 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The aim of the study was to determine the influence of sage extracts (0.02% and 0.05%) on the quality of turkey meatballs packed in a modified atmosphere (80% O2; 20% CO2) and stored at 4°C over a period of 9 days. Sage extract added to meatballs had a high antioxidant capacity due to a phenolic compounds: 47.92 mg of gallic acid (GAE)/100 g dw including high flavonoids contents (20.47 mg GAE/100 g dw) and phenolic acids (8.14 GAE/100 g dw) contents. During the storage, lipid oxidation and microbial growth increased rapidly and the results showed that the influence of sage extract on meatballs was the most noticeable on the last day of storage. Furthermore, analysis of the volatile compounds indicated that the addition of sage extract delayed formation of lipid-derived products of oxidation through storage of the turkey meatballs. Sage extract has a potential as a natural preservative in the meat industry.
RESUMEN
El presente estudio se propuso determinar cómo influye varios extractos de salvia (0.02% y 0.05%) en la calidad de albóndigas de pavo empacadas en una atmósfera modificada (80% O2; 20% CO2) y almacenadas a 4°C durante un periodo de nueve días. El extracto de salvia agregado a las albóndigas produjo una elevada capacidad antioxidante debido a los compuestos fenólicos: 47.92 mg de ácido gálico (GAE)/100 g ps (peso seco), incluyendo elevados contenidos de flavonoides (20.47 mg GAE/100 g ps) y ácidos fenólicos (8.14 GAE/100 g ps). Durante el almacenamiento, la oxidación de lípidos y el crecimiento microbiano aumentaron rápidamente; los resultados muestran que la influencia más notable del extracto de salvia en las albóndigas se registró durante el último día de almacenamiento. Asimismo, el análisis de los compuestos volátiles indicó que la adición de extracto de salvia postergó la formación de derivados de lípidos producidos por oxidación durante el almacenamiento de las albóndigas de pavo. Por ello se concluye que el extracto de salvia tiene posibilidades de servir como conservador natural en la industria cárnica.
PALABRAS CLAVE:
1. Introduction
One of the methods to reduce lipid oxidation, which influence the quality and acceptance of meat products, is the application of antioxidants (Rababah, Hettiarachchy, & Horax, Citation2004). Antioxidants are the chemical substances that block or delay oxidation process, by reducing the free radicals. Lipid oxidation leads to discoloration, texture deterioration, off-flavours and loss of nutrients and formation of toxic compounds (Jia, Kong, Liu, Xinping, & Xiufang, Citation2012; Min & Ahn, Citation2005). In addition, while lipids are hydrolyzed, free fatty acids are released, which can undergo further secondary changes of an oxidative character. These secondary products of oxidation of polyunsaturated fatty acids, especially volatile aldehydes and ketones, affect taste and smell of meat and meat products in a degradation way (Min & Ahn, Citation2005), this is what determines consumers’ acceptance of meat products.
Many researchers have indicated that lipid oxidation in meat and meat products can be controlled by the addition of commercial, synthetic or natural antioxidants (Chouliara, Karatapanis, Sawaidis, & Kontominas, Citation2007; Jia et al., Citation2012; Shah, Don Bosco, & Mir, Citation2014). However, compared with synthetic antioxidants, natural compounds are of great interest because of their safety and health characteristics (Fasseas, Mountzouris, Tarantilis, Polissiou, & Zervas, Citation2008). Good source of natural antioxidants are extracts from plants materials, such as vegetables, fruits, herbs and spices (Shah et al., Citation2014; Wolfe, Wu, & Liu, Citation2003). The antioxidant action of the spices is related to their phenolic compounds, that is why their action is similar to synthetic antioxidants. Herbs of the Labiaceae family, mainly rosemary (Rosmarinus officinalis L.), sage (Salvia officinalis L.) and oregano (Origanum vulgare L.), have been reported as having a significant antioxidant capacity (Fasseas et al., Citation2008; Velasco & Williams, Citation2011; Wojdyło, Oszmioński, & Czemerys, Citation2007). Several authors have demonstrated that rosemary and sage are an important contributor of the oxidative quality of meat products. The addition of rosemary extract to meatballs and chicken sausages delayed the oxidation of the lipid fraction and the appearance of a rancid taste in the products (Karpińska, Borowski, & Danowska-Oziewicz, Citation2000; Lee, Williams, Sloan, & Littell, Citation1997). Sage was found to protect minced chicken breast against lipid oxidation induced by high hydrostatic pressure during the subsequent chilled storage for 2 weeks (Mariutti, Orlien, Bragagnolo, & Skibsted, Citation2008).
Natural antioxidants are mainly used in the cold storage or freezing and due to recent contamination outbreaks associated with meat products. The effective method of a variety of foods, including fresh meat and poultry preservation is also modified atmosphere packaging (MAP) (Chouliara et al., Citation2007; Min & Ahn, Citation2005; Rojas & Brewer, Citation2007; Velasco & Williams, Citation2011). The combination of both modified atmosphere packaging and oregano essential oil extends the shelf life of fresh chicken breast meat to 5–6 days (Chouliara et al., Citation2007). Fresh meat is usually provided in a packaging with an increased O2/CO2 contents by utilizing modified atmosphere packaging (MAP). The usual gas mixtures used for retail sliced poultry meat under MAP are 20% CO2 and 80% O2 (Rossaint, Klausmann, & Kreyenschmidt, Citation2015). This atmosphere increases the shelf life of poultry to approximately 8 days depending on the microbiological quality, processing and condition of storage. The MAP with high O2 and with a cold storage may be insufficient to preserve minced poultry meat. Turkey meatis especially considered to be more prone to the development of oxidation as compared to the red meat (Rhee, Anderson, & Sams, Citation1996). According to Komprda et al. (Citation2002) and Mercier, Gatellier, Viau, Remignon, and Renerre (Citation1998), in spite of having moderately low fat content, turkey meat has a high content of PUFA and a high concentration of free iron.
