ABSTRACT
The use of polymeric matrices releasing active principles is a promising strategy to circumvent dipping treatments and reduce additive usage. Herein, we developed soy protein-based films containing 0, 10, 25 or 50% sodium sulfite on protein isolate basis (SPI), and determined the influence of the antioxidant on the color, thickness, opacity, solubility, moisture, water permeability and mechanical properties of the resulting materials. Raising sulfite concentration reduced the tensile strength, without affecting the Young’s modulus of the films. The formulation containing 50% sodium sulfite on SPI basis was selected based on the lighter color, lowest moisture and higher permeability of the resulting films to evaluate the efficacy of the materials to prevent apple browning. The incorporation of SO2-releasers in packages containing fresh-cut apples delayed fruit yellowing (increase of b* values) by 40%. This shows that sodium sulfite soy protein–based films may be useful to extend the postharvest life of fresh-cut apple.
RESUMEN
Las matrices poliméricas capaces de liberar principios activos son una estrategia prometedora para evitar aplicaciones de inmersión directa y minimizar los niveles de uso de algunos aditivos. En este trabajo se formularon películas en base a proteína de soja con 0, 10, 25 y 50% de sulfito de sodio en base al aislado proteico de soja (SPI) y se determinó la influencia del antioxidante sobre el color, el espesor, la opacidad, la solubilidad, la humedad, la permeabilidad al vapor de agua y las propiedades mecánicas del material. El aumento de la concentración de sulfito en la suspensión generadora de película redujo la resistencia a la tracción, pero no afectó el módulo de Young de los materiales resultantes. La formulación conteniendo 50% de sulfito de sodio en base a SPI por su color más claro, menor nivel de humedad inicial y mayor permeabilidad de las películas obtenidas se seleccionó y empleó para experimentos evaluando la eficacia de los materiales como liberadores de SO2 capaces de reducir el pardeamiento de manzana fresca cortada. La incorporación de las películas liberadoras de SO2 en envases conteniendo manzanas frescas cortadas retrasó el amarillamiento (aumento del valor b*) en un 40%. Esto muestra que las películas en base a proteínas de soja conteniendo sulfito de sodio podrían ser de utilidad para reducir el deterioro poscosecha de manzana fresca cortada.
PALABRAS CLAVE:
1. Introduction
The popularity of fresh-cut vegetables has increased in recent times due to changes in social and food consumption habits (Rico, Martín-Diana, Barat, & Barry-Ryan, Citation2007). Consumers demand premium quality and safe fresh-cut fruits, which have long shelf life and are easy to use. However, these products are normally more perishable than their unprocessed counterparts (Barry-Ryan, Martin-Diana, Rico, & Barat, Citation2007; Wilson, Stanley, Eyles, & Ross, Citation2017).
Enzymatic browning is one of the most common causes of deterioration in fresh-cut fruit (Golan-Goldhirsh & Whitaker, Citation1984). Strong antioxidants, such as sulfites have been long used to reduce this problem (Barry-Ryan et al., Citation2007). The release of SO2 in packed fruits has been also used to reduce in some cases decay (Saito & Xiao, Citation2017). However, a major drawback of sulfites is their allergenicity (Berry & Aked, Citation1997). Unfortunately, most alternative browning inhibitors, such as ascorbic, erythorbic and citric acids and ethylenediamine tetra-acetic acid (EDTA) are normally less effective than sulfites and provide far less residuality (Golan-Goldhirsh & Whitaker, Citation1984; Huawang & Yaguang, Citation2007). In fresh-cut produce sulfites are approved for products that would have a cooking step prior to consumption (He & Luo, Citation2007; Lichter, Zutahy, Kaplunov, & Lurie, Citation2008). Active research is being conducted to find novel, effective and safe browning inhibitors. Meanwhile, the search of strategies to minimize sulfite residues has great interest (Pretel, Martínez-Madrid, Matínez, Carreño, & Romojaro, Citation2006). Controlled delivery of SO2 into the headspace of packed products may be envisioned as a strategy to reduce sulfite use as compared to conventional dipping treatments.
