalgo, Mexico. Collected yellow–brown mature pods from different trees were mixed in a single pool of 52 kg. Pods were ground in one and two stages, using a 900 W blender (Nutribullet, Los Angeles, CA, USA). For one-stage milling process, an exhaustive milled pods batch of 15 kg was sieved in 30 and 80 mesh, producing three fractions: mesocarp-seed flour (MSF) which pass through 80 mesh, exocarp and seed coat bran (ExSCB) retained in the 80 mesh, and endocarp hard coat bran (EnHCB) retained in the 30 mesh. For two-stage milling process, a milled pods batch of 15 kg was sieved in 30 and 80 mesh, producing three fractions: mesocarp flour (MF) which pass through 80 mesh, exocarp bran (ExB) retained in the 80 mesh and intact endocarp retained in the 30 mesh. The intact endocarp was submitted to a second milling process, producing three fractions: seed flour (SF) which pass through 80 mesh, seed coat bran (SCB) retained in the 80 mesh, and EnHCB retained in the 30 mesh. Flours of commercial legume seeds purchased at local market, tamesi soy bean flour (SBF) (Glycine max) and flor de mayo common bean flour (CBF) (Phaseolus vulgaris) were prepared for comparative purposes.
2.2. Proximate composition
Proximate composition of the different pods flours and brans was assessed according the methods of the Association of Official Analytical Chemists (AOAC, Citation2000).
2.3. Free sugars content
Content of sugars in raw flours was determined by HPLC method, according to Akanni, du Preez, Steyn, and Kilian (Citation2015) using a Dionex-UltiMate 3000 equipment (Thermo Scientific, MA, USA) supplied with a REZEX RPM-Monosaccharide PB+2(8%) column (300 × 7.8 mm). Sugars separation was developed using water as mobile phase at 80°C column temperature and were detected by refractive index.
2.4. Amino-acid profile
The amino-acid content in raw flours was analyzed after acid hydrolysis, using a cation exchange separation column (LCA K06/Na, 4.6 × 150 mm; Sykam GmbH, Germany) with ninhydrin postcolumn derivatization, in an amino-acid analyzer (Sykam GmbH, Germany) (Li et al., Citation2012). Same method was used for sulfur-containing amino acids, using performic acid oxidation before hydrolysis (Li et al., Citation2012). Tryptophan was determined at 620 nm, after enzymatic hydrolysis and reaction with p-dimethylaminobenzaldehyde (Nielsen, Klein, & Hurrell, Citation1985).
2.5. Thermal treatment of flours
MF, SF, and MSF were hydrated with 1.5 mL of water per gram of flour and thermal treated, as in a baked process, in a convection oven (Thermo Scientific, MA, USA) at 190°C during 20 min. Mesocarp flour thermal treated (MFT), seed flour thermal treated (SFT), and mesocarpo-seed flour thermal treated (MSFT) were dried and ground.
2.6. Preparation of extracts
Phenolic compounds of raw flours, thermal-treated flours, and brans were extracted with aqueous ethanol, according the method of Kalia, Sharma, Singh, and Singh (Citation2008). Samples of 100 mg were extracted with one milliliter of 40% ethanol in water (v/v) and centrifuged at 12,000 rpm/10 min. The extract was removed and the extraction process was done again with the residual pellet. Both extracts were mixed and diluted with 40% ethanol to obtain a final volume of 25 mL.
2.7. Hydrolysis of extracts
For hydrolysis of phenolic glycosides, a replicate was prepared for each obtained extract. HCl hydrolysis method of Nuutila, Kammiovirta, and Oksman-Caldentey (Citation2002) was used for hydrolysis with an ethyl acetate aglycones recovery. The ethyl acetate was evaporated at 45°C overnight and the residue was diluted with 40% ethanol to obtain a final volume of 25 mL.
2.8. Ultraviolet analysis of Maillard reaction products
Analysis of MRP in extracts of raw and thermal-treated flours was performed using the spectrophotometric method reported by Yu, Zhao, Hu, Zeng, and Bai (Citation2012). Appropriate dilution of extracts were scanned from 240 to 320 nm using an ultraviolet–visible (UV–Vis) spectrophotometer (Genesys 10S, Thermo Scientific, MA, USA). The presence of MRP was evidenced by the increase in the UV absorbance between 270 and 290 nm.
