ABSTRACT
The biofunctionality of native and nanostructured starch obtained from blue corn was evaluated on prediabetic Wistar rats. The surface of both types of starch was analyzed by atomic force microscopy (AFM). Total polyphenols content, antioxidant activity and digestibility were also evaluated. Prediabetes was induced by feeding a diet high in fat, carbohydrates and the administration of streptozotocin. Experimental design included a control group, prediabetic group and two prediabetic groups, one supplemented with native starch and the other with nanostructured starch. AFM analysis showed nano-cavities <5 nm in nanostructured starch. Nanostructured starch also had a higher content of total polyphenols, higher antioxidant activity and higher percentage of slow digestibility starch compared to native starch. Glucose, triglycerides 34 and insulin in the plasma increased significantly in the prediabetic group. Nanostructured starch administration decreased the levels of glucose and insulin in the plasma and therefore has potential as functional ingredient.
RESUMEN
En este trabajo se evaluó la biofuncionalidad del almidón nativo y nanoestructurado del grano de maíz azul en ratas Wistar con prediabetes. En ambos almidones se analizó la superficie mediante microscopía de fuerza atómica (AFM), polifenoles totales, actividad antioxidante y digestibilidad. Prediabetes fue inducida con una dieta alta en grasa y carbohidratos y estreptozotocina. El estudio contó con un grupo control, con prediabetes, con prediabetes administrado con almidón nativo y almidón nanoestructurado. El análisis de AFM mostró nanocavidades < 5 mm en el almidón nanoestructurado. El almidón nanoestructurado tuvo un mayor contenido de polifenoles totales, actividad antioxidante y un mayor porcentaje de almidón de digestión lenta en comparación con el almidón nativo. Los niveles de glucosa, triglicéridos e insulina en plasma aumentaron significativamente en el grupo con prediabetes. La administración de almidón nanoestructurado disminuyó los niveles de glucosa e insulina en plasma. El almidón nanoestructurado tiene potencial de aplicación con ingrediente funcional.
Introduction
In recent years, the interest of the food industry on the development of bio-functional ingredients has increased, due to a raising number of consumers who demand healthier food products. Starch is a widely used ingredient in the production of foodstuffs, and currently, the industry is focusing on digestibility given the interest in foods with low contents of digestible starch; another goal is to lower digestibility in those products already containing starch. It is known that slowly digestible starch (SDS) releases glucose in a slow and longer manner onto the blood during hydrolysis; this effect helps patients with obesity and diabetes, two major public health concerns.
Prediabetes, also known as intermediate hyperglycemia or dysglycemia, is an altered basal glycemia (BG) that implies a higher risk of developing type 2 diabetes and cardiovascular disease. Altered BG is defined between the margin of 6.1–6.9 mmol/L, according to the World Health Organization, and of 5.55–6.94 mmol/L according to the American Diabetes Association, being an intermediate situation between normal BG and diabetes (American Diabetes Association, Citation2014). Prediabetic individuals also present impaired glucose tolerance (IGT) and/or insulin resistance (IR). Development of prediabetes is strongly influenced by the diet, particularly by starch-rich foods with high contents of rapidly digestible starch (RDS), and represents a risk factor for the onset of chronic diseases (Englyst, Kingman, & Cummings, Citation1992). The rapid release of glucose onto the bloodstream causes the metabolism to enter an out-of-control state, which favors the development of diabetes mellitus (Jenkins et al., Citation2002). Given the above, improvement the nutritional features of starch through by increasing the amounts of slowly digestible starch, is of particular interest for the food industry.
Corn is one of the many sources of starch; Mexico offers many variations of this cereal that are grouped in 59 races, amongst which some pigmented varieties rich in polyphenols, such as blue corn, are found (Salinas-Moreno, Vázquez-Carrillo, Aragón- Cuevas, & Velázquez-Cardelas, Citation2012). Animal studies in vivo have shown that polyphenols have a preventive effect in the development of diabetes (Ştefănuţ et al., Citation2013) and obesity (Tsuda, Horio, Uchida, Aoki, & Osawa, Citation2003). Furthermore, previous research indicates that blue corn has a lower glycemic index than white corn (Agama et al., Citation2013).
