 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study investigated possible differences in the suwari reaction among surimi and examined the possibility of using measurements obtained during the analysis of the suwari reaction as measures of quality. The changes in breaking strength during the suwari reaction of six kinds of frozen surimi, comprising high-grade and low-grade samples of three species – blue grenadier, Alaska pollock, and threadfin bream – were examined to determine the setting speed and activation energy. There was little difference in the setting speed between the grades of each fish species, likely due to the lower setting ability of the low-grade surimi which quickly reached the maximum breaking strength. In contrast, the activation energy of the high-grade surimi samples of all three species was lower by 1.3–2.5 times. Thus, the activation energy of the suwari reaction was demonstrated to be an effective measure of surimi setting ability and quality, which varies between species and surimi grade.
RESUMEN
El presente estudio investigó la existencia de posibles diferencias en la reacción suwari de diferentes calidades de surimi, examinando la posibilidad de utilizar como medida de su calidad las mediciones obtenidas durante el análisis de dicha reacción. En este sentido, durante la reacción suwari de seis variedades de surimi congelado, entre las que había muestras de alta y baja calidad de tres especies —merluza de cola, abadejo de Alaska y Baga tolu— se identificaron los cambios en la resistencia de ruptura, con el fin de determinar la velocidad de solidificación y la energía de activación. Se constató que existe poca diferencia en la velocidad de solidificación relacionada con la calidad de cada una de las especies de pescado, debido probablemente, a la reducida capacidad del surimi de baja calidad para solidificarse; rápidamente, éste alcanzó su máxima resistencia de ruptura. En cambio, la energía de activación de las muestras de surimi de alta calidad de las tres especies fue de 1.3 a 2.5 veces más baja. Ello demuestra que la energía de activación de la reacción suwari constituye una medida efectiva para determinar la capacidad de solidificación y la calidad del surimi; la misma varía según las especies y de acuerdo con la calidad de los surimis.
Introduction
Fish paste products, such as kamaboko and chikuwa, are traditional processed foods in Japan that take advantage of the properties of fish proteins. Increasing health consciousness among consumers outside Japan has increased the production and consumption of kamaboko, mainly for imitation crab meat (Tuji, Citation2003). Although the production of fish paste products has recently been on the decline in Japan, it still accounts for 31.5% (in 2015) of the overall processed food production, by types of processing, representing the highest percentage among all processed seafood products. This illustrates the importance of the fish paste industry in Japan.
Fish paste products have a characteristic elasticity and flexibility, referred to as ‘ashi’ in Japanese. Conferring ashi to fish pastes requires myosin, the major structural protein in fish meat, and involves the formation, by heating, of a network structure comprised mainly of myosin and actomyosin. The properties of fish paste are greatly affected by the heating process. Of particular importance is understanding the characteristic phenomenon of ‘suwari’ (structure-setting), observed during heating at temperatures between 20°C and 40°C, and ‘modori’ (structural disintegration), observed at temperatures around 50°C. The suwari reaction occurs as a result of the cross-linking polymerization of myosin molecules by the transglutaminase found in fish meat (Ni, Nozawa, & Seki, Citation1998, Citation1999; Numakura et al., Citation1985). Suwari behavior depends largely on the species of fish, its habitat (Asano, Itoh, Suwansakornkul, & Obatake, Citation2003; Benjakul, Chantarasuwan, & Visessanguan, Citation2003; Ishida, Kubota, & Toyohara, Citation2013; Matsuoka, Wan, & Ushio, Citation2013), and variations in myosin due to factors such as the season (Abe et al., Citation1996; Itoh, Maekawa, Suwansakornkul, & Obatake, Citation1995), pH, and the amount of salt (Makinodan, Nakagawa, Ando, & Matsuno, Citation1996; Ni, Nozawa, & Seki, Citation2001). In contrast, the modori reaction involves multiple endogenous proteases collectively referred to as modori-inducing proteinases (MIPs). And cathepsins (B, H, L, L-Like) and heat-stable alkaline proteinases act and cause modori (Hu et al., Citation2012; Liu, Yin, Zhang, Li, & Ma, Citation2008; Makinodan, Kitagawa, Toyohara, & Shimizu, Citation1987). Because the suwari and modori reactions are enzyme-catalyzed, they are greatly affected by temperature and time, and their behavior depends on the species of fish and the grade of surimi (minced fish meat) (Kinoshita, Citation1998; Kinoshita, Toyohara, & Shimizu, Citation1990; Ohkubo, Osatomi, Hara, Ishihara, & Aranishi, Citation2005a, Citation2005b; Tsuchiya & Sano, Citation1988).