2.9. Total phenolic compounds content
Total phenolic compounds content was determined in raw, thermal-treated and hydrolyzed extracts by the Folin–Ciocalteu method (Pekal & Pyrzynska, Citation2014). The absorbance was measured at 760 nm and the results were expressed as g of gallic-acid equivalents/kg (mg GAE/kg) of dry weight.
2.10. Total flavonoids content
Total flavonoid content was determined in raw, thermal-treated, and hydrolyzed extracts using two aluminum complexing assays reported by Pekal and Pyrzynska (Citation2014). First assay consisted in the reaction of extracts with an aluminum chloride (AlCl3) solution and the subsequent absorbance measurement at 425 nm. Results were reported as g of quercetin equivalents/kg (g QE/kg) of dry weight. Second assay consisted in the reaction of extracts, in the presence of sodium nitrite (NaNO2), with an aluminum chloride solution in alkaline media and the subsequent absorbance measurement at 510 nm. Results were reported as g of catechin equivalents/kg (g CE/kg) of dry weight. Total flavonoids content (g/kg) was reported as the sum of both assays.
2.11. DPPH radical scavenging capacity
The DPPH radical scavenging capacity of raw, thermal-treated, and hydrolyzed extracts was determined according to the Cardozo et al. (Citation2010) method. The absorbance was measured at 515 nm and the results were expressed as g of ascorbic acid equivalent/kg (g AAE/kg) of dry weight.
2.12. ABTS radical scavenging capacity
The ABTS radical scavenging capacity of raw, thermal-treated, and hydrolyzed extracts was determined according to the Pekal and Pyrzynska (Citation2014) method. The absorbance was measured at 734 nm and the results were expressed as mM of trolox equivalent/kg (mM TE/kg) of dry weight.
2.13. Statistical analysis
All assays were performed in triplicate, and expressed as means ± standard deviation. Data were submitted to analysis of variance (ANOVA) and means were compared by Tukey test (p ≤ 0.05) using Statsoft Statistica 8.0. (Citation2007). Correlation analysis (p ≤ 0.05) between absorbance at 290 nm and total phenolic compounds and free-radical scavenging capacity was developed using Statsoft Statistica 8.0. (Citation2007).
3. Results and discussion
3.1. Proximate composition
The proximate composition and pods milling fractions are shown in . The composition in weight of dry mature pods of P. laevigata was mesocarp, endocarp and exocarp, with 440, 350 and 210 g/kg, respectively, seed in the endocarp represents 160 g/kg. One-stage milling process of the pods produced three fractions; MSF, EnHCB and ExSCB, representing, 535, 215, and 250 g/kg, respectively. Two-stage milling process of the pods produced five fractions; MF, SF, ExB, EnHCB and SCB, representing, 230, 100, 405, 205, and 60 g/kg, respectively. The highest flour yield was obtained by one-stage milling process with 535 g/kg, including the mesocarp rich in sugars and the seed rich in protein. Similar flour yield have been reported previously for P. alba and P. pallida (Felker et al., Citation2003). The protein MF content reported here for P. laevigata (105 g/kg), is slightly higher than previous reported values for P. alba (Felker et al., Citation2003), P. glandulosa (Harden & Zolfaghari, Citation1988) and P. pallida (Felker et al., Citation2003) with 80, 70, and 100 g/kg, respectively. The protein content reported here for SF of P. laevigata (310 g/kg) is higher than previously reported values for P. Africana (Igwe et al., Citation2012) and similar to P. alba (Sciammaro et al., Citation2016), P. juliflora (Marangoni & Alli, Citation1988) and P. glandulosa (Harden & Zolfaghari, Citation1988). Similar whole pod flours (mesocarp-SF) protein content have been reported for P. chilensis (Astudillo et al., Citation2000), P. glandulosa (Harden & Zolfaghari, Citation1988), and P. tamarugo (Astudillo et al., Citation2000). Using one- or two-stage milling process is possible to obtain flour fractions rich in protein (seed), rich in carbohydrates (mesocarp) or a mix of both (mesocarp-seed).