Even though some cereals such as corn are a good source of SDS, food industry uses physical, chemical and enzymatic methods in order to increase SDS content (Bello, Contreras, Romero, Solorza, & Jiménez, Citation2002). Among the physical methods is nanotechnology, which is useful to develop food ingredients with novel nutritional properties that offer health benefits to the health of the consumer (Acosta-Domínguez, Hernández-Sánchez, Gutiérrez-López, Alamilla-Beltrán, & Azuara, Citation2016). Currently, innovations in the food area need to refocus attention from the macroscopic scale to the nanoscale, as well as to define the impact of the functionality of a food at the nutritional and physiological levels. Therefore, the aim of the present work was to evaluate the bio-functionality of native and nanostructured starch on induced prediabetes in a Wistar rat model.
Materials and methods
Materials
Blue corn for preparation of native starch ( was kindly provided by the Center for Interdisciplinary Research for Integral Development in Oaxaca, Mexico (CIIDIR-IPN, unidad Oaxaca). Corn was ground to obtain flour of uniform particle size, then it was soaked in water for 6 h and the sediment was washed repeatedly with water. It was left to stand for another 6 h and the supernatant was discarded. The paste obtained was dried in a forced air circulation oven at 45°C for 48 h. Later, it was ground again and sieved through an 80-μm (0.17 mm) US mesh (Medina et al., Citation2010).
Figure 1. Native (a) and nanostructured starch (b).
Figura 1. Almidón nativo (a) y nanoestructurado (b).
Nano-structured corn ( was obtained by applying a criogenic treatment (Acosta-Domínguez et al., Citation2016). Crystals obtained were lyophilized (Labconco, Look Lyph 4,5 EE. UU) for 3 days. Both types of starch were vacuum-packed to prevent moisture absorption.
Surface analysis by atomic force microscope microscopy
Samples were analyzed at the nanometric scale using an atomic force microscope (AFM), which is a scanning microscope with a very high resolution and is considered one of the main tools in the recording of images and measuring of properties at the nanometric scale. The model Multimode V Veeco, USA, and Software Nanoscope 7.3 were used in the present study. Samples were taken at a temperature of 25°C.
Nuclear magnetic resonance of low field
The time of length relaxation (T1) was quantified in the samples in a range of aw of 0.11–0.85 at 25°C, using a Bruker Minispec (mq20 Bruker Optik Gmbtt Rheinstetten Germany). In order to reduce the moisture content of the samples to a minimum, they were placed in a vacuum dessicator containing P2O5 for a week. Saturated salt solutions with a water activity of 0.1–0.85 were prepared according to Labuza, Kaanane, and Chen (Citation1985). Samples (2 g) were placed by triplicate in the saturated solutions and stored at 25°C until reaching equilibrium. Afterwards, relaxation time was measured.
Biofunctional properties
The Folin–Ciocalteu (Singleton & Rossi, Citation1965) method was used to quantify the content of total polyphenols in both native and nanostructured starch. Antioxidant activity was measured by the DPPH method (Brand-Williams, Cuvelier, & Berset, Citation1995). Total starch was analyzed by the method 76.13 of AACC (Citation2000), using the enzymatic kit Megazyme. Resistant starch was measured according to the AACC (Citation2000) and the Megazyme kit. Fractions of RDS and SDS were determined according to their degree of digestion in the small intestine as measured by glucose release after 20 min (RDS) and 120 min (SDS) (Englyst et al., Citation1992).