Under pro-oxidative conditions (grounding and mixing of meat, MAP with a high content of oxygen as well as a high PUFA content in turkey meat), the application of antioxidants in order to reduce lipid oxidation and deterioration of meat quality and even food wastage due to a short shelf life of the meat products is indicated. Considering the above, the objective of the present study is to determine the effect of addition of different levels of sage extracts (Salvia officinalis L.) on the quality of turkey meatballs packed in a modified atmosphere (80% O2; 20% CO2) and stored at 4°C, over a 9 day period.
2. Materials and methods
2.1. Materials and meat sample preparation
Fresh turkey breast (with typical pH values) was obtained from a farm situated in the north-east part of Poland from the company Indykpol S.A. The meat was transported to the laboratory in ice boxes, under chilled conditions (4°C ± 1°C) on the 3rd day after slaughter.
Powder extract of sage leaves (Salvia officinalis L.) was commercially purchased (Firmenish; Geneva, Switzerland). List of ingredients provided by the supplier: maize maltodextrin, E 1450 modified corn starch (7.9%), flavouring components, medium-chain triglycerides (from coconut fatty acids), canola oil, E 471 mono- and diglycerides of fatty acids (0.4%) and nutritional information (calculated on dry weigh basis; g/100 g product): energy (kcal/100 g) – 437, energy (kJ/100 g) – 1828, protein – 0.28, carbohydrates (excluding fibre) – 82.76, sugars (mono-and disaccharides – 5.22, fibre – 0.03, fat – 11.45, saturated 7.24, sodium 0.09.
The meat was minced (mincer – model PI-22-TU-T EDESA, Czosnow Poland) using an 8 mm plate and was divided randomly into three portions. Commercial sodium chloride was added to all portions (2.5% based on the finished product weight). The samples were subjected to the following treatments: group S1 – 0.02% of sage extract, group S2 – 0.05% of sage extract and group C – a control group. The samples (1 kg of meat per group) were prepared in accordance with the following formula: 79.5% (w/w) meat, 0.5% (w/w) NaCl (w/w) and 20.0% (w/w) of cooled water plant extracts. The control group contained 20.0% (w/w) of distilled water. Subsequently, meatballs (ready-to-use meat product) consisting of 20 ± 1 g of meat were formed. All meatballs in pairs were packed in a modified atmosphere (80% O2 and 20% CO2). The MAP was performed using a SEALPAC M3 semiautomatic gas traysealer (Sealpac International, Harderwijk, Nederland) in transparent trays with dimensions of 18.5 × 13.7 × 5 cm, OTR (oxygen transmition rate) of 10 cm3/m2 per day at 23 °C at 0% relative humidity and WVTR (water vapour transmition rate) of 15 g/m3 per day at 38 °C at 90% relative humidity. The sealing barrier film parameters were as follows: OTR of 3 cm3/m2 per day at 23 °C at 0% relative humidity and WVTR of 8 g/m3 per day at 38 °C at 90% relative humidity (Despol, Poznan, Poland). All samples were kept in the refrigerator (4°C ± 1°C) and analysed on the 1st, 3rd, 6th, and 9th day of storage.
2.2. Evaluation of natural extract
The total phenolic acids estimation was carried out according to the Arnov method (Polish Pharmacopoeia VI, Citation2002). The absorbance was measured by UV-Visible Spectrophotometer Hitachi U-2900 (Japan) at 490 nm. The total phenolic acid content was expressed as caffeic acid equivalent (CAE). Measurement of total flavonoid content in the investigated extracts was determined spectrophotometrically according to Polish Pharmacopoeia VII (Citation2006), using a method based on the formation of complex flavonoid-aluminium with the maximum absorptivity at 430 nm. The flavonoids content was expressed as a log of quercetin equivalents (QE) per g of dried extract (de), by using a standard graph. All measurements were carried out in five repetitions. Free radical scavenging effects of the extracts on DPPH (2,2-diphenyl-1-picrylhidrazyl) were estimated according to the method of Chen and Ho (Citation1997). Absorbance of the resulting solutions was measured spectrophotometrically by UV-Visible Spectrophotometer Hitachi U-2900 (Japan) at 517 nm.
The amount of tannin estimation was determined using Pharmacopoeia procedure (Polish Pharmacopoeia X, Citation2012). The content of tannins was expressed as dry weight percentage. All measurements were carried out in five repetitions.
2.3. Lipid oxidation analysis by TBARS (thiobarbituric acid reactive substances)
Secondary lipid oxidation products from raw meat were determined according to the method described by Brodowska et al. (Citation2016) and Gantner et al. (Citation2016). The minced meat (2.5 g) was mixed and homogenized with 25 ml of trichloroacetic acid solution (20%) and 1.25 ml of antioxidant (0.5% propyl gallate in ethyl alcohol/water, 1:1) for about 30 s at 1200 rpm (WT 500 homogenizer, Wiggenhauser, Germany). After centrifuged by 10 min at 8000 rpm (laboratory centrifuge MPW-251, MPW Med. Instruments, Poland) 5 ml 2-thiobarbitiric acid (0.02 mmol/l) was added to 5 ml of supernatant. Then, samples were heated in a water bath (90°C) for 40 min and next cooled in ice bath. The absorbance was measured at 532 nm, against a blank, using a UV-VIS spectrophotometer (Shimazu UV-1800, Japan). The recovery of samples and calibration curve was evaluated with the using of standard (1,1,3,3-tetramethoxypropane). The results were expressed as mg of MDA/kg meat.
2.4. Colour measurement
The measurement of the colour of meatballs was performed in the CIE L*a*b* system (L* – lightness, a* – colour axis ranging from greenness (−a*) to redness (+a*), b* – colour axis ranging from blueness (−b*) to yellowness (+b*). A Minolta chromameter (CR-400, Konica Minolta Inc., Tokyo, Japan) was used. The measuring head with a diameter of 8 mm, a D65 illuminant and a standard 2° observer was applied. The chromameter was calibrated using a white standard plate (L* = 98.45, a* = −0.10, b* = −0.13). The evaluation of colour parameters was carried out by measuring 10 different places on each sample. ∆E was calculated with the following equation:
∆E indicated the degree of overall colour changes in comparison to the colour values of the control group. Colour parameters were measured on the 1st, 3rd, 6th, and 9th day of the storage.