Systems such as the Hazard Analysis Critical Control Point (HACCP) have been introduced for the sanitation management of fish paste products (Tzouros & Arvanitoyannis, Citation2000). However, currently, no clear standards have been established to evaluate the quality of frozen surimi. Manufacturers prepare heat-induced gel samples of their products and evaluate their safety and quality based on their own standards.
Thus, the present study focused on ‘suwari,’ a phenomenon unique to frozen surimi, and attempted a food engineering approach for the analysis of its behavior. The same approach was employed by Katoh, Hashimoto, Nozaki, and Arai (Citation1984) to demonstrate the differences in the quality of fish paste made from the Alaska pollock, white croaker, and tilapia (Katoh et al., Citation1984), although the effectiveness of this approach in showing the differences in quality among the same species of fish of varying grades has not been examined in showing the differences in quality among the same species of fish of varying grades. The present study thus examined frozen surimi of different grades made from the Alaska pollock, blue grenadier, and threadfin bream. The Alaska pollock is a species of cold-water fish of the family Gadidae in the order Gadiformes, and is abundantly distributed in the North Pacific. It is the world’s largest source of surimi ingredients. Therefore, Alaska pollock and the properties of surimi derived from it have been studied most extensively. It has been reported that the suwari reaction in Alaska pollock surimi requires a long time, and that the maximum breaking strength is observed at 20°C and the second highest at 30°C (Kitakami et al., Citation2004; Konno, Imamura, & Yuan, Citation2011; Numakura et al., Citation1987; Seki, Nozawa, & Ni, Citation1998; Seki et al., Citation1990; Wan & Seki, Citation1992). The blue grenadier is a cold-water fish of the family Merlucciidae in the order Gadiformes, distributed in the Southern Hemisphere, such as near New Zealand. The breaking strength of blue grenadier surimi continues to increase up to 30°C, following which the gel structure begins to disintegrate at 40°C or above. While blue grenadier frozen surimi has properties similar to those of Alaska pollock surimi, it is distinctive in that its protein degradation proceeds relatively quickly, as does the suwari reaction (Lee et al., Citation1990b). The threadfin bream belongs to the family Nemipteridae in the order Perciformes, and is distributed in temperate and tropical waters. It has traditionally been used as an ingredient in fish balls produced in Southeast Asia. The suwari reaction in threadfin bream surimi is minimally active at 10°C and begins to occur at 20°C; the optimum temperature has been reported to be between 37°C and 38°C. Threadfin bream surimi is more resistant to degradation by high-temperature heating compared to the surimi made from cold-water fish species (Kato, Nakagawa, & Terui, Citation1989; Lee et al., Citation1990a). To examine whether these measures can effectively differentiate the quality among the different species and grades, the present study used different grades of commercially available frozen surimi made from these three fish species to determine the setting speed, based on changes in the breaking strength during the suwari reaction, and to calculate the activation energy of the suwari reaction.
Materials and methods
Materials
Frozen surimi of the Alaska pollock (SA and RA grades), blue grenadier (FA and KA grades), and threadfin bream (SA and KA grades) were purchased from Nichimou Co., Ltd. (Tokyo). In this study, the SA-grade Alaska pollock, FA-grade blue grenadier, and SA-grade threadfin bream represented the higher-grade samples, and the RA-grade Alaska pollock, KA-grade blue grenadier, and KA-grade threadfin bream were the lower-grade samples. The blocks were divided into approximately 1 kg samples and were kept at −25°C until used.