Soy protein films have been suggested to be useful as biodegradable packaging materials for several packing solutions (Salgado et al., Citation2017). Their use seems is less promising for fresh fruits and vegetables, given that under the high relative humidity (RH) required for keeping quality the mechanical properties of these biopolymeric materials are poor and their gas permeability is high (Chinma, Ariahu, & Alakali, Citation2015; Rachtanapun & Wongchaiya, Citation2012). Ortiz, Mauri, and Vicente (Citation2013) took advantage of high RH induced changes in protein films, to generate pads able to release the gaseous inhibitor of fruit ripening 1-methylcyclopropene (1-MCP) in the headspace of tomato packages. The 1-MCP releasers proved effective to reduce fruit softening and decay. Bio-polymeric matrices have been also used to release volatile compounds, such as essential oils and improve food conservation (Montero-Prado, Rodriguez-Lafuente, & Nerin, Citation2011; Raybaudi-Massilia, Rojas-Graü, Mosqueda-Melgar, & Martín-Belloso, Citation2008; Tzortzakis, Citation2007). In this work, we evaluated the physical properties of soy protein films activated with sodium sulfite and provided an initial evaluation of the efficacy to prevent color modification in fresh-cut apples.
2. Materials and methods
2.1. Materials
A commercial soy protein isolate (SPI) SUPRO 500E (protein 85 ± 2%; carbohydrates 11 ± 1%; ash 2.4 ± 0.2%; and lipid ≤ 1%), kindly supplied by DuPont N & H (Brazil), was used as raw material. SPI protein solubility (39.5 ± 2.8%) was determined by the Bradford method (Bradford, Citation1976). Glycerol and sodium sulfite (Sigma, St Louis USA) were used as film plasticizer and active additive, respectively. All the other reagents used in this study were of analytical grade.
2.2. Film production
Films were prepared by casting, dispersing SPI (5% w/v) and glycerol (1.5% w/v) in distilled water with the addition of different concentrations of sodium sulfite (0, 10, 25, 50 g sodium sulfite/100 g SPI) at pH 7.0. Ten milliliters of each film-forming dispersion were poured on polystyrene Petri dishes (64 cm2) and then dehydrated at 60°C for 3 h in an oven with air flow circulation (Yamato, DKN600, U.S.A). The dry films were conditioned 48 h at 20°C and 58% RH in desiccators with saturated solutions of NaBr, before being peeled from the casting surface for further characterization.
2.3. Film properties
2.3.1. Thickness
Film thickness was measured with a digital coating thickness gauge (Check Line DCN-900, U.S.A). Three films were evaluated per each formulation and 10 measurements were done on each film and averaged.
2.3.2. Opacity
The films were cut in pieces of 0.5 cm × 5 cm and the absorbance at 500 nm was evaluated in a UV-vis spectrophotometer (Beckman Model 1240-UV Mini, U.S.A) according to Condés, Añón, Mauri, and Dufresne (Citation2015). Measurements were done in triplicate and results were expressed in absorbance units per mm (UA mm−1).
2.3.3. Color
Color was determined with a chromameter Minolta (Model CR 400, Osaka, Japan) to determine the L*, a*, b* values. The color variation (∆E*) was calculated as: (∆L*2 ∆a*2+∆b*2)1/2. Nine measurements were done on different areas of each film. Three independent films were evaluated per each formulation.
2.3.4. Moisture content (MC)
Film MC was evaluated according to the method reported by the American Society for Testing and Materials (ASTM D644-94, Citation1994).
2.3.5. Solubility
Film solubility in water was determined according to Ortiz, De Moraes, Vicente, Laurindo, and Mauri (Citation2017). Results were expressed in percentage and the measurements were done in triplicate.
2.3.6. Water-vapor permeability (WVP)
WVP tests were determined according to Salgado, Molina Ortiz, Petruccelli, and Mauri (Citation2010). The films were inserted in within the window (0.00185 m2) of a permeation cell and incubated in a dessicator containing anhydrous silica. In order to have a gradient of 75% RH the permeation cell contained a saturated NaCl solution. Measurements were done in triplicate and results were expressed in g H2O m−1 Pa−1 s−1.
2.3.7. Mechanical properties
Film mechanical properties were determined by performing tensile tests according to Salgado et al. (Citation2010). The tensile strength (σr), Young’s modulus (E), and the elongation at break (εr). Six measurements were done for each film formulation.