2.5. Volatile profile analysis
Volatile organic compounds (VOC) profile was determined by an electronic nose analyzer based on an ultra-fast gas chromatography (UFGC) Heracles II (Alpha M.O.S., France). Tests were carried out with 2.5 g samples. Analysed samples, closed in 20 ml glass vial, were incubated at temperature of 55°C in HeadSpace thermostat for 15 min. Samples of 3500 µl of air from above each meat sample were automatically transferred to injection chamber of gas chromatograph (GC). GC was equipped with two steel capillary columns of different polarities: DB-5 and DB-1701. Analysis was performed according to the method described by Gantner et al. (Citation2016). The VOC profiles of samples were analyzed in three repetitions on the 1st, 3rd, 6th and 9th day of the storage. Identification of volatile compounds was carried automatically based on Kovats indexes. A standard mixture of alkanes from C6 to C16 was used for calibration.
2.6. Microbiological analysis
The total number of aerobic bacteria was calculated by Sanitary-Epidemiological Station in Warsaw according to PN-EN ISO 4833-1 recommendations ISO-4833-2). L-agar plates with 0.1% glucose were used in the experiments. Microbiological analyses were performed on the fresh meat and on the meat stored for 3, 6 and 9 days. Each sample was examined in three repetitions. The results were presented as log10 CFU/g meat.
2.7. Sensory analysis
The sensory evaluation was carried out to assess the sensory properties of control and treated turkey patties. Chosen descriptors of sensory quality were: colour, odour specific for turkey, herbal odour, tenderness, juiciness, salty taste, taste specific for turkey and herbal taste. The analysis was performed in accordance with the ISO 6658 (Citation1998) recommendations. Panel was composed of 10 members who were recruited from the staff of Department of Technique and Food Development at Warsaw University of Life Sciences, using an unstructured continuous 10 cm-long scale for each sensory trait. Turkey patties used for sensory evaluation were previously roasted for 20 min in a convection steam oven (model CPE 110, Küppersbuch, Großkuchentechnik, Galsenkirchen, Germany) at a temperature of 180°C. Roasted samples were cooled to room temperature and immediately cut into small pieces of about 2.5 cm of thickness. Mixture of apple juice and water (1:5) was provided to clear the palate from flavours between the samples. The whole procedure was performed in a laboratory environment in order to maintain accurate, repeatable and reliable results.
2.8. Statistic data analysis
The normality of data distribution was tested using the Shapiro-Wilk test. The analysis was performed by Sanitary – Epidemiological Station in Warsaw according to PN-EN ISO 4833-1 (Citation2013) recommendations. The significance level was determined as α = 0.05. All analyses were performed using Statistica Software version 8.0 (StatSoft, Tulsa, Oklahoma, USA).
The chromatogram profiles data sets such as a data matrix based on time and relative area of peaks were analysed using the statistical software AlfaSoft delivered with Heracles II. Automatic data reduction was used to select the most significant variables for the application. Statistical package allowed to trace VOC profile changes using principal component analysis (PCA) and the Euclidean distances.
3. Results and discussion
3.1. Antioxidant potential of salvia officinalis extract
The presence of secondary metabolites contents in dry extracts from sage was recorded. The content of phenolic compounds in total stood at 47.92 mg of gallic acid (GAE)/100 g dry weight (dw) extract, phenolic acids 8.14 mg GAE/100 g dw extract, flavonoids 20.47 mg GAE/100 dw extract, and the amount of tannins was equal to 4.43%. The potent radical scavenging activity of sage extract (1 mg/1) was 11.03%. So, it is possible to conclude that the antioxidant activity of dry extracts of sage is in fact modified by the number of phenolic and flavonoid compounds. Our results are almost similar to those reported by Roby, Khaled, Khalel, and Khalela (Citation2013) that total phenols extracted from sage ranged from 4.65 to 5.95 (mg GAE/100 g dw) depending on the type of solvents (methanol, ethanol, diethyl ether, and hexane) used in extracting. According to Wojdyło et al. (Citation2007), the amount of total phenolics measured by Folin-Ciocalteu method varied widely in herb materials and ranged from 0.00 to 15.2 mg GAE/100 g dry weight (dw) and herb of Salvia officinalis contained the highest level of phenolics as compared to other herbs of the Labiatae family, such as Origanum vulgare or Rosmarinus vulgare. Pizzale, Bortolomeazzi, Vichi, Überegger, and Conte (Citation2002) described that the total phenolic compound content in sage extracts from leaves ranged from a minimum of 46.4 g kg−1 for a sample of S. officinalis to a maximum of 113.4 g kg−1 for a sample of S. fructicosa with no significant difference between the two species.
Antioxidant properties of sage were found to be related with presence of rosmarinic acid and carnosic acid, salvianolic acid and its deriverates (Karakaya, Bayrak, & Ulusoy, Citation2011). It was also confirmed by other authors (Berdahl & McKeague, Citation2015). Sage is particularly prolific in the production of flavonoids and other phenolic derivatives, including those structurally related to rosmarinic acid. Wang et al. (Citation1998) identified in sage 15 diterpenoids, 7 flavonoids, 17 triterpenes, steroids and related compounds. According to Shan, Cai, and Corke (Citation2005), a total phenolic content of sage, oregano, mint, rosemary and sweet basil from Labiatae family was higher in comparison with other tested spices, such as coriander, cumin, parsley, dill, caraway extracts from Apiacae family. The same situation concerned with a total antioxidant capacity (TEAC). The spice extract samples that had a high antioxidant capacity showed a tendency to have a high phenolic content. It was due to the fact that the spice extracts in the Labiatae family were found to have rosmaric acid with a very powerful antioxidant activity.