Preparation of setting thermal gels
The frozen surimi kept at −25°C was thawed overnight in a refrigerator set at 4°C. The thawed surimi was ground in a food processor (MK-K48P, Panasonic, Osaka, Japan) or a high-speed food cutter (UMC-5, Stephan, Chiba, Japan) for approximately 1 min. Next, 30% water was added, and the mixture was ground for approximately 1 min. Finally, 2.5% salt was added and the mixture was again ground for approximately 1 min to produce the meat paste. This meat paste was stuffed into a casing film (Φ23 mm) and heated at 20°C, 25°C, and 30°C (only the threadfin bream was heated at 23°C) in a water bath (THERMO MINDER SM-05R, TAITEC, Saitama, Japan) to produce the setting thermal gel (Suwari gel).
Measurement of breaking strength
The setting thermal gels prepared at different temperatures and times were measured to determine the breaking strength (gf) with a rheometer (NRM-3002D, Rheotech, Tokyo, Japan) using a cylindrical plunger (5 mm in diameter) at a stage speed of 6.0 cm/min. The sample was divided into 2.5-cm thicknesses, and the average of the three samples was used as the measurement value.
Measurement of setting speed
The data obtained from the breaking strength measurements were plotted on the y-axis and the setting time on the x-axis of a semilogarithmic graph, and an approximate curve was drawn. The time (T1/2) to reach half of the maximum breaking strength (BSmax) was determined, and the reciprocal was taken as the setting speed K (s−1).
Analysis of activation energies
The logarithmic value of the setting speed was plotted against the reciprocal value (1/T) of the absolute temperature (Arrhenius plot). The activation energy of each result was calculated using the following equation:
where R is the gas constant.
Electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 12.5% commercial polyacrylamide gels (e-PAGEL, ATTO Corporation, Tokyo, Japan) according to the method of Laemmli (Citation1970). The sample (0.2 g) and protein dissolution solution (3.75 µL) consisting of 20 mM Tris–HCl (pH 8.0)–2% SDS–8 M urea–2% B-mercaptoethanol were mixed and boiled for 2 min. The samples were then dissolved by stirring overnight. Prestained molecular weight markers (Bio-rad) were used as the standard. The gel was stained with Coomassie Brilliant blue (CBB) R-250 after electrophoresis for 1.5 h and then destained overnight with 30% methanol containing 10% acetic acid.
Results and discussions
Measurement of breaking strength
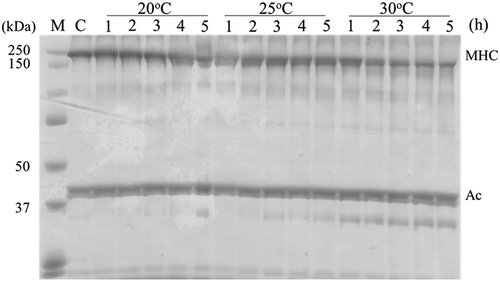
Fish gel samples were prepared from six kinds of frozen surimi, and changes in their breaking strength were examined during heat-induced setting at 20°C, 25°C, and 30°C. As an example, shows changes in the breaking strength of the FA-grade blue grenadier surimi along the trend lines. The breaking strength was measured similarly for the other five kinds of surimi, although the results are not presented here. The results showed that the breaking strength increased over the course of heating at all tested temperatures in surimi samples of all grades prepared from all species, resulting in the formation of a suwari gel. The breaking strengths of the FA-grade blue grenadier surimi reached their maximum at 24 h at all tested temperatures, measuring 1308, 1790, and 1464 gf at setting temperatures of 20°C, 25°C, and 30°C, respectively. The KA-grade blue grenadier demonstrated a breaking strength trend similar to that shown by the FA-grade blue grenadier, with the maximum values of 1140, 1322, and 1140 gf at 20°C, 25°C, and 30°C, respectively. The SA- and RA-grade Alaska pollock also showed