2.4. Use of soy protein films containing sulfite as So2-releasers to prevent browning in fresh-cut apple
Apples (Malus domestica cv. Red Delicious) produced in Río Negro Argentina were harvested at a commercial maturity (163 days after full bloom, firmness of 77 N, 12.4% w/w soluble solids content, 2.9 g/L titratable acidity and a 40% of starch degradation). Fruit was transported to the laboratory and visually inspected to eliminate those having defects or physical damages and washed with water containing 150 mg L−1 sodium hypochlorite (pH 6.5). Fruit was subsequently manually peeled and de-cored, cut and packaged (ca. 200 g) in plastic (polyethylene therephtalate, PET) trays (10 cm × 20 cm × 4 cm) containing one sulfite releasing pad (64 cm2 with 50% w/w relative to SPI). The releasing pads were glued to the package walls to avoid direct contact with the fruit. Corresponding trays with soy protein pads without sulfite were used as controls. Samples were stored at 5°C for 7 days. After this period the trays were opened and immediately analyzed. Fruit surface color was measured with a colorimeter (Model CR-400, Minolta, Osaka, Japan) to obtain L*, a* and b* values. To minimize the error caused by intra-fruit variance 4 measurements were conducted on each fruit piece and averaged it an evaluated 20 pieces from 3 replicate packages. Fruit pH was evaluated potentiometrically. For titratable acidity, 10 g of ground tissue were put in a beaker. One hundred milliliter of distilled water was added and samples were titrated with 0.1 N NaOH until pH 8.2 (AOAC, Citation1980). Results were expressed as meq. H+ kg−1. For sugar analysis fruit was frozen in liquid N2 and 1 g of the resulting powder was extracted with 10 mL ethanol 96% v/v. The homogenate was vortexed and centrifuged at 10.000 × g for 10 min at 4°C. The supernatant was saved and the pellet was re-extracted and centrifuged as described above. The supernatants were pooled and brought to 100 mL with distilled water. Sugars dosage was made with the anthrone assay. Briefly, 1 mL of 0.5 g L−1 anthrone, prepared in 98% (w/w) H2SO4, was added slowly to the test tubes in a water-ice bath containing 100 μL of sample extract and 150 μL of distilled water. Samples were boiled at 100°C for 10 min, cooled in water and then the absorbance at 620 nm was measured. Four measurements were done for each treatment. Glucose was used as a standard and results were expressed as g of glucose equivalents per kilogram on dry basis.
2.5. Statistical analysis
Results were subjected to analysis of variance (ANOVA) and were expressed as mean ± standard deviation. Means were tested with the Tukey test for paired comparison, at a significance level of α = 0.05, using the SYSTAT v. 12 software (Systat Software, Inc., Chicago, U.S.A).
3. Results and discussion
3.1. Effect of sodium sulfite addition soy protein films properties
We tested the effect of four different levels of sodium sulfite incorporation (0, 10, 25 and 50% w/w SPI) on the properties of soy protein films. As no previous work has reported the addition of sulfites in similar materials, the doses were selected to cover a broad concentration range, which would still allow complete solubilization of glycerol and Na2SO3 in the soy protein suspension and yield continue films upon drying. The formulation contained glycerol as a plasticizer to overcome the brittleness of pure protein films and increase the permeability to gases (Echeverría, Eisenberg, & Mauri, Citation2014). The films were formulated at pH 7.0 to minimize and/or retard sulfite hydrolysis, which would have caused higher SO2 release during processing (Berry & Aked, Citation1997).
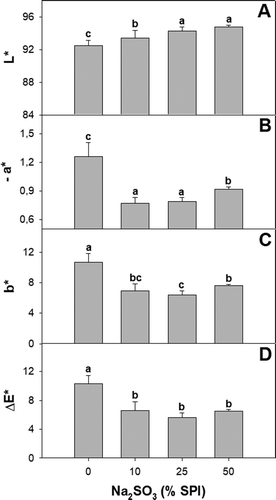
The films were continuous regardless of the sulfite concentration used (). Increasing sodium sulfite level up to 25% SPI did not affect film thickness. However, the biopolymeric materials with the highest sulfite levels were thicker (). Sulfites are known to reduce disulfide bonds (Petrucelli & Añón, Citation1995). This may increase protein–protein spacing and contributed explain, at least in part, the higher thickness of sulfite-containing films. The addition 50% SPI of sodium sulfite increased film opacity, indicating that a portion of the antioxidant added may have precipitated into the matrix (). The incorporation of sulfite also changed film color as depicted from the higher ∆E* values (). Control films showed a yellow-brown color which could be partially due to the presence of phenolics in the protein isolates, which could oxidize during drying (Salgado et al., Citation2017). Sulfite-containing films were whiter (higher L*). The addition of the antioxidant also reduced the yellow (b*) and red (a*) color components of the films. The bleaching effect was already observed with the use of 10% sulfite SPI. Besides their effectiveness as inhibitors of both enzymatic and non-enzymatic browning, sulfites have been shown to bleach readily oxidized phenolics (Prabhakar & Mallika, Citation2014). The lighter color of the films prepared with formulations containing sulfite may be related to a reduction of quinones to reduced non-colored. This may explain the sulfite elimination of the typical yellow-brown hues of soy protein films (Ortiz et al., Citation2017). No differences in color were detected in films containing higher antioxidant concentration.