3.2. Lipid oxidation
The effect of adding spice sage extracts on the lipid oxidation in the raw turkey patties during 9 days of the storage was determined by TBARS values (). TBARS values of all samples increased during the long term of the storage (p < 0.05). The exponential rise in TBARS was observed between the 3rd and the 6th day of the storage. Significant effect of the addition of sage (S1 and S2 group) to turkey meat on the levels of TBARS was stated only on the 6th day (p < 0.05). On the 8th day inhibition of lipid oxidation products was not significant The antioxidant activity of sage plants extract is related to the levels of antioxidant bioactive compounds. The antioxidant effects of sage extract is mainly due to presence of phenolic compounds. The main phenolic compounds of sage are carnosol, carnosic acid, rosmarinic acid, rosmadial, rosmanol, epirosmanol and methyl carnosate (Mariutti, Nogueira, & Bragagnolo, Citation2011; Pizzale et al., Citation2002). Especially, carnosol and carnosic acid are peroxyl and hydroxyl radical-scavenging. According to Lu and Yeap (Citation2001) rosmarinic acid derivatives are major polyphenols in sage and the superoxide scavenging activities of the rosmarinic acid derivatives were 15-20 times stronger than trolox (water-soluble analog of vitamin E). Salvia officinalis may limit lipid oxidation in food.
Figure 1. The TBARS values in samples with addition of Salvia officinalis during storage at 4 ± 1°C.
C: control samples with addition of 0.5% NaCl.
S1: samples with addition of 0.5% NaCl and 0.02% of sage extract.
S2: samples with addition of 0.5% NaCl and 0.05% of sage extract.
Figura 1. Valores TBARS en muestras adicionadas con Salvia officinalis durante el almacenamientos a temperaturas de 4 ± 1°C.
C: muestras adicionadas con 0.5% NaCl.
S1: muestras adicionadas con 0.5% NaCl y 0.02% de extracto de salvia.
S2: muestras adicionadas con 0.5% NaCl y 0.05% de extracto de salvia.
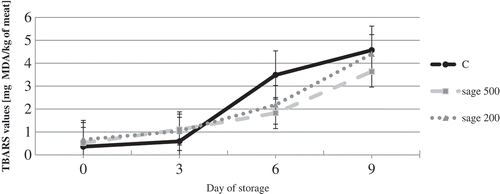
Results are in accordance with the study of Fasseas et al. (Citation2008), who demonstrated for minced bovine an antioxidant effect (measured by TBARS method) of sage essential oils. The results were significant at the last days of storage (on the day 8th and 12th). McCarthy, Kerry, Kerry, Lynch, and Buckely (Citation2001) showed that the addition of sage powder 0.05% to pork meat decreased lipid oxidation of patties manufactured from frozen pork for 9 days, as effectively as the rosemary and synthetic BHA. Strong antioxidant properties of sage confirm effective in minimizing the lipid oxidation in presence of salt as prooxidant (0.5%). With the addition 0.1% of dry sage similar results for the chicken breast were found (Mariutti et al., Citation2008).
In case of turkey, the addition of antioxidant substances is particularly important due to the inefficient accumulation of dietary vitamin E in tissue (Mielnik, Olsen, Vogt, Adeline, & Skrede, Citation2006). Due to the low content of dietary vitamin E in meat, an addition of antioxidants is required during the processing of turkey meat.
3.3. Principal component analysis
The analysis of the PCA received all 35 components. The first two components accounted for 55% of variation. The visualization was presented in as PC1 plotted versus PC2. The relative position of the triangles shows how similar the tested samples are. The PC1 allows to differentiate samples by additive content of sage – an increase in the PC1 value means that content of sage decreases. The PC2 allows to differentiate samples by storage time – a decrease in the PC2 value means that storage time increases.
Figure 2. PCA analysis (score plot for main variation) of volatile chromatographic profiles of turkey samples C, S1, S2 stored 1, 3, 6 and 9 days.
C : control samples with addition of 0.5% NaCl.
S1: samples with addition of 0.5% NaCl and 0.02% sage extract.
S2: samples with addition of 0.5% NaCl and 0.05% of sage extract.
Figura 2. Análisis PCA (diagrama de datos para la variación principal) de perfiles cromatográficos volátiles de muestras de pavo C, S1 y S2 almacenadas durante 1, 3, 6 y 9 días.
C : muestras adicionadas con 0.5% NaCl.
S1: muestras adicionadas con 0.5% NaCl y 0.02% de extracto de salvia.
S2: muestras adicionadas con 0.5% NaCl y 0.05% de extracto de salvia.
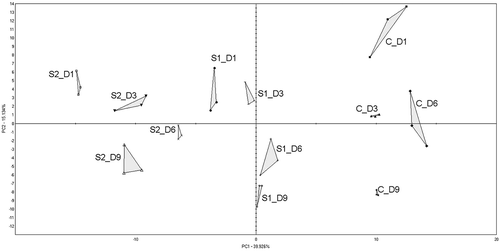
shows the distance values with p ≤ 0.05. The greatest spread within the groups was observed for the group C – the distance between the control sample from the day 1 and the sample stored for 9 days was 20.55. The smallest distance (12.25) between the day 1 and day 9 was observed in group S2. In S1 group, the distance between the day 1 and the day 9 was 14.81. A relationship was observed: an increase in an additive content of sage causes a decrease in changes of volatile compounds profile within the tested samples.
Table 1. The Euclidian distance between the volatile chromatographic profiles of turkey groups (C: control, S1: the sage addition 0.02%, S2: the sage addition 0.05%) stored 1, 3, 6 and 9 days (D1, D3, D6, D9); p < 0,05.
Tabla 1. Distancia euclidiana entre los perfiles cromatográficos volátiles de grupos de pavo (C: control, S1: adición de salvia 0.02%, S2: adición de salvia 0.05%) almacenados durante 1, 3, 6 y 9 días (D1, D3, D6, D9); valor p < 0,05.