Table 1. Prosopis laevigata pods milling fractions and proximate composition (g/kg).
Tabla 1. Fracciones de molienda de vainas de P. laevigata y composición proximal (g/kg).
3.2. Free sugars content
The contents of free sugars in raw flours are shown in . Sucrose and three monosaccharides (glucose, fructose and xylose) were found in MF, MSF, and SF. Sucrose was the main sugar followed by glucose, fructose and xylose. Total sugars in MF, MSF, and SF were 447.81, 304.97 and 95.99 g/kg, respectively. No previous reports were found for sugars content in P. laevigata. Felker et al. (Citation2013) found sucrose (382 g/kg) as the main sugar, followed by fructose (96 g/kg), glucose (g/kg), and xylose (2 g/kg) in mesocarp of P. alba. Particular importance have monosaccharides in foods as substrates for Maillard reactions and fermentation (Yu et al., Citation2012).
Table 2. Contents of free sugars in Prosopis laevigata pods flours (g/kg).
Tabla 2. Contenido de azúcares en harinas de vaina de P. laevigata (g/kg).
3.3. Amino-acid profile
The essential amino-acid contents in raw mesocarp, seed and mesocarp-SF are shown in . Considering the FAO recommended amino-acid scoring patterns for humans older than 3 years (FAO, Citation2013), limiting amino acids were Ile in MF, Ile, Trp, and Val in SF and mesocarp-SF. Particularly, high values of Lys and sulfur-containing amino acids (Met+Cys) were found in the three raw flours. Previously, Felker and Bandurski (Citation1977) reported Val, Thr, Ile, Lys, Trp, and sulfur-containing amino acids as the limiting amino acids in seeds of P. chilensis and P. juliflora, meanwhile, whole pods of P. juliflora was limited only in sulfur-containing amino acids, suggesting that whole pod has a better amino-acid profile than seed. Marangoni and Alli (Citation1988) and Felker et al. (Citation2013) reported sulfur-containing amino acids as the limiting amino acids in pods of Prosopis species, without differencing seed from mesocarp. Thr was the limiting amino acid in seed of P. Africana (Igwe et al., Citation2012). Barba de la Rosa et al. (Citation2006) using a single method for amino-acid analysis, reported a good balance of essential amino acids in P. laevigata whole pod flour, with limited content of sulfur-containing amino acids and particularly high values of Trp, an amino-acid profile that contrast with the results presented here for the same species, which could be explained by differences in the used methodology. Particular methodology must be used for amino acids that are unstable during acid hydrolysis (Met, Cys, and Trp) (Li et al., Citation2012; Nielsen et al., Citation1985).
Table 3. Essential amino-acid content in Prosopis laevigata pods flours (g/kg of protein).
Tabla 3. Contenido de aminoácidos esenciales en harinas de vainas de P. laevigata (g/kg de proteína).