Figure 1. (a) Appearance, (b) thickness and (c) opacity of soy protein films activated with 0; 10; 25; y 50% w/w sodium sulfite on SPI basis. Different letters indicate significant differences based on a Tukey test at a level of significance of P < 0.05.
Figura 1. (a) Apariencia, (b) grosor y (c) opacidad de las películas de proteína de soya activadas con 0; 10; 25; y 50% p/p sulfito de sodio con base en el SPI (aislado de proteína de soya). Las distintas letras indican la presencia de diferencias significativas con base en una prueba de Tukey a un nivel de significancia de P < 0.05.
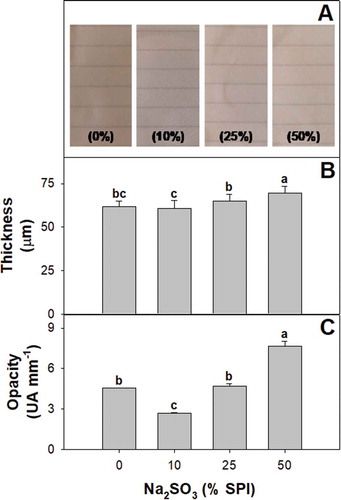
Figure 2. (a) Lightness, (b) -a*, (c) b* and (d) color variation of soy protein films activated with 0; 10; 25; y 50% w/w sodium sulfite on SPI basis. Different letters indicate significant differences based on a Tukey test at a level of significance of P < 0.05.
Figura 2. (a) Luminosidad, (b) -a*, (c) b*, y (d) variación de color de las películas de proteína de soya activadas con 0; 10; 25; y 50% p/p de sulfito de sodio con base en el SPI. Las distintas letras indican la presencia de diferencias significativas con base en una prueba de Tukey a un nivel de significancia de P < 0.05.
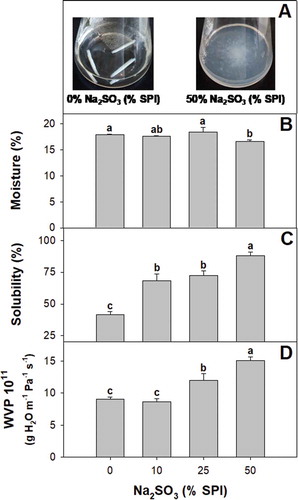
The initial water content of soy protein films was unaffected by sodium sulfite incorporation up to 25% w/w SPI ((b)). The films containing the highest sulfite levels showed the lowest water content. The solubility ((c)) and WVP ((d)) of the films was positively correlated with the level of sulfite in the formulation. Zhang and Sun (Citation2008) reported that disulfide bridges of glycinin the main soy protein fraction were reduced by NaHSO3 levels in the concentration range tested in the present work. The increased solubility and permeability of sulfite-containing films may be then likely due to changes in the redox potential and disulfide-thiol equilibrium.
Figure 3. (a) Appearance of soy protein films with 0 g and 50 g of sodium sulfite per 100 g of SPI after incubation in water for 24 h. (b) Moisture content, (c) solubility and (d) water vapor permeability of soy protein films activated with 0; 10; 25; y 50% w/w sodium sulfite on SPI basis. Different letters indicate significant differences based on a Tukey test at a level of significance of P < 0.05.
Figura 3. (a) Apariencia de las películas de proteína de soya con 0 g y 50 g de sulfito de sodio por 100 g de SPI, después de su incubación en agua durante 24 h. (b) Contenido de humedad, (c) solubilidad y (d) permeabilidad al vapor de agua de las películas de proteína de soya activadas con 0; 10; 25; y 50% p/p con base en el SPI. Las distintas letras indican la presencia de diferencias significativas con base en una prueba de Tukey a un nivel de significancia de P < 0.05.
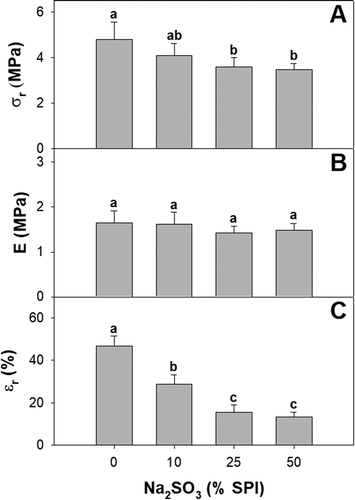
The breaking stress of the films decreased at the maximum concentration of sulfite evaluated ((a)). In contrast, incorporating the antioxidant had no effect on the Young’s modulus ((b)). This is in accordance with the results reported by Min, Song, and Zheng (Citation2008) in thermoformed gluten biopolymers. The elongation at break dropped as sulfite load was increased ((c)). Whereas this may have been caused in part by changes in protein interactions, it is also plausible that precipitated salt particles, especially at the highest sulfite load may yield heterogeneous regions acting as tension concentrating nodes, which may cause early film rupture in elongation tests (Wihodo & Moraru, Citation2013). The disaggregation of sulfite films after immersion in water for 24 h, as opposed to control films that maintained integrity ((a)), supports this. Meanwhile, sulfite may induce novel protein interactions, which may mask the effects of disulfide bond disruption in tension and modulus values (Mauri & Añón, Citation2008).