similar setting behavior, with the maximum breaking strength at 48, 24, and 3 h at 20°C, 25°C, and 30°C, respectively. In contrast to the blue grenadier, for SA- and RA-grade Alaska pollock, large differences were observed in the time taken to reach the maximum breaking strength at different temperatures setting. In the threadfin bream, the time required to reach the maximum breaking strength was equivalent in all the SA- and KA-grade samples, measuring 24, 24, and 48 h at 20°C, 25°C, and 30°C, respectively, except for the KA-grade sample at 30°C. For all fish species, the maximum breaking strength of the suwari gel was greater for the surimi samples of higher grade when compared to the lower grade. The maximum breaking strength was approximately twice as great, or even greater, in the high-grade samples compared to that in the low-grade surimi samples prepared from Alaska pollock and threadfin bream. In contrast, regarding blue grenadier, the maximum breaking strength of their high-grade surimi samples was 1.1–1.3 times greater than that of their low-grade surimi samples. The breaking strength of the KA-grade threadfin bream sample heated at 30°C reached its maximum in a short period of time and had a significantly lower value than that of the sample heated at 25°C, which suggests the involvement of ‘modori,’ another unique phenomenon observed in seafood surimi. Thus, changes in the protein composition in the KA-grade threadfin bream gel samples heated to set at 20°C, 25°C, and 30°C for 1, 2, 3, 4 and 5 h were examined using SDS-PAGE (). The results showed no significant changes in the myosin monomers at 20°C and 25°C, and no bands indicating molecular weights smaller than myosin monomers were observed. In contrast, the bands representing the myosin monomers began to fade over time when heated at 30°C. No bands of large molecular weight, indicative of polymerization, were observed at 30°C, while new bands of molecular weight lower than myosin monomers were observed, suggesting that, in the KA-grade threadfin bream surimi sample heated at 30°C, the modori reaction occurred at the same time as the suwari reaction, resulting in the weakening of the gel structure. Possible causes include insufficient rinsing of the KA-grade threadfin bream samples in water, as well as the presence of proteases that function at relatively low temperatures. Furthermore, it suggested acidic acting cathepsins (B, H, L, L-Like) and alkaline acting heat-stable alkaline proteinases (HAP) acted and caused modori. The effects of the modori reaction were expected to be significant if the results at 30°C were included in further analyses of the KA-grade threadfin bream samples. Therefore, the suwari behavior was also examined at 23°C, instead of using the results at 30°C. At 23°C, the breaking strength reached its maximum, 357 gf, at 48 h.
Figure 1. Approximate breaking strength of FA-grade blue grenadier. ![]()
Figura 1. Resistencia de ruptura aproximada de merluza de cola de calidad FA.![]()
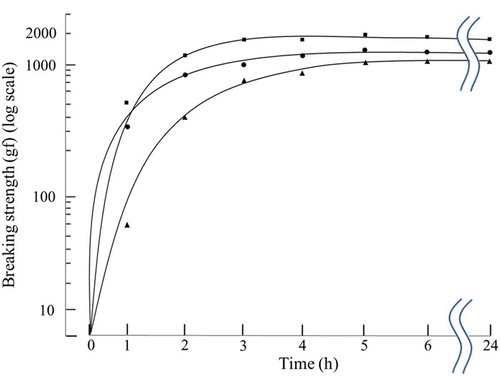
Figure 2. SDS-PAGE patterns of KA-grade threadfin bream during suwari setting at 20°C, 25°C, and 30°C.M: molecular weight marker; C: control; MHC: myosin heavy chain; Ac: actin; C: Heating time of 0; 1–5: The heating times were 1, 2, 3, 4, and 5 h, respectively.
Figura 2. Patrones SDS-PAGE de Baga tolu de calidad KA durante la solidificación suwari a temperaturas de 20, 25 y 30°C.M: Marcador de peso molecular; C: Control; MHC: cadena pesada de miosina; Ac: actina.C. Tiempo de calentamiento de 0 1–5. Los tiempos de calentamiento fueron 1, 2, 3, 4 y 5 h, respectivamente.