Animal model
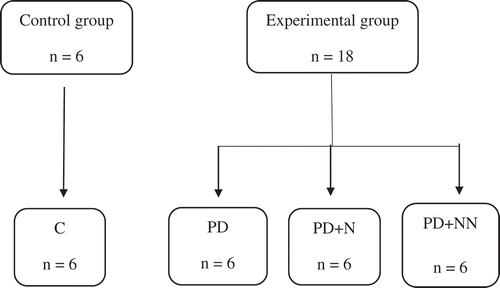
For the animal model, 24 male Wistar rats (Harlan Teklab), with 21 days old and newly weaned, were used. Animals maintenance were in accordance with the laboratory animals specifications provided by the Official Mexican Standard NOM 062-ZOO-1999 and the National Institute of Health “Guide for the Care and Use of Laboratory Animals” (National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Citation2011). Rats were housed in individual boxes at room temperature, with 12-h light/12-h dark cycle and fed ad libitum with standard diet (Rodent Laboratory Chow®). Once the desired weight was achieved (250–300 g), the animals were divided in two groups: control (C) (n = 6) that received a standard diet of Rodent Lan Chow®, and the experimental group (n = 18), in which prediabetes was induced by receiving a high-fat and carbohydrate diet for 6 weeks. This enriched diet contained 68% standard feed, 20% sugar, 0.5% cholesterol (Sigma Aldrich) and 11.5% vegetable shortening from the local market. Once this feeding period was over, 35 mg/kg of streptozotocin was injected via intraperitoneal to induce prediabetes (PD group). Serum glucose was measured in order to ensure that animal model was according to specifications.
Experimental diet
For the experimental diet supplemented with blue corn starch, the total of 24 rats was divided into four groups: control (C, n = 6) which received a standard diet; prediabetic group (PD), receiving a high-fat, high-carbohydrate diet; prediabetic group with a high-fat, high-carbohydrate diet supplemented with native starch (PD + N, n = 6) at the amount of 1.8 g/kg of body weight; and finally the prediabetic group with a high-fat, high-carbohydrate diet supplemented with nanostructured starch (PD + NN, n = 6) at 1.8 g/kg of body weight ().
Figure 2. Experimental group. Control group (C) fed with a standard diet and water ad libitum; group with pre-diabetes induced by streptozotocin and fed with a high-fat, high-carbohydrate diet and water ad libitum (PD); group with prediabetes induced by streptozotocin and fed with a high-carbohydrate, high-fat diet, water ad libitum and native starch (PD + N), and nanostructured starch (PD + NN).
Figura 2. Grupo control (C) administrado con dieta estándar y agua ad libitum, grupo con prediabetes inducida con estreptozotocina y alimentado con una dieta alta en carbohidratos y grasa y agua ad libitum (PD), grupo con prediabetes inducida con estreptozotocina alimentado con una dieta alta en carbohidratos y grasa, agua ad libitum y almidón nativo (PD+N) y almidón nanoestructurado (PD+ NN).
Administration of both types of starch was carried out with an orogastric tube. The animals were put down after 4 weeks of experimental diets and their plasma, adipose tissue and organs were collected, weighed and frozen in liquid nitrogen for further analysis.
Biochemical parameters in the plasma
Glucose levels in the blood were measured with the enzymatic colorimetric method glucose – LQ GOD-POD; the concentration of insulin was estimated by the kit Rat Insulin Elisa No. 80-INSERT-E0 while total and HDL cholesterol were analyzed with the enzymatic colorimetric method cholesterol – LQ CHOD-POD; and triglycerides – LQ GOP-POD was used for triglycerides.
Homeostasis method: IR (HOMA-RI) was calculated using the concentrations of glucose and serum insulin levels (Matthews et al., Citation1985). On the other hand, IR was calculated using the concentration of glucose and serum insulin.
Statistical analysis
A Lilliefors test of normality based on the Kolmogorov–Smirnov test was carried out on each variable in order to verify if data fit a normally distributed population. Effects of both starches were tested using one-way ANOVA followed by Tukey post-hoc tests for multiple mean comparison test. Results are expressed as means ± standard error and the level of significance was set at p < 0.05. Data were analyzed using MINITAB 17 statistical software.
Results and discussion
Microscopy analysis
In order to verify that starch was properly nanostructured (, the surface of both starches was analyzed with an atomic force microscope. ) shows that native starch has very few pores as compared to the nanostructured sample. Furthermore, the surface of nanostructured starch ( is made of small homogeneous peaks of nanometric size. AFM images show that native starch has a heterogenous distribution with cavities of ≥100 nm ( while nanostructured starch has a homogenous distribution with nanocavities of 2 nm.
Figure 3. Microscopy atomic analysis of nanostructured (a, b) and native (c, d) of blue corn starch.
Figura 3. Análisis de microscropía atómica del almidón nanoestructurado (a.b) y nativo (c,d) de maíz azul.