Figure 4. (a) Tensile strength (σr), (b) Young’s modulus (E), and (c) elongation at break (εr) of soy protein films activated with 0; 10; 25; y 50% w/w sodium sulfite on SPI basis. Different letters indicate significant differences based on a Tukey test at a level of significance of P < 0.05.
Figura 4. (a) Resistencia a la tracción (σr), (b) Módulo de Young (E), y (c) elongación de ruptura (εr) de las películas de proteína de soya activadas con 0; 10; 25; y 50% p/p de sulfito de sodio con base en el SPI. Las distintas letras indican la presencia de diferencias significativas con base en una prueba de Tukey a un nivel de significancia de P < 0.05.
3.2. Effect of soy protein based SO2-releasing pads on browning prevention
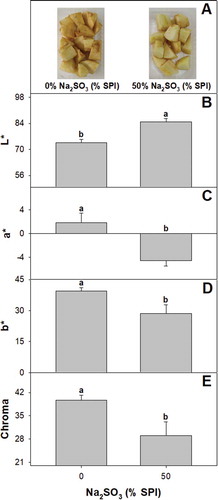
Enzymatic browning is caused by endogenous polyphenol oxidase leading to the formation of melanin brown pigments is one of the main factors limiting the postharvest life of fresh-cut apples (Hemachandran, Anantharaman, Mohan, & Mohan, Citation2017). Though ascorbic and citric acid have been used to reduce browning in apple fruit these compounds do not have a long-lasting action (Bosch et al., Citation2013; De´Nobili et al., Citation2016). Consequently should be used at high concentrations and combined with other antioxidants which are in some cases not available for commercial use (Gil, Gorny, & Kader, Citation1998; Salminen & Russotti, Citation2017). Coating formulations with ascorbic and ferulic acid at 4.0 g L−1 and pH 7.0 have been evaluated to extend the shelf life of fresh-cut apples, but were less effective than AA dips (Alves, Gonçalves, & Rocha, Citation2017). Sulfites are highly effective browning inhibitors and usually show high residual effect (Artés-Hernández, Tomás-Barberán, & Artés, Citation2006; Jiang & Qu, Citation2016). Finding alternative strategies to deliver low SO2 levels may be a valuable as novel anti-browning solutions are launched. The films containing with 50% sulfite SPI showing lighter color, lowest initial MC and higher permeability were selected to evaluate the efficacy to control browning in fresh-cut apples. Control apples stored at 5°C for 7 d showed advanced browning symptoms. In contrast, fruit held in packages containing SO2 releasers showed markedly higher visual quality ((a)). The improved color maintenance of fruit packed in trays containing SO2 releasing pads was also depicted by the higher fruit lightness and lower b* values (). Browning in fresh-cut apple has been primarily determined by the loss of cellular integrity during cutting operations, and the main effects of SO2 has been repeatedly related to its ability to inhibit PPO (through reducing copper in its active site) and revert quinone polymeration (Fennema, Citation2008). Interestingly recent results by Xue and Yi (Citation2017) showed that SO2 exposure may also induce the accumulation of internal antioxidants through the accumulation of PAL and elicit enzymes that have been associated with control against pathogens, such as chitinases and may also likely exert an antimicrobial effect.
Figure 5. (a) Appearance, (b) lightness, (c) b*, (d) a* and (e) chroma of fresh cut apples, control or treated with soy protein SO2 releasers stored for 7 days at 5°C. Different letters indicate significant differences based on a Tukey test at a level of significance of P < 0.05.
Figura 5. (a) Apariencia, (b) ligereza (c) b*, (d) a*, y (e) croma de manzanas recién cortadas, controladas o tratadas con liberadores SO2 de proteína de soya, almacenadas durante 7 días a 5°C. Las distintas letras indican la presencia de diferencias significativas con base en una prueba de Tukey a un nivel de significancia de P < 0.05.