The time taken by surimi samples of different fish species to reach the maximum breaking strength was compared, which revealed that the threadfin bream surimi samples needed more time for the suwari reaction at all temperatures when compared to the surimi samples of other two species. This likely reflects the temperature dependence of the transglutaminase activity, attributable to the habitat temperature.
Significant differences were observed in how the breaking strength changed over time among the samples of different grades of Alaska pollock and threadfin bream but not for the blue grenadier, suggesting species-dependent differences in quality among different grades. The grade of frozen surimi is determined by factors such as the freshness of the fish meat used as ingredients and the frequency of rinsing in water (Ueki, Citation2017; Ueki, Wan, & Watabe, Citation2016). This suggests the effects of the reaction products associated with deterioration and the amount of water-soluble substances and proteins eliminated by rinsing. In addition, previous reports on the properties of two-step heated surimi gel indicate greater breaking strength for the surimi made from fish species such as threadfin bream that live in temperate waters when compared to the surimi made from fish species that live in cold waters (Kato et al., Citation1989; Kitakami et al., Citation2004; Konno et al., Citation2011; Lee et al., Citation1990a, Citation1990b; Numakura et al., Citation1987; Seki et al., Citation1998, Citation1990; Wan & Seki, Citation1992). In contrast, the results of the present study showed the surimi made from blue grenadier, a cold-water species, has the greatest breaking strength. As suwari gel displays behaviors different from those of a two-step heated gel, it is considered effective for representing the characteristics of the suwari reaction for determining the grade of frozen surimi. Comparison among the fish species indicated that the breaking strength did not increase in proportion to the length of the setting time, but varied greatly according to species.
Measurement of setting speed
From the changes in the breaking strength during the suwari reaction, 1/2BSmax, half of the breaking strength, and T1/2, the time required to reach 1/2BSmax, were obtained to determine the setting speed, which is the inverse of T1/2 (). When the setting speed was compared among different grades of surimi prepared using the same species, no clear differences in the maximum breaking strength were observed in the Alaska pollock and blue grenadier. Similarly, in the threadfin bream, no clear differences between the grades were observed in the setting speeds measured at 20°C and 25°C, excluding the results at 30°C, in which the effects of the modori reaction were observed.
Table 1. Analytical parameters of the six frozen surimi samples used in this study.
Tabla 1. Parámetros analíticos de las seis muestras de surimi congelado usadas en este estudio.
The setting speed was then compared among species. In the high-grade samples at 20°C and 25°C, the setting speed was fastest in the blue grenadier, followed by the Alaska pollock and threadfin bream. At 30°C, the setting speed was fastest in the Alaska Pollock, followed by the blue grenadier and threadfin bream. In the low-grade samples, for setting temperatures of 20°C, 25°C, and 30°C, the setting speed was fastest in the blue grenadier followed by the Alaska Pollock and threadfin bream, although the results lack accuracy because the results at 30°C need to be interpreted by considering the modori reaction in the threadfin bream.
The faster setting speeds observed in the low-grade samples are likely attributable to the inferior setting ability of the low-grade samples, resulting in less time required to reach the maximum breaking strength. Comparison of the setting speed among species in the surimi samples of equivalent grades revealed faster setting speeds for species living in cold waters, such as the Alaska pollock and blue grenadier, and slower setting speeds for species living in temperate waters, such as the threadfin bream. Overall, the usefulness of the setting speed in comparing the quality of products among grades and species was limited.