3.4. Ultraviolet analysis of Maillard reaction products
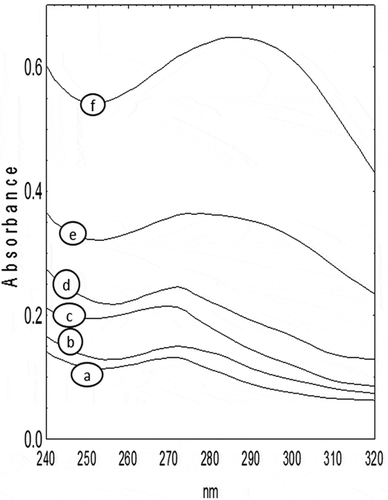
The UV–Vis spectra of extracts of raw and thermal-treated flours of P. laevigata pods are shown in . The absorbance ratios (thermal treated/raw) at 290 nm for seed (0.2/0.13), mesocarp (0.35/0.09), and mesocarp-seed (0.65/0.11) extracts were 1.53, 3.88 and 5.9, respectively (). The increment in absorbance at 290 nm after thermal treatment was highest in mesocarp-SF, followed by mesocarp and seed. The Maillard reaction between reducing sugars and amines (amino acids, peptides or proteins) during thermal treatments of foods produces low-molecular-weight heterocycles, resulting in a maximum of UV absorbance around 280–294 nm (Sahin, Topuz, & Pischetsrieder, Citation2009; Yu et al., Citation2012). MRPs are complex mixture of intermediate compounds (such as aldehydes, reductones, and heterocyclic compounds) and final compounds (such as melanoidin), which, respectively, contribute to the flavor and color of foods (Mondaca-Navarro et al., Citation2017; Vhangani & Van-Wyk, Citation2016; Yu et al., Citation2012). Necessary reagents for Maillard reaction development are free amino groups from amino acids, peptides or proteins and reducing sugars as hexoses (glucose and fructose) or pentoses (ribose, xylose, and arabinose) (Yu et al., Citation2012). The presence of any combination of these components in foods, during thermal treatment, produces MRP which can be detected by increases in UV absorbance at 290 nm (Yu et al., Citation2012). In the present work, highest absorbance at 290 nm after thermal treatment were found in mesocarp-SF, followed by MF, samples with a high content of hexoses (glucose and fructose) and pentoses (xylose) (). Seed with a low content of reducing sugars () had the lowest absorbance at 290 nm after thermal treatment. The highest absorbance at 290 nm was found in mesocarp-SF, a sample with important quantities of reducing sugar and protein (), another important component for Maillard reaction development. In some cases MRP may generate negative effects on human health, resulting in anti-nutritional properties, such as the loss of essential amino acids and formation of mutagenic compounds (Mondaca-Navarro et al., Citation2017). In recent years, research on MRP has focused on their relationship with bioactive properties of foods, such as antioxidant, chelating, antimicrobial, anti-inflammatory, prebiotic and antihypertensive activities (Mondaca-Navarro et al., Citation2017; Sahin et al., Citation2009; Yu et al., Citation2012). In many instances, MRP exhibited higher radical scavenging and reducing power than BHA (Vhangani & Van-Wyk, Citation2016). The MRP detected by UV spectroscopy has important free-radical scavenging capacity, which correlates with the reduction of Folin–Ciocalteu reagent in the quantification of total phenolic compounds (Sahin et al., Citation2009). MRP with antioxidant activity could be obtained from the reaction between amino acids and reducing sugars (hexoses or pentoses) (Yu et al., Citation2012). Pods of P. laevigata contain a diverse source of amino groups () and a mix of reducing sugars, including glucose, fructose and xylose (). The composition of P. laevigata pods made their flours very prone to Maillard reaction development when are thermal treated.
Table 4. Absorbance at 290 nm of raw and thermal-treated Prosopis laevigata pods flours and its correlation with phenolic compounds content and DPPH radical scavenging capacity.
Tabla 4. Absorbancia a 290 nm de harinas crudas y térmicamente tratadas de vaina de P. laevigata y su correlación con el contenido de compuestos fenólicos totales y la capacidad captadora de radical DPPH.
Figure 1. UV–Vis spectra of extract of thermal treated and non-thermal-treated samples. a) mesocarp flour (MF), b) mesocarp-seed flour (MSF), c) seed flour (SF), d) seed flour thermal treated (SFT), e) mesocarp flour thermal treated (MFT), f) mesocarp-seed flour thermal treated (MSFT).
Figura 1. Espectro UV–Vis de extractos de muestras térmicamente tratadas y no térmicamente tratadas. a) harina de mesocarpo (MF), b) harina de mesocarpo y semilla (MSF), c) harina de semilla (SF), d) harina de semilla térmicamente tratada (SFT), e) harina de mesocarpo térmicamente tratada (MFT), f) harina de mesocarpo y semilla térmicamente tratada (MSFT).