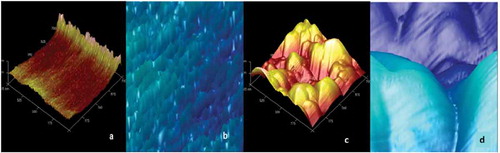
According to other authors (Azuara & Beristain, Citation2006; Pascual-Pineda et al., Citation2014), in order to improve the physical, chemical and microbiological stability of a food, it is necessary to increase the number of micropores (nanocavities with a diameter of ≤5 nm) of the solid matrix, as observed in the nanostructured starch (. The nanostructured sample showed a more homogeneous surface with cavities smaller than 2 nm. Furthermore, it has been reported that the amount of water molecules adsorbed in these nanocavities has a maximum degree of ordering (minimum entropy), which increases the stability of a food since water is less available for spoilage reactions (Pascual-Pineda et al., Citation2014).
Relaxation length time
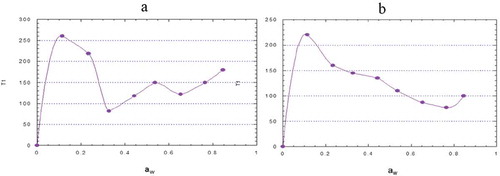
(b) shows that adsorbed water molecules in nanostructured blue corn starch have a lower molecular mobility as compared to those adsorbed by native starch in the estimated range of water activity. This is due to the nanocavities <5 nm in nanostructured starch, where molecules are bonded with higher surface energy as compared to native starch. This lower mobility contributes to a higher stability of the compounds in a food, such as bioactive starch compounds.
Figure 4. Relaxation length time (T1) of water in native (a) and nanostructured (b) starch of blue corn using the solids NMR technique.
Figura 4. Tiempo de relajación longitudinal (T1) del agua en el almidón nativo (a) y nanoestructurado (b) de grano de maíz azul utilizando la técnica de RMN de sólidos.
Chemical analysis
Values for total polyphenols and antioxidant activity were higher in nanostructured starch (). The higher content of polyphenols and anthocyanins is attributed to the higher order degree of water molecules adsorbed in the nanocavities (minimal entropy), which increases stability of starch components since water is less available for reactions (Pascual-Pineda et al., Citation2014). This effect has the potential to improve the bio-functional properties of this widely used ingredient.
Table 1. Polyphenol content, antioxidant activity and digestibility of native and nanostructured blue starch.
Tabla 1. Contenido de polifenoles, actividad antioxidante y digestibilidad del almidón azul nativo y nanoestructurado.
The percentage of total starch and resistant starch was similar for both samples; however, resistant starch has lower values than those reported by the literature. Previous studies indicate that drying methods employed in production starch affect its digestibility (Ozturk, Koksel, & Ng, Citation2009). In the present study, the analysis of rapidly and slowly digestible fractions showed significant differences between native and nanostructured starch; since RDS was of 25.6% for native and 23% for nanostructured, while SDS was of 54.7% for native and 60.2% for nanostructured (). It could be attributed to a loss of structure of the starch during the extraction process, caused by the exposure to low temperatures during long time.
From these results, it can be noted that the content of SDS was higher in the nanostructured sample, which is considered beneficial in the dietetic treatment of common chronic diseases such as obesity, diabetes and cardiovascular disease (Ludwig, Citation2002; Wolever & Mehling, Citation2002).
It is also noteworthy that besides digestibility, functional properties of starches are also affected by the method employed in their production, such as lyophilization (Tao et al., Citation2015). According to studies made by our group, the method selected for starch nanostructuration of blue corn starch improved some of its properties, such as water absorption index, water solubility index, and swelling power as compared to native starch (data not shown). This may also impact the biofunctionality of nanostructured starch.