Analysis of activation energies
Analysis of the setting speed revealed interspecies differences in the samples of similar quality, but it failed to demonstrate clear differences among the grades. We, therefore, used the setting speeds to calculate the activation energies of the respective suwari reactions for comparison. The setting speeds obtained at the three temperatures were plotted on the y-axis of a semilogarithmic plot, and the inverse of the absolute temperatures was plotted on the x-axis, with a straight line drawn through each set of three points (). A straight line was successfully drawn through the three points representing the setting temperatures of 20°C, 25°C, and 30°C in the SA- and RA-grade Alaska pollock samples, FA- and KA-grade blue grenadier samples, and SA-grade threadfin bream samples. As discussed previously, the inclusion of the setting temperature of 30°C for the KA-grade threadfin bream samples results in the inability to draw a straight line connecting the point at 30°C with the points at 20°C and 25°C, which confirms the effects of the modori reaction at 30°C (figure not shown). Thus, in the KA-grade threadfin bream samples, the three setting temperatures of 20°C, 23°C, and 25°C were used to draw a straight line, using which the activation energy was determined ()).
Figure 3. Approximate line of setting speed.(a) Alaska pollock ![]()
Figura 3. Línea aproximada de la velocidad de solidificación.(a) Abadejo de Alaska ![]()
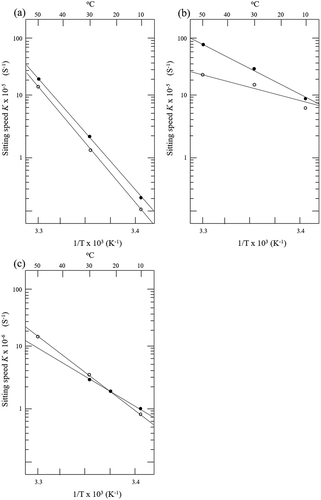
summarizes the activation energy values calculated for the respective frozen surimi samples. Activation energy is the minimum energy required to excite the reactants to convert from the ground state to the transition state. In the present study, the activation energy was calculated as a measure of setting ability. That is to say, the greater the value of the activation energy, the lower the expected ability of the surimi to set.
Table 2. Activation energies of the frozen surimi used in this study.
Tabla 2. Energías de activación del surimi congelado utilizado en este estudio.
When compared between the grades within each species, the activation energy of low-grade samples was greater by 1.3, 2.2, and 2.5 times that of the high-grade samples of Alaska pollock, blue grenadier, and threadfin bream, respectively, indicating clear inter-grade differences.
When compared among species, the activation energy in the high-grade samples was greatest in the Alaska Pollock, followed by the threadfin bream and blue grenadier, whereas, in the low-grade samples, the activation energy was greatest in the threadfin bream, followed by the Alaska Pollock and blue grenadier. These results indicate that, among the three species studied, the blue grenadier surimi has the greatest ability to set. In the present study, the values of food engineering properties, such as the setting speed and the activation energy, were calculated to examine whether they can be used as quality measures for frozen surimi. Although the setting speed did not reflect differences in the quality, the activation energy was demonstrated to be effective in presenting the inter-grade differences in quality in addition to the interspecies differences, as previously reported. The structure-setting ability is a critical factor in the processing of surimi, and the activation energy of the suwari reaction quantifies this ability as a value unique to the kind of surimi. The activation energy is thus expected to be useful in determining the grade of surimi. We intend to further investigate the potential of the activation energy as a measure of quality in surimi samples using a wider range of fish species of varying grades.
Conclusions
In the present study, the setting speed and the activation energy were determined by measuring the breaking strength during the heat-induced suwari reaction, in order to examine whether these measures effectively indicate the differences in quality among the different fish species and grades. The results suggest that the activation energy of the suwari reaction is an effective measure for representing the differences in quality. Moreover, the results of the KA-grade threadfin bream samples studied at 30°C indicate that an inappropriate setting temperature may induce the modori reaction, which adversely affects the calculation. The setting temperature needs to be specified so that the modori reaction can be prevented, although a temperature that is too low may interfere with the progression of the suwari reaction, which suggests the need to establish an appropriate setting temperature based on the species and grade.