3.5. Total phenolic compounds content
Total phenolic compounds contents in raw, thermal-treated, and hydrolyzed samples are shown in . Total phenolic compounds contents in raw flours were 8.87, 6.57, and 8.36 g GAE/kg for MF, SF, and MSF, respectively. These values are higher than previous reported values of free phenolic compounds for pods of P. nigra (3.3–6.6 g GAE/kg) and P. alba (2.2–4.6 g GAE/kg) (Perez et al., Citation2014). Thermal treatment of MF, SF, and MSF increases significantly (40%, 17%, and 58%, respectively) the total phenolic compound content when compared with their respective raw flours. This increased values could be attributed to the generation of s, which can be evidenced by the increased absorbance between 270 and 290 nm in thermal-treated samples (). Total phenolic compounds content showed high linear correlation (r = 0.76) with absorbance at 290 nm (), suggesting the reduction of Folin–Ciocalteu reagent by MRP generated during thermal treatment. Folin–Ciocalteu assay is not specific for phenolic compounds and other compounds like MRP can also reduce the Folin–Ciocalteu reagent, a phenomena previously described in carob pods flours thermal-treated (Sahin et al., Citation2009). Major increases in UV absorbance were found in thermal-treated flours with high levels of monosaccharides (), a substrate for Maillard reaction. Previous works reported the total phenolic compounds content in flours of Prosopis species using the Folin–Ciocalteu reagent method (Bernardi et al., Citation2010; Briones-Labarca et al., Citation2011; Cardozo et al., Citation2010; Cattaneo et al., Citation2016; Gallegos-Infante et al., Citation2013; Perez et al., Citation2014; Schmeda-Hirschmann et al., Citation2015; Sciammaro et al., Citation2016) with variability in the values, which can be due to different samples, species and sample treatment. Sample treatment during drying could enhance Maillard reaction activity, indirectly modifying the total phenolic compound content of Prosopis flours. Acid hydrolysis of extracts of MF, SF, and MSF decreases significantly (11%, 60% and 13%, respectively) the total phenolic compound content when compared with their respective raw flours. During acid hydrolysis free phenolic acids and flavonoids can be partially degraded, while flavonoids as quercetin and kaempferol are considerate acid resistant (Nuutila et al., Citation2002). Total phenolic compounds content in brans ranged from 3.46 to 7.50 g GAE/kg in raw samples (ExB, EnHCB, SCB, and ExSCB) and from 0.69 to 5.71g GAE/kg in hydrolyzed samples (ExBH, EnHCBH, SCBH, and ExSCBH). No previous reports were found for total phenolic compounds content in Prosopis brans. Total phenolic compounds contents of SBF and CBF, raw and hydrolyzed are shown in . For both legume seeds, their values represents between 10% and 20% of the respective mesquite pods flours. Regarding the phenolic compounds content, mesquite pods are more dense foods than soybean and common bean.
Table 5. Total phenolic compounds, total flavonoids and free-radical scavenging capacity of Prosopis laevigata pods flours and brans.
Tabla 5. Compuestos fenólicos totales, flavonoides totales y capacidad captadora de radicales libres de harinas y salvados de vainas de P. laevigata.
3.6. Total flavonoids content
The contents of total flavonoids in raw, thermal-treated, and hydrolyzed extracts are shown in . Thermal treatment of MF, SF, and MSF flour had a slightly effect on total flavonoids contents when compared with their respective raw flours. Acid hydrolysis of extracts of MF, SF, and MSF increases significantly (300%, 32%, and 180%, respectively) the total flavonoids contents when compared with their respective raw flours. Food flavonoids exist mainly in the form of glycosides which are less able to form complex with aluminum. Acid hydrolysis of glycosides produce free aglycones with higher capacity to interact with aluminum, increasing the sensibility of the assays (Denni & Mammen, Citation2012; Pekal & Pyrzynska, Citation2014). The assay conducted in neutral media without NaNO2, must be used only to determine the content of flavonoles and the assay in the presence of NaNO2 in alkaline medium must be used for rutin, catechins, and some phenolic acids, being less specific than the first assay (Pekal & Pyrzynska, Citation2014). Together both assays represent a better and complemented measurement of the total flavonoids content in food samples. Major increases after hydrolysis were found in neutral media, suggesting the presence of flavonoid glycosides of the flavonol subgroup. Previous studies reported the presence of glycosides of two flavonoids, apigenin, and quercetin in mesocarp and seed of P. alba (Cattaneo et al., Citation2016; Perez et al., Citation2014), P. nigra (Perez et al., Citation2014), and P. chilensis (Schmeda-Hirschmann et al., Citation2015). Total flavonoids content in brans ranged from 1.41 to 3.16 g/kg in raw samples (ExB, EnHCB, SCB and ExSCB) and from 0.13 to 3.68 g/kg in hydrolyzed samples (ExBH, EnHCBH, SCBH and ExSCBH). No previous reports were found for total flavonoids content in Prosopis brans. Total flavonoids content of SBF and CBF, raw, and hydrolyzed are shown in . For both legume seeds, their values represents between 5% and 15% of the respective mesquite pods flours. Regarding the total flavonoids content, mesquite pods are more dense foods than soybean and common bean.