Experimental model
The prediabetic model (PD group) was achieved by the administration of high-fat, high-carbohydrate diet and streptozotocin in male Wistar rats for 6 weeks. shows serum concentrations of impaired fasting glucose (IFG), insulin, triglycerides, total cholesterol, HDL, VLDL and in control and prediabetic rats. Significant differences were observed in glucose, insulin, cholesterol and triglycerides when comparing control versus PD groups. In the present work, the PD group showed values of impaired fasting glucose of 6.21 mmol/L. In addition, PD group also showed higher values of insulin than the control group. Prediabetes is associated to the following conditions: IFG, IGT, IR and/or partial pancreatic β-cell (Kanat, DeFronzo, & Abdul-Ghani, Citation2015). So, it can be said that the administration of high-fat high-sucrose diet and streptozotocin generated a prediabetic model. This prediabetic model also was characterized by high levels of plasma triglycerides, total cholesterol and insulin, as well as a significantly increased epididymal and abdominal fat and liver weight (). No significant differences were found in body weight among control and PD groups, both in the initial and final body weight (). In addition, no significant differences were observed in diary food intake between control and PD groups ().
Table 2. Serum parameters in control and experimental groups.
Tabla 2. Parámetros séricos en el grupo control y grupos experimentales.
Table 3. Adipose tissue and liver weight in control and experimental groups.
Tabla 3. Peso de tejido adiposo e hïgado del grupo control y grupos experimentales.
Table 4. Food intake and body weight in rat control and experimental groups.
Tabla 4. Ingesta de alimento y peso de la rata del grupo control y grupos experimentales.
Experimental diets
When compared to the PD group, serum glucose concentration was lower (20%) when nanostructured starch was administered (PD + NN group) (). Serum insulin concentrations and HOMA-IR index also decreased in the PD + NN group (48% and 53%, respectively) as compared to PD group. In addition, no significant differences were found in serum glucose, insulin and HOMA-IR index parameters when comparing to the control group. It is known that intake of high-SDS foods helps control appetite and therefore decreases food consumption, which results in a lower amount of glucose entering the bloodstream. In the same way, this stimulates the pancreas more slowly, producing a lower insulin secretion (Péronnet et al., Citation2015).
Polyphenol content is another factor that may contribute to a lower glucose, insulin and glycemic index in the prediabetes group supplemented with nanostructured starch. Recently, it has been reported that purple corn anthocyanins improve IR in adipocytes by activating insulin signaling and increasing GLU4 translocation (Mazewski, Liang, & Gonzalez, Citation2017). In the present study, anthocyanins from nanostructured starch may also help to the lower levels of glucose and insulin in the plasma (Guzmán-Gerónimo et al., Citation2017).
No significant differences were observed in triglyceride plasma concentrations among PD and experimental groups (PD + N and PD + NN groups). However, triglyceride levels were higher in the PD + N group (20%) as compared to the PD group (). The elevation of serum levels of triglycerides in PD + N group could be attributed to a higher content of RDS of native blue corn, as well to a lower concentration of polyphenols as compared to the nanostructured blue starch.
shows no significant differences in abdominal fat weight between PD, PD + N and PD + NN groups. However, significant differences were found in pericardial fat weight among control, native and nanostructured starch groups (p < 0.05) (). In other words, PD + N and PD + NN groups showed a significant decrease of pericardial fat weight as compared to the PD group (42% and 23%, respectively; p < 0.05). Epididymal fat weight was lower in the PD + NN group (17%), as compared to the prediabetic group (PD). This agrees with a previous study on the effect of blue corn extract on rats with metabolic syndrome, where a decrease in epididymal fat was also observed and was attributed to the effect of polyphenols and anthocyanins (Guzmán-Gerónimo et al., Citation2017). Furthermore, it has been reported that intake of foods with a higher fraction of SDS reduces the risk of accumulating fat due to a reduction in the amount of food ingested and a lower postprandial insulinemia (Lee & Lee, Citation2016; Seal et al., Citation2003).
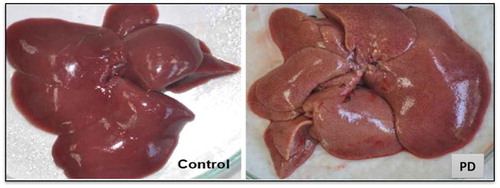
Regarding liver weight (), differences were observed when comparing control versus PD groups. A typical fatty liver condition was observed in prediabetic groups and as expected, this condition was not found in the control group (). Even though a slight decrease (8%) between the weight of the liver of PD and PD + NN groups was observed, it was not statistically different.