3.4. Volatiles
Alcohol 3-methyl-1-butanol, aldehydes: propanal, 2-methylpentanal and ketone – cyclohexanone were found in the control group on the 1st, 3rd, 6th and 9th day of storage. Ethanol was present in the group C on the 3rd, 6th and 9th day. Terpenes: α-pinene, β-pinene, 1,8-cineole and linalool were found in all of the samples with additive content of sage (S1 and S2). These are the characteristic compounds for sage Ozek et al., Citation2010). Apart from terpenes, 3-methyl-1-butanol, propanal, 2-methylpentanal were found in the groups S1 and S2 on the 1st, 3rd, 6th and 9th day of the tests. Linear ketones originated from lipid oxidation. Linear aldehydes, such as propanal, 2-methylpentanal, are derived from the auto-oxidation of unsaturated fatty acids (Pignoli, Bou, Rodrigez-Estrada, & Decker, Citation2009). Alcohols, such as ethanol and 3-methyl-1-butanol are formed during the process of lipid oxidation as a result of reduction of aldehydes due to the microbial activity and biochemical reactions (Leroy, Vasilopoulos, Van Hemelryck, Falcony, & De Vuyst, Citation2009). 3-methyl-1-butanol is the typical alcohol detected in a raw meat (Casaburi et al. Citation2011). According to Smit, Smit, and Engels (Citation2005), 3-methyl-1-butanol is produced from leucine catabolism. Ethanol and cyclohexanone were not found in the groups S1 and S2. Absence of these compounds could be associated by partial inhibition of microbiological growth due to addition of sage which is confirmed in presented study.
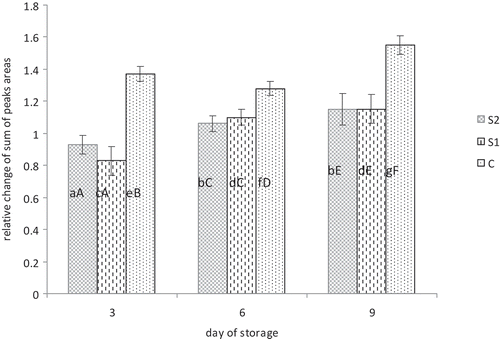
shows changes in volatile compounds in forms of relative change of sums of peaks areas of all volatiles compounds, excluding terpenes, compared to the sums of peaks areas from the first day of storage. Significant statistical differences between relative change of volatile compounds content between S1, S2 and C groups in consecutive days of storage (p < 0.05) were found. A slower relative increase in volatile compounds in consecutive days of storage was observed for the groups containing additive content of sage which may indicate that lipid oxidation processes are inhibited in contrast to the control group. Marques Pino et al. (Citation2013) found that 0.1% sage addition to chicken meatballs inhibited the development of hexanal, considered as an indicator of oxidation processes. Aldehydes such as hexanal and pentanal are considered as products of oxidation of PUFA (Pignoli et al., Citation2009; Romeu-Nadal, Castellote, & López-Sabater, Citation2004). Pentanal was the most abundant aldehyde in our study. The highest value of pentanal was observed in the control samples in contrast to the S1 and S2 samples with addition of sage extract. The rate of increase of pentanal was higher in the S1 group than in the S2 group. Similarly, Mielnik, Sem, Egelandsdal, and Skrede (Citation2008) reported low values of selected volatile aldehydes, such as propanal, pentanal, hexanal, heptanal, octanal, nonanal, decadienal, in turkey thighs marinated with sage as compared to control.
Figure 3. Changes in total amount of lipid-derived volatile compounds of turkey samples C, S1, S2 stored 3, 6 and 9 days in comparison to the first day of storage. Significant statistical differences (P ≤ 0,05) within the groups (S1: the addition 0.02%, S2: the addition 0.05%, C: control group) were denoted by different small letters. Significant statistical differences between the data for certain days of storage were denoted by different capital letters.
C: control samples with addition of 0.5% NaCl.
S1: samples with addition of 0.5% NaCl and 0.02% of sage extract.
S2: samples with addition of 0.5% NaCl and 0.05% of sage extract.
Figura 3. Cambios en la cantidad total de compuestos volátiles derivados de lípidos encontrados en muestras de pavo C, S1 y S2 almacenadas durante 3, 6 y 9 días, en comparación con el primer día de almacenamiento. Las diferencias estadísticamente significativas (P ≤ 0,05) en los grupos (S1: la adición de 0.02%, S2: la adición de 0.05%, C: grupo de control) están indicadas por letras minúsculas diferentes. Las diferencias estadísticamente significativas entre los datos observados en ciertos días del periodo de almacenamiento están indicadas por letras mayúsculas diferentes.
C: muestras de control adicionadas con 0.5% NaCl.
S1: muestras adicionadas con 0.5% NaCl y 0.02% de extracto de salvia.
S2: muestras adicionadas con 0.5% NaCl y 0.05% de extracto de salvia.
3.5. Effect of salvia officinalis extract on instrumental colour value
The changes in the instrumental colour values of turkey meatballs during the storage are presented in . The value of L* parameter on 3rd day of storage was statistically significant higher in S1 group compare to S2 group. On 9th day of storage the values of L* parameter both in group S1 and S2 were statistically higher compare to control group. The polyphenols contained in Salvia officinalis are likely to be oxidized to corresponding quinines by polyphenol oxidases, which are widespread in plant materials. Such quinines may condense to form darkened which results in an intense colour of meat (Liu, Tsaur, Lin, Jan., & Tan, Citation2009; Zhang, Lin, Leng, Huang, & Zhou, Citation2013).
Table 2. The mean and standard deviation of colour components of turkey samples enhanced with antioxidant solution depending on the storage time (C: control, S1: the sage addition 0.02%, S2: the sage addition 0.05%) stored 1, 3, 6 and 9 days (D1, D3, D6, D9).
Tabla 2. Media y desviación estándar de los componentes de color de muestras de pavo mejoradas con una solución antioxidante, dependiendo de la duración del almacenamiento (C:control, S1: adición de salvia 0.02%, S2: adición de salvia 0.05%) almacenadas durante 1, 3, 6 y 9 días (D1, D3, D6, D9).
At 9th day of storage, parameter a* was statistically significant lower as compared to parameter a* at 1st and 3rd day of storage for S2 group. Statistically significant differences between all analysed samples for b* parameter at 9th day of storage were observed. Addition of natural antioxidants may improve oxidative stability of meat what can be the reason for smaller changes in meat colour (Hanczakowska, Świątkiewicz, & Grela, Citation2015).