3.7. Radical scavenging capacity
The radical scavenging capacities of raw, thermal-treated, and hydrolyzed extracts are shown in . Thermal treatment of MF, SF, and MSF increases significantly (35%, 15%, and 80%, respectively) the free-radical scavenging capacity when compared with their respective raw flours. These increased values could be attributed to the Maillard reactions involving reducing sugars and amino acids, leading to the formation of a variety of byproducts, intermediates, and brown pigments, which can be evidenced by the increased absorbance between 270 and 290 nm in thermal-treated samples (). Mayor increases in absorbance were found in flours samples thermal-treated rich in monosaccharides (), a necessary substrate for Maillard reaction. Free-radical scavenging capacity (DPPH) showed high linear correlation (r = 0.93) with absorbance at 290 nm (), suggesting the participation of MRP generated during thermal treatment on free-radical scavenging capacity. MRPs have been described as compounds with radical scavenging capacity and its production during thermal treatment can increase the radical scavenging capacity in flours of carob pods (Sahin et al., Citation2009) and synthetic media (Mondaca-Navarro et al., Citation2017; Vhangani & Van-Wyk, Citation2016; Yu et al., Citation2012). Major food antioxidants are phenolic compounds, but ascorbic acid, MRP and other compounds can reduce DPPH and ABTS radicals (Mondaca-Navarro et al., Citation2017; Vhangani & Van-Wyk, Citation2016; Yu et al., Citation2012). Thermal treatment of Prosopis pods flour have been related to brown color development, due to Maillard reaction (Felker et al., Citation2013). Acid hydrolysis of extracts of MF had a slightly positive effect in free-radical scavenging capacity, while in SF and MSF acid hydrolysis decreases significantly (40% and 55%, respectively). The reduction of free-radical scavenging capacity in hydrolyzed extracts could be attributed mainly to phenolic acids destruction. In hydrolyzed extracts of thermal-treated seed and MSF radical scavenging capacity increases significantly, an effect attributed to MRP generated during treatment. Hydrolysis of brans extracts decreases the radical scavenging capacity. No previous reports were found for radical scavenging capacity in Prosopis brans. The radical scavenging capacities of SBF and CBF, raw, and hydrolyzed are shown in . For both legume seeds, their values represents between 20% and 35% of the respective mesquite pods flours. Regarding the radical scavenging capacity, mesquite pods are more dense foods than soybean and common bean.
4. Conclusions
One- or two-stage milling process of P. laevigata pods can produce flours and brans able to be used as ingredient in human food products. These flours are compositionally similar to other Prosopis species. Brans can be considered as a valuable fiber-rich raw material. Raw flours are a good source of Lys and sulfur-containing amino acids. Raw flours and brans are a good source of active phenolic compounds with higher radical scavenging capacity than soybean and common bean. Thermal treatment of P. laevigata flours increases the apparent total phenolic compounds content and radical scavenging capacity, an effect associated with the generation of MRPs. Prosopis laevigata pods flours are very prone to Maillard reaction development, due to the presence of free sugars. Particular importance have MRP due to their recent described properties on health. The identification and quantification of specific antioxidant compounds produced during thermal treatment of flours would be further conducted. Total flavonoid content can be better defined using both aluminum complexation assays after acid hydrolysis, but given the important role in human health of these compounds, analytical definition must be further conduced for specific flavonoids identification. The results presented here highlight the alimentary traits of a currently underutilized biological resource.