The comparisons in the present study may have been relatively easy because they were between samples of the highest and lowest grades of surimi. Further investigation is required to demonstrate the effectiveness of this approach in a wider range of species and grades.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abe, Y., Yasunaga, K., Kitakami, S., Murakami, Y., Ota, T., & Arai, K. (1996). Quality of kamaboko gels from walleye pollack frozen surimis of different grades on applying additive containing TGase. Nippon Suisan Gakkaishi, 62(3), 439–445.
- Asano, Y., Itoh, Y., Suwansakornkul, P., & Obatake, A. (2003). Effects of manufacturing processes on myosin heavy chain degradation of shortfin lizardfish Saurida elongate meat paste at around 40°C. Nippon Suisan Gakkaishi, 69(3), 393–398.
- Benjakul, S., Chantarasuwan, C., & Visessanguan, W. (2003). Effect of medium temperature setting on gelling characteristics of surimi from some tropical fish. Food Chemistry, 82, 567–574.
- Hu, Y., Ji, R., Jiang, H., Zhang, J., Chen, J., & Ye, X. (2012). Participation of cathepsin L in modori phenomenon in carp (Cyprinus carpio) surimi gel. Food Chemistry, 134, 2014–2020.
- Ishida, T., Kubota, M., & Toyohara, H. (2013). Biochemical studies on the strong setting property of southern blue whiting. Nippon Suisan Gakkaishi, 79(3), 410–415.
- Itoh, Y., Maekawa, T., Suwansakornkul, P., & Obatake, A. (1995). Seasonal variation of gel-forming characteristics of three lizardfish species. Fisheries Science, 61(6), 942–947.
- Kato, N., Nakagawa, N., & Terui, S. (1989). Changes in myofibrillar protein in surimi during grounding with NaCl in relation to operating condition of a continuous mixer. Nippon Suisan Gakkaishi, 55(7), 1243–1251.
- Katoh, N., Hashimoto, A., Nozaki, H., & Arai, K. (1984). Effect of temperature on the rate for the setting of meat pastes from Alaska pollack, White croaker and Tilapia. Bulletin of the Japanese Society of Fisheries, 50(12), 2093–2101.
- Kinoshita, M. (1998). Studies on modori-inducing proteases. Nippon Suisan Gakkaishi, 64(4), 593–596.
- Kinoshita, M., Toyohara, H., & Shimizu, Y. (1990). Diverse distribution of four distinct types of Modori (gel-degradation)-inducing proteinases among fish species. Nippon Suisan Gakkaishi, 56(9), 1485–1492.
- Kitakami, S., Murakami, Y., Koseki, S., Abe, Y., Yasunaga, K., & Arai, K. (2004). Gel forming ability of walleye pollack salt-ground meat and its heating temperature dependence. Nippon Suisan Gakkaishi, 70(3), 354–364.
- Konno, K., Imamura, K., & Yuan, C. H. (2011). Myosin denaturation and cross-linking in Alaska pollack salted surimi during its preheating process as affected by temperature. Food Science and Technology Research, 17(5), 423–428.
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 277(5259), 680–685.
- Lee, N. H., Seki, N., Kato, N., Nakagawa, N., Terui, S., & Arai, K. (1990a). Gel forming ability and cross-linking ability of myosin heavy chain in salted meat paste from threadfin bream. Nippon Suisan Gakkaishi, 56(2), 329–336.
- Lee, N. H., Seki, N., Katoh, N., Nagasawa, N., Terui, S., & Arai, K. (1990b). Changes in myosin heavy chain and gel forming ability of salt-ground meat from Hoki. Nippon Suisan Gakkaishi, 56(12), 2093–2101.
- Liu, H., Yin, L., Zhang, N., Li, S., & Ma, C. (2008). Isolation of cathepsin B from the muscle of silver carp (Hypophthalmichthys molitrix) and comparison of cathepsins B and L actions on surimi gel softening. Food Chemistry, 110, 310–318.
- Makinodan, Y., Kitagawa, T., Toyohara, H., & Shimizu, Y. (1987). Participation of muscle alkaline proteinase in himodori of kamaboko. Nippon Suisan Gakkaishi, 53(1), 99–101.