Cruz-Romero, Kelly, and Kerry (Citation2007) classified overall colour changes into seven groups: ∆E value between 0 and 0.2 means imperceptible colour change; 0.2 and 0.5 means a very small difference; 0.5 and 1.5 means a small difference; 1.5 and 3.0 means a slight significant change; 3.0 and 6.0 means a significant change; 6.0 and 12.0 means a very significant change; >12 means a great change. According to the mentioned classification after nine days of storage, S1 group was characterized as samples with significant colour changes and the observer perceived a clear colour difference. It means that observer can perceive two different colours. An S2 sample was characterized as a sample with slight significant changes and not experienced observer can perceive colour differences.
3.6. Microbiological analysis
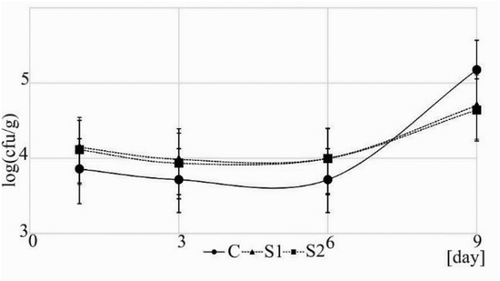
To verify antibacterial effect of the addition of the sage extract to stored turkey meatballs packed under MAP, the total number of aerobic bacteria in the samples was analysed. In the , the total number of aerobic bacteria in the turkey meatballs with or without the addition of the sage extract is presented. Obtained data showed that the sage extract inhibits growth of aerobic bacteria compared to control after the 7th day of the storage of the meatballs. Karpińska-Tymoszczyk (Citation2007) also indicated that addition of sage extract (1000 ppm) to turkey meatballs packed under vacuum inhibits microbial growth. Furthermore, our results revealed that both the concentrations (0.02% and 0.05%) of the sage extract in the meatballs have similar effect on the growth of aerobic bacteria. These results correspond with the observations made by the other authors, showing that different concentrations of sage extract have the similar effect on the growth of E. coli O157:H7 (Sağdıç, Kuşçu, Özcan, & Özçelik, Citation2002). The sage extracts inhibited growth of food born pathogens such as Shigella flexneri, Shigella sonnei, Salmonella typhi, Staphylococcus aureus and vancomycin-resistant enterococci (Horiuchi et al., Citation2007; Shirazi et al., Citation2008). Marchev et al. (Citation2014) presented that antibacterial activity has terpenes and polyphenols of sage extract. Terpenes are able to inhibit bacterial growth and their proliferation due to binding with enzymes and conformational changes in the bacterial cell (Calsamiglia, Busquet, Cardozo, Castillejos, & Ferret, Citation2007). In the study, terpenes were identified in S1 and S2 samples from groups S1 and S2. The chemical structure and concentration of the terpenes are associated with the sensitivity of bacteria to them (Longaray Delamare, Moschen-Pistorello, Artico, Atti-Serafini, & Echeverrigaray, Citation2007).
Figure 4. Effect of the sage extract on the total number of aerobic bacteria in turkey meatballs during storage.
C: control samples with addition of 0.5% NaCl.
S1: samples with addition of 0.5% NaCl and 0.02% of sage extract.
S2: samples with addition of 0.5% NaCl and 0.05% of sage extract.
Figura 4. Efecto del extracto de salvia en el número total de bacterias aeróbicas presentes en albóndigas de pavo durante el almacenamiento.
C: muestras de control adicionadas con 0.5% NaCl.
S1: muestras adicionadas con 0.5% NaCl y 0.02% extracto de salvia.
S2: muestras adicionadas con 0.5% NaCl y 0.05% de extracto de salvia.
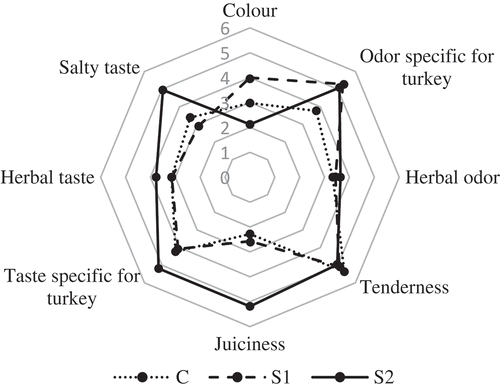
3.7. Sensory evaluation
Results of sensory analysis are presented in . No significant difference in 7 out of 8 discriminants was observed. It was only for juiciness that the difference was noted between the groups. The highest scores for this attribute were obtained for meat treated with 0.05% of sage and scores were almost twice higher for this attribute than for the control group and for the meat with the addition of 0.02% of sage. Contradictory, Karpińska-Tymoszczyk (Citation2007) did not observe the differences in juiciness between control and sage groups, while noted a significant differences in taste and flavour to the detriment of meat with sage addition. Similarly, Mielnik et al. (Citation2008) reported higher scores for flavour and odour attributes for meat with sage than for untreated meat. It is assumed that those differences may occur from the different doses of sage used in three researches mentioned above. Therefore, we can conclude that the addition of sage in a concentration of 0.05% does not adversely affect sensory quality of turkey meatballs.
Figure 5. Sensory panel ratings (for eight descriptors) of various treatments assessed by trained sensory panel. Rated treatments: C: control, S1: 0.02% of sage extract, S2: 0.05% of sage extract.
Figura 5. Calificaciones del panel sensorial (para ocho descriptores) de varios tratamientos evaluados por un panel sensorial de personas capacitadas. Tratamientos evaluados: C: control, S1: 0.02% de extracto de salvia, S2: 0.05% de extracto de salvia.
4. Conclusions
The results of this study indicated that the addition of sage extract to turkey meatballs was effective in controlling lipid oxidation and the microbial growth, during the refrigerated storage of pre-cooked turkey meatballs. Therefore, the addition of sage extracts can improve the oxidative stability of lipids and result in a better commercial acceptance of the product. To conclude, this study shows that a sage extract, which is rich in flavonoids and phenolic acids, may have application as a natural food preservative in the meat industry.