Figure 5. PD group showing the typical fatty liver condition.
Figura 5. Grupo PD mostrando la típica condición de hígado graso.
Differences were observed in diary food intake between groups, specifically those rats consuming nano-structured starch showed a decreasing in the intake (28.3 %) as compared to the prediabetic group (p < 0.05) (). It is well known that SDS is strongly linked with glucose metabolism, giving a lengthy hydrolysis and absorption, producing a sensation of satiety (Han & BeMiller, Citation2007). In the present work, the lower food intake of the experimental PD + NN group is due to the higher SDS amount – given by nanostructured starch – in their diet. No significant differences were found in body weight among all groups, both in the initial and final body weight ().
Conclusion
This research showed that supplementation of nanostructured blue corn has a beneficial effect by decreasing levels of glucose and insulin in plasma of prediabetic rats. Nanostructured blue corn starch has a good potential as a functional ingredient in the food industry. According to our knowledge, this is the first report on the bio-functionality of nanostructured starch extracted from blue corn in alterations related to prediabetes.
Acknowledgments
Authors thank (SINAREFI): [Grant Number BEI-MAI-10-32] for the financial support provided for completion of this research project.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AACC. (2000). Approved methods of the American association of cereal chemists (pp. 1). St. Paul, MN: The Association.
- Acosta-Domínguez, L., Hernández-Sánchez, H., Gutiérrez-López, G. F., Alamilla-Beltrán, L., & Azuara, E. (2016). Modification of the soy protein isolate surface at nanometric scale and its effect on physicochemical properties. Journal of Food Engineering, 168, 105–112. doi:10.1016/j.jfoodeng.2015.07.031
- Agama-Acevedo, E., Juárez-García, E., Evangelista-Lozano, S., Rosales-Reynoso, O. L., & Bello-Pérez, L. A. (2013). Características del almidón de maíz y relación con las enzimas de su biosíntesis. Agrociencia, 47, 1–12.
- American Diabetes Association. (2014). Diagnosis and classification of diabetes, mellitus. (2014). Diabetes Care, 37(Suppl. Supplement_1), S81–S90. doi:10.2337/dc14-S081
- Azuara, E., & Beristain, C. I. (2006). Enthalpic and entropic mechanisms related to water sorption of yogurt. Drying Technology, 24, 1501–1507. doi:10.1080/07373930600961173
- Bello, P., Contreras, S., Romero, R., Solorza, J., & Jiménez, A. (2002). Propiedades químicas y funcionales del almidón modificado de plátano (Musa paradisiaca L. var. Macho). Agrociencia, 36, 169–180.
- Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology, 28, 25–30. doi:10.1016/S0023-6438(95)80008-5
- Englyst, H. N., Kingman, S. M., & Cummings, J. H. (1992). Classification and measurement of nutritional important starch fractions. European Journal of Clinical Nutrition, 46, 33–50.
- Guzmán-Gerónimo, R. I., Alarcón-Zavaleta, T. M., Oliart-Ros, R. M., Meza-Alvarado, J. E., Herrera-Meza, S., & Chávez-Servia, J. L. (2017). Blue maize extract improves blood pressure, lipid profiles, and adipose tissue in high-sucrose diet-induced metabolic syndrome in rats. Journal of Medicinal Food, 20, 110–115. doi:10.1089/jmf.2016.0087
- Han, J.-A., & BeMiller, J. N. (2007). Preparation and physical characteristics of slowly digesting modified food starches. Carbohydrate Polymers, 67, 366–374. doi:10.1016/j.carbpol.2006.06.011
- Jenkins, D., Kendall, C. W., Augustin, L., Franceschi, S., Hamidi, M., Marchie, A., & Jenkins, A. (2002). Glycemic index: Overview of implications in health and disease. The American Journal of Clinical Nutrition, 76, 266S–273. doi:10.1093/ajcn/76.1.266S
- Kanat, M., DeFronzo, R. A., & Abdul-Ghani, M. A. (2015). Treatment of prediabetes. World Journal of Diabetes, 6, 1207–1222. doi:10.4239/wjd.v6.i12.1207
- Labuza, T. P., Kaanane, A., & Chen, J. Y. (1985). Effect of temperature on the moisture sorption isotherms and water activity shift of dehydrated food. Journal of Food Science, 50, 358–391.