Acknowledgments
The first author acknowledges to CONACYT for PhD fellowship.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Akanni, G. B., Du Preez, J. C., Steyn, L., & Kilian, S. G. (2015). Protein enrichment of an Opuntia ficus-indica cladode hydrolysate by cultivation of Candida utilis and Kluyveromyces marxianus. Journal of the Science of Food and Agriculture, 95, 1094–1102.
- AOAC. (2000). Official methods of analysis of AOAC international (17th ed.). Gaithersburg, MD: Association of Analytical Communities.
- Astudillo, L., Schmeda-Hirschmann, G., Herrera, J. P., & Cortes, M. (2000). Proximate composition and biological activity of Chilean Prosopis species. Journal of the Science of Food and Agriculture, 80, 567–573.
- Barba de la Rosa, A. P., Frias-Hernandez, J. T., Olalde-Portugal, V., & Gonzalez-Castañeda, J. (2006). Processing, nutritional evaluation and utilization of whole mesquite flour (Prosopis laevigata). Journal of Food Science, 71, 315–320.
- Bernardi, C., Sanchez, H., Freyre, M., & Osella, C. (2010). Gluten-free bread formulated with Prosopis ruscifolia (vinal) seed and corn flours. International Journal of Food Sciences and Nutrition, 61, 245–255.
- Briones–Labarca, V., Muñoz, C., & Maureira, H. (2011). Effect of high hydrostatic pressure on antioxidant capacity, mineral and starch bioaccessibility of a non-conventional food: Prosopis chilensis seed. Food Research International, 44, 875–883.
- Cardozo, M. L., Ordoñez, R. M., Zampini, I. C., Cuello, A. S., Dibenedetto, G., & Isla, M. I. (2010). Evaluation of antioxidant capacity, genotoxicity and polyphenol content of non-conventional foods: Prosopis flour. Food Research International, 43, 1505–1510.
- Cattaneo, F., Costamagna, M. S., Zampini, I. C., Sayago, J., Alberto, M. R., Chamorro, V., … Isla, M. I. (2016). Flour from Prosopis alba cotyledons: A natural source of nutrient and bioactive phytochemicals. Food Chemistry, 208, 89–96.
- Denni, M., & Mammen, D. (2012). A critical evaluation on the reliability of two aluminum chloride chelation methods for quantification of flavonoids. Food Chemistry, 135, 1365–1368.
- FAO. (2013). Dietary protein quality evaluation in human nutrition; Report of an FAO expert consultation (FAO Food and Nutrition Paper 92). Italy: Rome.
- FAO. (2016). Pulses; Nutritious seeds for a sustainable future. Italy: Rome.
- Felker, P., & Bandurski, R. (1977). Protein and amino acids composition of tree legume seeds. Journal of the Science of Food and Agriculture, 28, 791–797.
- Felker, P., Grados, N., Cruz, G., & Prokopiuk, D. (2003). Economic assessment of production of flour from Prosopis alba and P. pallida pods for human food applications. Journal of Arid Environments, 53, 517–528.
- Felker, P., Takeoka, G., & Dao, L. (2013). Pod mesocarp flour of North and South American species of leguminous tree Prosopis (mesquite): Composition and food applications. Food Reviews International, 29, 49–66.
- Gallegos-Infante, J. A., Rocha-Guzman, N. E., Gonzalez-Laredo, R. F., & Garcia-Casas, M. A. (2013). Efecto del procesamiento térmico sobre la capacidad antioxidante de pinole a base de vainas de mezquite (Prosopis laevigata). CyTA – Journal of Food, 11, 162–170.
- George, C., Lochner, A., & Huisamen, B. (2011). The efficacy of Prosopis glandulosa as antidiabetic treatment in rat models of diabetes and insulin resistance. Journal of Ethnopharmacology, 137, 298–304.
- Harden, M. L., & Zolfaghari, R. (1988). Nutritive composition of green and ripe pods of honey mesquite (Prosopis glandulosa). Economic Botany, 42, 522–532.