Acknowledgments
We would like to acknowledge the Project BIOFOOD – innovative, functional products of animal origin no. POIG.01.01.02-014-090/09 co-financed by the European Union from the European Regional Development Fund within the Innovative Economy Operational Programme 2007-2013 (made available on equipment).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Berdahl, D. R., & McKeague, J. (2015). Rosemary and sage extracts as antioxidants for food preservation. In F. Shahidi (Eds.), Handbook of antioxidants for food preservation. Woodhead Publishing Series in Food Science, Technology and Nutrition. ISBN: 978-1-78242-089-7. doi:10.1016/B016/B978-1-78242-089-7.00008-7
- Brodowska, M., Guzek, D., Kołota, A., Głąbska, D., Górska-Horczyczak, E., Wojtasik-Kalinowska, I., & Wierzbicka, A. (2016). The effect of diet on oxidation and profile of volatile compounds of Semimembranosus after freezing storage. Journal of Food and Nutrition Research, 55, 40–47.
- Calsamiglia, S., Busquet, M., Cardozo, P., Castillejos, L., & Ferret, A. (2007). Essential oils as modifiers of rumen microbial fermentation: A review. Journal of Dairy Science, 90, 2580–2595.
- Casaburi, A., Nasi, A., Ferrocino, I., Di Monaco, R., Mauriello, G., Villani, F., & Ercolini, D. (2011). Spoilage-related activity of Carnobacterium maltaromaticum strains in airstored and vacuum-packed meat. Applied and Environmental Microbiology, 77, 7382–7393.
- Chen, J. H., & Ho, C. T. (1997). Antioxidant activities of caffeic acid and its related hydroxycinnaminic acid compounds. Journal Of Agricultural And Food Chemistry, 45, 2374–2378.
- Chouliara, E., Karatapanis, A., Sawaidis, I. N., & Kontominas, M. G. (2007). Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4°C. Food Microbiology, 24, 607–617.
- Cruz-Romero, M., Kelly, A. L., & Kerry, J. P. (2007). Effects of high-pressure and heat treatments on physical and biochemical characteristics of oysters (Crassostrea gigas). Innovative Food Science and Emerging Technologies, 8, 30–38.
- Fasseas, M. K., Mountzouris, K. C., Tarantilis, P. A., Polissiou, M., & Zervas, G. (2008). Antioxidant activity in meat treated with oregano and sage essential oils. Food Chemistry, 106, 1188–1194.
- Gantner, M., Guzek, D., Najda, A., Brodowska, M., Górska-Horczyczak, E., Wojtasik-Kalinowska, I.,E., & Godziszewska, J. (2016). Oxidative and microbial stability of poultry meatballs added with coriander extracts and packed in cold modified atmosphere. International Journal of Food Properties, 20(11), 2527–2537.
- Hanczakowska, E., Świątkiewicz, M., & Grela, E. R. (2015). Effect of dietary inclusion of a herbal extract mixture and different oils on pig performance and meat quality. Meat Science, 108, 61–66.
- Horiuchi, K., Shiota, S., Hatano, T., Yoshida, T., Kuroda, T., & Tsuchiya, T. (2007). Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biological and Pharmaceutical Bulletin, 30, 1147–1149.
- ISO-4833-2. (2013). Microbiology of the food chain. Horizontal method for the enumeration of microorganisms. Part 2: Colony count at 30 degrees C by the surface plating technique (PN-EN ISO 4833-1), 12 pp.
- ISO-6658. (1998). Sensory Analysis. Methodology. General Guidance [Analiza sensoryczna. Metodologia Wytyczne ogólne] (PN-ISO 6658), 21 pp. (in Polish).
- Jia, N., Kong, B., Liu, Q., Xinping, D., & Xiufang, X. (2012). Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Science, 91, 533–539.
- Karakaya, M., Bayrak, E., & Ulusoy, K. (2011). Use of natural antioxidants in meat and meat products. Journal of Food Science and Engineering, 1, 1–10.
- Karpińska, M., Borowski, J., & Danowska-Oziewicz, M. (2000). Antioxidative activity of rosemary extract in lipid fraction of minced meatballs during storage in the freezer. Die Nauhrung, 44, 38–41.
- Karpińska-Tymoszczyk, M. (2007). Effects of sage extract (Salvia officinalis L.) and a mixture of sage extract and sodium isoascorbate on the quality and shelf life of vacuum-packed turkey meatballs. Journal of Muscle Foods, 18, 420–434.
- Komprda, T., Šarmanová, I., Kelenka, J., Bakaj, P., Fialová, M., & Fajmonová, E. (2002). Effect of sex and age on cholesterol and fatty acid content in turkey meat. Archiv Für Geflügelkunde, 66, 263–273.
- Lee, T. G., Williams, S. K., Sloan, D., & Littell, R. (1997). Development and evaluation of a chicken breakfast sausage manufactured with mechanically deboned chicken meat. Poultry Science, 76, 415–421.
- Leroy, F., Vasilopoulos, C., Van Hemelryck, S., Falcony, G., & De Vuyst, L. (2009). Volatile. analysis of spoiled, artisan-type, modified-atmosphere-packaged cooked ham stored under different temperatures. Food Microbiology, 26, 94–102.
- Liu, D. C., Tsaur, R. T., Lin, Y. C., Jan., S. S., & Tan, F. J. (2009). Effect of various levels of rosemary of Chinese mohogany on the quality of fresh chicken sausage during refrigerates storage. Food Chemistry, 117(1), 106–113.
- Longaray Delamare, A. P., Moschen-Pistorello, I. T., Artico, L., Atti-Serafini, L., & Echeverrigaray, S. (2007). Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chemistry, 100, 603–608.
- Lu, Y., & Yeap, F. (2001). Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chemistry, 75, 197–202.
- Marchev, A., Haas, C., Schulz, S., Georgiev, V., Steingroewer, J., Bley, T., & Pavlov, A. (2014). Sage in vitro cultures: A promising tool for the production of bioactive terpenes and phenolic substances. Biotechnology Letters, 36, 211–221.
- Mariutti, L., Nogueira, G., & Bragagnolo, N. (2011). Lipid and cholesterol oxidation in chicken meat are inhibited by sage but not by garlic. Journal of Food Science, 76, 909–915.