Other attributes related to apple fruit quality, such as pH acidity and sugars were not affected by the use of the SO2-releasing pads (). Sortino et al. (Citation2017) also found very subtle change in SSC and TA in grapes subjected to SO2 treatments. Also in grape proper dose of sulfur dioxide significantly inhibited the respiration rate (Yiqiang, Weiyi, Ying, & Qiang, Citation1997). Modifications in the film formulation may be envisioned as a strategy to modulate SO2 release. Giménez, Gómez-Guillén, López-Caballero, Gómez-Estaca, and Montero (Citation2012) and Echeverría, López-Caballero, Gómez-Guillén, Mauri, and Montero (Citation2016), among others, showed that incorporation of different nanoclays into film formulation affected the volatilization of eugenol (from clove essential oil) from the film matrix. Mascheroni, Chalier, Gontard, and Gastaldi (Citation2010) showed the same behavior when using wheat gluten/montmorillonite based system as carvacrol carrier. In addition, cellulose nanofibers are still being explored for this type of applications in which control is sought for the diffusion and release of active compounds (Lavoine, Guillard, Desloges, Gontard, & Bras, Citation2016; Sun-Waterhouse & Waterhouse, Citation2016). Overall, results from this work show that sulfite-containing soy protein films may be useful to control oxidative browning in fresh-cut apple. Future work characterizing the kinetics of SO2 release in response to varying temperature and RH conditions within the fruit packages would be useful.
Table 1. pH, acidity and soluble sugars in fresh cut apples stored in container system containing active soy protein films with and without 50% w/w sodium sulfite (Na2SO3). Different letters within each column indicate significant differences at P < 0.05.
Tabla 1. pH, acidez y azúcares solubles en manzanas frescas cortadas almacenadas en un sistema de contenedores que incluye películas de proteína de soya, con y sin 50% p/p sulfito de sodio (Na2SO3). Las distintas letras que figuran en cada columna indican la presencia de diferencias significativas a un nivel de P < 0.05.
4. Conclusions
The development of technological packaging solutions will be critical in responding to commercial trends towards the production of more fresh-cut fruits having good eating quality, longer storage capacity and are safe. In this work, we determined the influence of sodium sulfite on the properties of soy protein films. Increasing sulfite concentration resulted in whiter, thicker films. The incorporation of sodium sulfite in the formulation reduced MC and increased WVP and solubility probably by reducing the formation of disulfide bridges within the protein matrixes, as well as by interfering with intermolecular interactions by salt precipitation especially at high sulfite loads. Sulfite addition decreased film’s tensile strength but did not affect the Young’s modulus. The incorporation of sulfite containing protein as SO2 releasing pads in trays containing fresh-cut apple, markedly improved fruit color maintenance. Though future studies are needed to establish and eventually modulate SO2 release, results show that sulfite containing soy protein films may be effective antioxidant releasers to control browning in packed fruits.
Acknowledgments
The authors thank the CONICET (PIP-0098) and the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2012-2803) for financial support. C.M.O. is a Posdoctoral Scholars from CONICET, Argentina. A.N.M. and A.R.V. are research members of the CONICET, Argentina.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alves, M. M., Gonçalves, M. P., & Rocha, C. M. R. (2017). Effect of ferulic acid on the performance of soy protein isolate-based edible coatings applied to fresh-cut apples. LWT Food Science and Technology, 80, 409–415.
- AOAC. (1980). Methods of analysis (13th ed.). Washington, DC: Association of Official Analytical Chemists.
- Artés-Hernández, F., Tomás-Barberán, F. A., & Artés, F. (2006). Modified atmosphere packaging preserves quality of SO2-free aSuperior seedless’ table grapes. Postharvest Biology and Technology, 39, 146–154.
- ASTM D644-94. (1994). Standard test methods for moisture content of paper and paperboard by oven drying. In Annual book of ASTM standards (pp. 1–2). Philadelphia, PA: American Society for Testing Materials.
- Barry-Ryan, C., Martin-Diana, A., Rico, D., & Barat, J. (2007). Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends in Food Science & Technology, 18, 373–386.
- Berry, G., & Aked, J. (1997). Controlled atmosphere alternatives to the post-harvest use of sulphur dioxide to inhibit the development of Botrytis cinerea in table grapes. In Proceedings of the CA’97, postharvest horticulture series no. 19, vol. 3 (pp. 160–164). Davis, USA: University of California.
- Bosch, V., Cilla, A., García-Llatas, G., Gilabert, V., Boix, R., & Alegría, A. (2013). Kinetics of ascorbic acid degradation in fruit-based infant foods during storage. Journal of Food Engineering, 116, 298–303.