- Lee, K. Y., & Lee, H. G. (2016). Comparative effects of slowly digestible and resistant starch from rice in high-fat diet-induced obese mice. Food Science and Biotechnology, 25, 1443–1448. doi:10.1007/s10068-016-0224-2
- Ludwig, D. S. (2002). The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. Jama, 287, 2414–2423. doi:10.1001/jama.287.18.2414
- Matthews, D. R., Hosker, J. P., Rudenski, A. S., Naylor, B. A., Treacher, D. F., & Turner, R. C. (1985). Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 28, 412–419. doi:10.1007/BF00280883
- Mazewski, C., Liang, K., & Gonzalez, D. M. E. (2017). Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. Journal of Functional Foods, 34, 254–265. doi:10.1016/j.jff.2017.04.038
- Medina, C., Paredes, A., Rodríguez, M. E., Camacho, D., García, D., & Ojeda, C. (2010). Evaluación de dos métodos de extracción de almidón a partir de cotiledones de mango”. Bioagro, 22, 67–74.
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. (2011). Guide for the care and use of laboratory animals. Washington (DC): National Academies Press.
- Ozturk, S., Koksel, H., & Ng, P. K. W. (2009). Characterization of resistant starch samples prepared from two high-amylose maize starches through debranching and heat treatments. Cereal Chemistry Journal, 86, 503–510. doi:10.1094/CCHEM-86-5-0503
- Pascual-Pineda, L. A., Flores-Andrade, E., Alamilla-Beltrán, L., Chanona, P. J. J., Beristain, C. I., Gutiérrez-López, G. F., & Azuara, E. (2014). Micropores and their relationship with carotenoids stability: A new tool to study preservation of solid foods. Food and Bioprocess Technology, 7, 1160–1170. doi:10.1007/s11947-013-1162-0
- Péronnet, F., Meynier, A., Sauvinet, A., Normand, S., Bourdon, E., Mignault, D., … Vinoy, S. (2015). Plasma glucose kinetics and response of insulin and GIP following a cereal breakfast in female subjects: Effect of starch digestibility. European Journal of Clinical Nutrition, 69, 740–745. doi:10.1038/ejcn.2015.50
- Salinas-Moreno, Y. J. J., Vázquez-Carrillo, P.-A. G., Aragón- Cuevas, F., & Velázquez-Cardelas, G. A. (2012). Antocianinas y actividad antioxidante en maíces (Zea mays L.) de las razas Chalqueño, Elotes Cónicos y Bolita. Agrociencia, 47, 815–825.
- Seal, C. J., Daly, M. E., Thomas, L. C., Bal, W., Birkett, A. M., Jeffcoat, R., & Mathers, J. C. (2003). Postprandial carbohydrate metabolism in healthy subjects and those with type 2 diabetes fed starches with slow and rapid hydrolysis rates determined in vitro. British Journal of Nutrition, 90, 853–864. doi:10.1079/BJN2003972
- Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
- Ştefănuţ, M., Căta, A., Pop, R., Tănasie, C., Boc, D., Ienaşcu, I., & Ordodi, V. (2013). Anti-hyperglycemic effect of bilberry, blackberry and mulberry ultrasonic extracts on diabetic rats. Plant Foods for Human Nutrition, 68, 378–384. doi:10.1007/s11130-013-0380-y
- Tao, H., Yan, J., Zhao, J., Tian, Y., Jin, Z., & Xu, X. (2015). Effect of multiple freezing/thawing cycles on the structural and functional properties of waxy rice starch. Plos One, 10, 1–11. doi:10.1371/journal.pone.0127138
- Tsuda, T., Horio, F., Uchida, K., Aoki, H., & Osawa, T. (2003). Dietary Cyanidin 3-O-β-D-Glucoside-Rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. The Journal of Nutrition, 133, 2125–2130. doi:10.1093/jn/133.7.2125
- Wolever, T. M., & Mehling, C. (2002). High-carbohydrate-low-glycaemic index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. British Journal of Nutrition, 87, 477–487. doi:10.1079/BJN2002568