- Mariutti, L. R. B., Orlien, V., Bragagnolo, N., & Skibsted, L. H. (2008). Effect of sage and garlic on lipid oxidation in high-pressure processed chicken meat. European Food Research and Technology, 227, 337–344.
- Marques Pino, L., Cavaleiro, C., Conceição Castilho, M., Bismara Regitano D’arce, M. A., Da Silva Torres, E. A., & Ramos, F. (2013). The use of natural antioxidants (oregano and sage) to reduce hexanal production in pre-cooked chicken during chill storage. Vitae, 20, 105–111.
- McCarthy, T. L., Kerry, J. P., Kerry, J. F., Lynch, P. B., & Buckely, D. J. (2001). Evaluation of the antioxidant potential of natural food plant extract as compared with synthetic antioxidant and vitamin E in raw and cooked pork patties. Meat Science, 57, 45–52.
- Mercier, Y., Gatellier, P., Viau, M., Remignon, H., & Renerre, M. (1998). Effect of dietary fat and vitamin E on colour stability and on lipid and protein oxidation in Turkey meat during storage. Meat Science, 48, 301–318.
- Mielnik, M. B., Olsen, E., Vogt, G., Adeline, D., & Skrede, G. (2006). Grape seed extract as antioxidant in cooked, cold stored turkey meat. Lebensmittel-Wissenschaft Und Technologie, 39, 191–198.
- Mielnik, M. B., Sem, S., Egelandsdal, B., & Skrede, G. (2008). By-products from herbs essential oil production as ingredient in marinade for turkey thighs. LWT - Food Science and Technology, 41, 93–100.
- Min, B., & Ahn, D. U. (2005). Mechanism of lipid peroxidation in meat and meat products – A revive. Food Science and Biotechnology, 14, 152–163.
- Ozek, G., Demirci, F., Ozek, T., Tabanca, N., Wedge, D. E., Khan, S. I., … Hamzaoglu, E. (2010). Gas chromatographic–Mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm., and evaluation for biological activity. Journal of Chromatography A, 1217, 741–748.
- Pignoli, G., Bou, R., Rodrigez-Estrada, M. T., & Decker, E. A. (2009). Suitability of saturated aldehydes as lipid oxidation markers in washed turkey meat. Meat Science, 83, 412–416.
- Pizzale, L., Bortolomeazzi, R., Vichi, S., Überegger, E., & Conte, L. S. (2002). Antioxidant activity of sage (Salvia officinalis and S. fruticosa) and oregano (Origanum onites and O. indercedens) extracts related to their phenolic compound content. Journal of the Science of Food and Agriculture, 82, 1645–1651.
- Polish Pharmacopoeia VI. PTFarm. (2002). Warsaw, Poland.
- Polish Pharmacopoeia VII. PTFarm. (2006). Warsaw, Poland.
- Polish Pharmacopoeia X. PTFarm. (2012). Warsaw, Poland.
- Rababah, T. M., Hettiarachchy, N. S., & Horax, R. (2004). Total phenolics and antioxidant activities of fenugreek, green tea, black tea, grape seed, ginger, rosemary, gotu kola, and ginkgo extracts, vitamin E, and tert-butylhydroquinone. Journal of Agricultural and Food Chemistry, 52, 5183–5186.
- Rhee, K. S., Anderson, L. M., & Sams, A. R. (1996). Lipid oxidation potential of beef, chicken, and pork. Journal of Food Science, 61, 8–12.
- Roby, M. H. H., Khaled, A. S., Khalel, A. S., & Khalela, I. (2013). Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Industrial Crops and Products, 43, 827–831.
- Rojas, M. C., & Brewer, M. S. (2007). Effect of natural antioxidants on oxidative stability of cooked, refrigerated beef and pork. Journal of Food Science, 72, 5282–5288.
- Romeu-Nadal, M., Castellote, A. I., & López-Sabater, M. C. (2004). Headspace gas chromatographic method for determining volatile compounds in infant formulas. Journal of Chromatography A, 1046, 235–239.
- Rossaint, S., Klausmann, S., & Kreyenschmidt, J. (2015). Effect of high-oxygen and oxygen-free modified atmosphere packaging on the spoilage process of poultry breast fillets. Poultry Science, 94, 93–103.
- Sağdıç, O., Kuşçu, A., Özcan, M., & Özçelik, S. (2002). Effects of Turkish spice extracts at various concentrations on the growth of Escherichia coli O157:H7. Food Microbiology, 19, 473–480.
- Shah, M. A., Don Bosco, S. J., & Mir, S. A. (2014). Plant extracts as natural antioxidants in meat and meat products. Meat Science, 98, 21–33.
- Shan, B., Cai, Y. Z., & Corke, H. (2005). Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of Agricultural and Food Chemistry, 53, 7749–7759.
- Shirazi, M., Ranjbar, R., Eshraghi, S., Amin, G., Nouri, M. S., & Bazzaz, N. (2008). Inhibitory effects of Sage extract on the growth of enteric bacteria. Pakistan Journal of Biological Sciences, 11, 487–489.
- Smit, G., Smit, B. A., & Engels, W. J. M. (2005). Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiology Review, 29, 591–610.
- Velasco, V., & Williams, P. (2011). Improving meat quality though natural antioxidants. Chilean Journal of Agricultural Research, 71, 313–322.
- Wang, H., Li, J., Rangarajan, M., Shao, Y., LaVoie, E. J., Huang, T. C., & Ho, C. T. (1998). Antioxidative phenolic compounds from sage (Salvia officinalis). Journal of Agricultural and Food Chemistry, 46, 4869–4873.
- Wojdyło, A., Oszmioński, J., & Czemerys, R. (2007). Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chemistry, 105, 940–949.
- Wolfe, K., Wu, X., & Liu, R. H. (2003). Antioxidant activity of apple peels. Journal of Agricultural and Food Chemistry, 45, 1039–1044.
- Zhang, L., Lin, Y. H., Leng, X. J., Huang, M., & Zhou, G. H. (2013). Effect of sage (Salvia officinalis) on the oxidative stability of Chinese-style sausage during refrigerated storage. Meat Science, 95, 145–150.