- Makinodan, Y., Nakagawa, T., Ando, M., & Matsuno, S. (1996). Reinforcement of ashi (elasticity) of low salt kamaboko by setting and observation of kamaboko-structure by electron microscope. Nippon Suisan Gakkaishi, 62(4), 654–658.
- Matsuoka, Y., Wan, J., & Ushio, H. (2013). Thermal gelation properties of white croaker, walleye pollack and deep sea bonefish surimi after suwari treatment at various temperatures. Fisheries Science, 79(4), 715–724.
- Ni, S., Nozawa, H., & Seki, N. (1998). Effect of microbial transglutaminase on thermal gelation of carp actomyosin sol. Fisheries Science, 64(3), 434–438.
- Ni, S., Nozawa, H., & Seki, N. (1999). The combined effect of transglutaminase and protease inhibitors on the thermal gelation of actomyosin sol from carp and salmon muscles. Fisheries Science, 65(4), 606–612.
- Ni, S., Nozawa, H., & Seki, N. (2001). Effect of pH on the gelation of walleye pollack surimi and carp actomyosin pastes. Fisheries Science, 67, 920–927.
- Numakura, T., Seki, N., Kimura, I., Toyoda, K., Fujita, T., Takama, K., & Arai, K. (1985). Cross-linking reaction of myosin in the fish paste during setting. Nippon Suisan Gakkaishi, 51(9), 1559–1565.
- Numakura, T., Seki, N., Kimura, I., Toyoda, K., Fujita, T., Takama, K., & Arai, K. (1987). Changes in the SDS-gel filtration patterns on muscle proteins in salted fish meat paste during setting (suwari). Nippon Suisan Gakkaishi, 53(11), 2045–2049.
- Ohkubo, M., Osatomi, K., Hara, K., Ishihara, T., & Aranishi, F. (2005a). Myofibrillar proteolysis by myofibril-bound serine protease from white croaker. Argyrosomus Argentatus. Fisheries Science, 71, 1143–1148.
- Ohkubo, M., Osatomi, K., Hara, K., Ishihara, T., & Aranishi, F. (2005b). A novel type of myofibril-bound serine protease from white croaker (Argyrosomus argentatus). Comparative Biochemistry and Physiology, Part B: Biochemistry & Molecular Biology, 141, 231–236.
- Seki, N., Nozawa, H., & Ni, S. (1998). Effect of transglutaminase on the gelation of heat-denatured surimi. Fisheries Science, 64(6), 959–963.
- Seki, N., Uno, H., Lee, N. H., Kimura, I., Toyoda, K., Fujita, T., & Arai, K. (1990). Transglutaminase activity in Alaska pollack muscle and surimi, and its reaction with myosin B. Nippon Suisan Gakkaishi, 56(1), 125–132.
- Tsuchiya, T., & Sano, T. (1988). Gel-forming characteristics of fish meat proteins. Nippon Shokuhin Kogyou Gakkaishi, 35(5), 367–376.
- Tuji, M. (2003). Internationalization of frozen surimi and its subject industry. Gyogyou Keizai Kenkyu, 48(1), 19–41.
- Tzouros, N. E., & Arvanitoyannis, I. S. (2000). Implementation of hazard analysis critical control point (HACCP) system to the fish/seafood. Food Reviews International, 16(3), 273–325.
- Ueki, N. (2017). A case study in food industry ―Processing of high-quality surimi-based products referring to the traditional techniques of craftsperson―. Nippon Suisan Gakkaishi, 83(1), 90.
- Ueki, N., Wan, J., & Watabe, S. (2016). Deterioration of white croaker (Pennahia argentata) meat thermally-induced gel products caused by proteolytic enzymes in the contaminated intestine and kidney. Food Chemistry, 199, 416–422.
- Wan, J., & Seki, N. (1992). Effects of salts on transglutaminase-mediated cross-linking of myosin in suwari gel from Walleye pollack. Nippon Suisan Gakkaishi, 58(11), 2181–2187.