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
- Chinma, C. E., Ariahu, C. C., & Alakali, J. S. (2015). Effect of temperature and relative humidity on the water vapour permeability and mechanical properties of cassava starch and soy protein concentrate based edible films. Journal of Food Science and Technology, 52(4), 2380–2386.
- Condés, M. C., Añón, M. C., Mauri, A. N., & Dufresne, A. (2015). Amaranth protein films reinforced with maize starch nanocrystals. Food Hydrocolloids, 47, 146–157.
- De´Nobili, M. D., Soria, M., Martinefski, M. R., Tripodi, V. P., Fissore, E. N., & Rojas, A. M. (2016). Stability of L-(+)-ascorbic acid in alginate edible films loaded with citric acid for antioxidant food preservation. Journal of Food Engineering, 175, 1–7.
- Echeverría, I., Eisenberg, P., & Mauri, A. N. (2014). Nanocomposites films based on soy proteins and montmorillonite processed by casting. Journal of Membrane Science, 449, 15–26.
- Echeverría, I., López-Caballero, M. E., Gómez-Guillén, M. C., Mauri, A. N., & Montero, M. P. (2016). Structure, functionality, and active release of nanoclay–soy protein films affected by clove essential oil. Food and Bioprocess Technology, 9, 1937–1950.
- Fennema, O. R. (2008). Fennema´s food chemistry (4th ed.). Eds., Damodaran, S., Parkin, K.L., Fennema, O.R. Boca Raton, FL: CRC Press, Taylor and Francis Group.
- Gil, M. I., Gorny, J. R., & Kader, A. A. (1998). Responses of ´Fuji´apple slices to ascorbic acid treatments and low-oxygen atmospheres. HortScience, 33, 305–309.
- Giménez, B., Gómez-Guillén, M. E., López-Caballero, J., Gómez-Estaca, J., & Montero, P. (2012). Role of sepiolite in the release of active compounds from gelatin-egg white films. Food Hydrocolloids, 27, 475–486.
- Golan-Goldhirsh, A., & Whitaker, J. R. (1984). Effect of ascorbic acid, sodium bisulfite, and thiol compounds on mushroom polyphenol oxidase. Journal of Agricultural and Food Chemistry, 32, 1003–1009.
- He, Q., & Luo, Y. (2007). Enzymatic browning and its control in fresh-cut produce extensive browning in fresh-cut fruits. Stewart Postharvest Review, 6, 3.
- Hemachandran, H., Anantharaman, A., Mohan, S., & Mohan, G. (2017). Unraveling the inhibition mechanism of cyanidin-3-sophoroside on polyphenol oxidase and its effect on enzymatic browning of apples. Food Chemistry, 227, 102–110.
- Huawang, H. F., & Yaguang, L. (2007). Control of browning and microbial growth on fresh-cut apples by sequential treatment of sanitizers and calcium ascorbate. Journal of Food Science, 72, M1–M6.
- Jiang, Y., & Qu, D. H. (2016). Browning: Enzymatic browning. In B. Caballero, P. M. Finglas, & F. Toldrá (Eds.), Encyclopedia of food and health (pp. 508–514). London, UK: Academic Press.
- Lavoine, N., Guillard, V., Desloges, I., Gontard, N., & Bras, J. (2016). Active bio-based food-packaging: Diffusion and release of active substances through and from cellulose nanofiber coating toward food-packaging design. Carbohydrate Polymers, 149, 40–50.
- Lichter, A., Zutahy, Y., Kaplunov, T., & Lurie, S. (2008). Evaluation of table grape storage in boxes with sulfur dioxide-releasing pads with either an internal plastic liner or external wrap. HortTechnology, 18, 206–214.
- Mascheroni, E., Chalier, P., Gontard, N., & Gastaldi, E. (2010). Designing of a wheat gluten/montmorillonite based system as carvacrol carrier: Rheological and structural properties. Food Hydrocolloids, 24, 406–413.
- Mauri, A. N., & Añón, M. C. (2008). Mechanical and physical properties of soy protein films with pH modified microstructures. Food Science and Technology International, 14, 119–125.
- Min, Z., Song, Y., & Zheng, Q. (2008). Influence of reducing agents on properties of thermo-molded wheat gluten bioplastics. Journal of Cereal Science, 48, 794–799.
- Montero-Prado, P., Rodriguez-Lafuente, A., & Nerin, C. (2011). Active label-based packaging to extend the shelf-life of “Calanda” peach fruit: Changes in fruit quality and enzymatic activity. Postharvest Biology and Technology, 60, 211–219.
- Ortiz, C. M., De Moraes, J. O., Vicente, A. R., Laurindo, J. B., & Mauri, A. N. (2017). Scale-up of the production of soy (Glycine max L.) protein films using tape casting: Formulation of film-forming suspension and drying conditions. Food Hydrocolloids, 66, 110–117.