- Huisamen, B., George, C., Dietrich, D., & Genade, S. (2013). Cardioprotective and anti-hypertensive effects of Prosopis glandulosa in rat model of pre-diabetes. Cardiovascular Journal of Africa, 24, 10–16.
- Igwe, C. U., Ojiako, O. A., Anugweje, K. C., Nwaogu, L. A., & Ujowundu, C. O. (2012). Amino acid profile of raw and locally processed seeds of Prosopis africana and Ricinus communis: Potential antidotes to protein malnutrition. Functional Foods in Health and Disease, 2, 107–119.
- Kalia, K., Sharma, K., Singh, H., & Singh, B. (2008). Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea lodd and quantification of its phenolic constituents by RP-HPLC. Journal of Agricultural and Food Chemistry, 56, 10129–10134.
- Li, P., Zeng, Z., Wang, D., Xue, L., Zhang, R., & Piao, X. (2012). Effects of the standardized ileal digestible lysine to metabolizable energy ratio on performance and carcass characteristics of growing-finishing pigs. Journal of Animal Science and Biotechnology, 3, 9.
- Marangoni, A., & Alli, I. (1988). Composition and properties of seeds and pods of the tree legume Prosopis juliflora. Journal of the Sciences of Food and Agriculture, 44, 99–110.
- Mondaca-Navarro, B. A., Ávila-Villa, L. A., Gonzalez-Córdova, A., Lopez-Cervantes, J., Sanchez-Machado, D., Campas-Baypolia, O. C., & Rodríguez-Ramírez, R. (2017). Antioxidant and chelating capacity of Maillard reaction products in amino acid sugar model systems: Applications for food processing. Journal of the Sciences of Food and Agriculture. doi:10.1002/jsfa.8206
- Nielsen, H., Klein, A., & Hurrell, R. (1985). Stability of tryptophan during food processing and storage. British Journal of Nutrition, 53, 293–300.
- Nuutila, A. M., Kammiovirta, K., & Oksman-Caldentey, K. M. (2002). Comparison of methods for the hydrolysis of flavonoids and phenolic acids from onion and spinach for HPLC. Food Chemistry, 76, 519–525.
- Palacios, R. A. (2006). Los mezquites mexicanos: Biodiversidad y distribución geográfica. Boletín De La Sociedad Argentina De Botánica, 41, 99–121.
- Pekal, A., & Pyrzynska, K. (2014). Evaluation of aluminium complexation reaction for flavonoid content assay. Food Analytical Methods, 7, 1776–1782.
- Perez, M. J., Cuello, A. S., Zampini, I. C., Ordoñez, R. M., Alberto, M., Quispe, C., … Isla, M. (2014). Polyphenolic compounds and anthocyanin content of Prosopis nigra and Prosopis alba pods flour and their antioxidant and anti-inflammatory capacities. Food Research International, 64, 762–771.
- Sahin, H., Topuz, A., & Pischetsrieder, M. (2009). Effect of roasting process on phenolic, antioxidant and browning properties of carob powder. European Food Research and Technology, 30, 155–161.
- Schmeda-Hirschmann, G., Quispe, C., Soriano, M. P., Theoduloz, C., Jimenez, A. F., Cuello, A. S., & Isla, M. I. (2015). Chilean Prosopis mesocarp flour: Phenolic profiling and antioxidant activity. Molecules, 20, 7017–7033.
- Sciammaro, L., Ferrero, C., & Puppo, M. C. (2016). Chemical and nutritional properties of different fractions of Prosopis alba pods and seeds. Journal of Food Measurement and Characterization, 10, 103–112.
- Statistica 8.0. (2007). Statistics computer program for Windows (Vol. 1). Tulsa: StatSoft Inc. Software.
- Vhangani, L. N., & Van-Wyk, J. (2016). Antioxidant activity of Maillard reaction products (MRPs) in a lipid-rich model system. Food Chemistry, 208, 301–308.
- Yu, X., Zhao, M., Hu, J., Zeng, S., & Bai, X. (2012). Correspondence analysis of antioxidant activity and UV-Vis absorbance of Maillard reaction products as related to reactants. LWT - Food Science and Technology, 46, 1–9.