- Ortiz, C. M., Mauri, A. N., & Vicente, A. R. (2013). Use of soy protein based 1-methylcyclopropene-releasing pads to extend the shelf life of tomato (Solanum lycopersicum L.) fruit. Innovative Food Science and Emerging Technologies, 20, 281–287.
- Petrucelli, S., & Añón, M. C. (1995). Thermal aggregation of soy protein isolates. Journal of Agricultural and Food Chemistry, 43(12), 3035–3041.
- Prabhakar, K., & Mallika, E. N. (2014). Preservatives. Permitted preservatives - sulfur dioxide. In C. A. Batt (Eds.), Encyclopedia of Food Microbiology (2nd ed., pp. 108–112). London, UK: Academic Press.
- Pretel, M. T., Martínez-Madrid, M. C., Matínez, J. R., Carreño, J. C., & Romojaro, F. (2006). Prolonged storage of ‘Aledo’ table grapes in a slightly CO2 enriched atmosphere in combination with generators of SO2. LWT Food Science & Technology, 39, 1109–1116.
- Rachtanapun, P., & Wongchaiya, P. (2012). Effect of relative humidity on mechanical properties of blended chitosan-methylcellulose film. Chiang Mai Journal of Science, 39(1), 133–137.
- Raybaudi-Massilia, R. M., Rojas-Graü, M. A., Mosqueda-Melgar, J., & Martín-Belloso, O. (2008). Comparative study on essential oils incorporated into an alginate-based edible coating to assure the safety and quality of fresh-cut Fuji apples. Journal of Food Protection, 71, 1150–1161.
- Rico, D., Martín-Diana, A. B., Barat, J. M., & Barry-Ryan, C. (2007). Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends in Food Science and Technology, 18, 373–386.
- Saito, S., & Xiao, C. L. (2017). Evaluation of sulfur dioxide-generating pads and modified atmosphere packaging for control of postharvest diseases in blueberries. Acta Horticulturae, 1180, 123–128.
- Salgado, P. R., Molina Ortiz, S. E., Denavi, G. A., Bosch, M. A., Añón, M. C., & Mauri, A. N. (2017). Chapter 4: Influence of initial protein structure on the properties of soybean protein edible films. In V. K. Thakur, M. K. Thakur, & M. R. Kessler (Eds.), Soy-based bioplastics (pp. 75–98). London, UK: Smithers Rapra Publisher.
- Salgado, P. R., Molina Ortiz, S. E., Petruccelli, S., & Mauri, A. N. (2010). Biodegradable sunflower protein films naturally activated with antioxidant compounds. Food Hydrocolloids, 24, 525–533.
- Salminen, W. F., & Russotti, G. (2017). Synergistic interaction of ascorbic acid and green tea extract in preventing the browning of fresh-cut apple slices. Journal of Food Processing and Preservation, 41, e13192.
- Sortino, G., Allegra, A., Passafiume, R., Gianguzzi, G., Gullo, G., & Gallotta, A. (2017). Postharvest application of sulphur dioxide fumigation to improve quality and storage ability of “red globe” grape cultivar during long cold storage. Chemical Engineering Transactions, 58, 403–408.
- Sun-Waterhouse, D., & Waterhouse, G. I. N. (2016). Chapter 2: Recent advances in the application of nanomaterials and nanotechnology in food research. In A. M. Grumezescu (Ed.), Novel approaches of nanotechnology in food (pp. 21–66). London, UK: Academic Press.
- Tzortzakis, N. G. (2007). Maintaining postharvest quality of fresh produce with volatile compounds. Innovative Food Science and Emerging Technologies, 8, 111–116.
- Wihodo, M., & Moraru, C. I. (2013). Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. Journal of Food Engineering, 114, 292–302.
- Wilson, M. D., Stanley, R. A., Eyles, A., & Ross, T. (2017). Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit Rev Food Sci Nutr, 11, 1–12.
- Xue, M., & Yi, H. (2017). Induction of disease defense responses by SO2 during the preservation of ‘Red Globe’ grapes. Chinese Journal of Applied and Environmental Biology, 23, 806–810.
- Yiqiang, G., Weiyi, Z., Ying, C., & Qiang, Y. (1997). Effect of sulfur dioxide on the respiration and the hormone levels of postharvest table grapes. Acta Horticulturae Sinica, 24, 120–124.
- Zhang, L., & Sun, X. S. (2008). Effect of sodium bisulfite on properties of soybean glycinin. Journal of Agricultural and Food Chemistry, 56, 11192–11197.
