 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Jicama roots have been studied as an alternative starch source. However, reports about the extraction yield are scarce, as well as publications about the use of pretreatment techniques for this material. Hence, the aim of this study was to analyze the effect of sonication on the starch yield as well as on the granule structure. Ultrasound was observed to have a positive effect on the starch yield, with the largest value obtained at a sonication time of 10 min (24.76 ± 2.4%). This represents a 46.9% increase over a non-treated sample (16.86 ± 0.9%), and the starch granule structure was unaffected, as confirmed by microscopy and an X-ray diffraction analysis The imperviousness of the starch granule to the sonication process is attributed to the protective effect of soluble fiber and a change in viscosity caused by the material released from parenchyma cells. More studies are required in this area.
RESUMEN
Las raíces de jícama han sido estudiadas como una fuente alternativa de almidón. Sin embargo, los datos sobre el rendimiento de extracción apenas se informan y las publicaciones sobre el uso de técnicas de pretratamiento en este material son pocas. Por lo tanto, el objetivo de este trabajo fue analizar el efecto de la sonicación sobre el rendimiento del almidón así como sobre la estructura del gránulo. Se observó que el ultrasonido tuvo un efecto positivo sobre el rendimiento de almidón, obteniendo el mayor valor a un tiempo de sonicación de 10 min (24,76 ± 2.4%), lo que representa un incremento del 46.9% con respecto a la muestra no tratada (16,86 ± 0.9%), sin afectar la estructura del gránulo de almidón, como se observó por microscopía y análisis de XRD. La resistencia del gránulo de almidón al proceso de sonicación se atribuye a un efecto protector de la fibra soluble y al cambio en la viscosidad provocada por el material liberado por las células de parénquima. Se requieren más estudios en esta área.
PALABRAS CLAVE:
Introduction
Currently, there is a widespread interest in biological biodegradable materials such as starch (Fakhouria, Martellia, Caonc, Velasco, & Innocentini Mei, Citation2015; Jiménez, Fabra, Talens, & Chiralt, Citation2012; Olivato, Müller, Carvalho, Yamashita, & Grossmann, Citation2014), pectin (Álvarez-Pérez, Montañez, Aguilar, & Rojas, Citation2015; Gaona-Sánchez et al., Citation2016), chitosan (Escamilla-García et al., Citation2013), zein (Gaona-Sánchez et al., Citation2015) and their combinations because they have been considered as potential resources to replace petroleum-based commodities such as plastics (Jiménez et al., Citation2012). Among these materials, starch is one of the most abundant biopolymers in nature and is commonly extracted from cereals (e.g. rice, wheat and maize) and tubers (e.g. potatoes and cassava) and is normally used in many food and nonfood applications.
Table 1. Experimental design and results.
Tabla 1. Diseño experimental y resultados.
Many other sources of starch such as sweet potato, sago, mung bean, jicama tubers, acorns, arrowroot, arracacha, bananas, yams and others are found worldwide. Among these, Jicama (Pachyrhizus erosus) root has been considered as a potential raw material for starch (Mali, Grossmann, García, Martino, & Zaritzky, Citation2005; Santana & Meireles, Citation2014) due to its chemical composition (Amaya-Llano, Martínez-Bustos, Martínez-Alegría, & Zazueta-Morales, Citation2011; Forsyth & Shewry, Citation2002; Nursandi, Machmudi, Santoso, & Indratmi, Citation2017; Stevenson, Jane, & Inglett, Citation2007). However, the data reported about the starch content differ (20–50 g/100 g dry basis) depending on the study (Ramos-de-la-Peña, Renard, Wicker, & Contreras-Esquivel, Citation2013; Santayana et al., Citation2014). In addition, only a few starch extraction yield percentages have been published, and their values range from 2.5% to 68% (db) (Flores-Farías, Citation2004; Galván-Mendoza, Citation2002; Mélo, Krieger, & Montenegro, Citation1994). These differences could be related to the jicama structure or to the extraction procedure, but no explanation has been reported.
On the other hand, publications indicate that pretreatments such as ultrasound and freezing are useful tools to improve the extraction yields of starch from different sources. Sit, Deka, and Misra (Citation2014) analyzed the effect of combining ultrasound and enzymatic pretreatments using cellulase and xylanase to obtain panchamukhi taro starch and obtained the best yield (20.81%) by sonicating the sample for 20 min. In another study, Park, Bean, Wilson, and Schober (Citation2006) proved that ultrasound in combination with buffers was an effective method for rapidly isolating sorghum starch, while Pinyo, Luangpituksa, Suphantharika, Hansawasdi, and Wongsagonsup (Citation2017) found that the ultrasonic pretreatment increased the yield extraction by more than 20% compared to a non-treated sample. However, none of these methods have been applied to extract starch from jicama tubers.
Freezing is another technology that could be employed to improve the effect of ultrasound pretreatment on starch extraction. The formation of large ice crystals in foods with a high-water content can cause mechanical damage, facilitating the release of material when the cells are sonicated, damaging the cell wall structure (Xin, Zhang, & Adhikari, Citation2014). Therefore, the aim of this study was to analyze the effect of different sonication pretreatments on the starch extraction yield and its relationship with the changes in the granule structure, evaluated by means of microscopic (confocal laser scanning microscopy [CLSM], scanning electron microscope [SEM], light and polarized light) and spectroscopic techniques (X-ray diffraction analysis [XRD]), as well as thermal properties measured by differential scanning calorimetry (DSC), to find an explanation that helps to clarify the reason for the differences in the reported values of the starch extraction yields.
Materials and methods
Jicama starch extraction
Starch was extracted from jicama tubers (P. erosus L. Urban, variety “San Juan”, Nayarit, Mexico), based on the methodology proposed by Novelo-Cen and Betancur-Ancona (Citation2005) with some modifications. Briefly, a jicama tuber was rinsed, cut homogeneously (1 × 1 × 1 cm3), and subsequently soaked for 0, 15 or 30 min, depending on the experimental design, in a 1500 ppm sodium bisulfite solution (1:3, tuber-solution ratio, w/v). Subsequently, the jicama cubes were placed in a plastic bag and immersed in a dry ice/acetone bath for 10 min, then stored at −24°C in a horizontal freezer (Kenmore, 12,512, USA) for 24 or 48 h (based on the experimental design). A sample that was not frozen was also obtained. After the required freezing storage time was reached, the samples were allowed to thaw at room temperature (12 h, 25°C) and then were ground (1:3 jicama-water ratio w/v) in a blender at maximum speed for 60 s (Oster® Blender with 10 Speeds, 450 W). The resulting suspension was processed in an ultrasonic bath (Cole-Parmer 08895–69, 40 kHz, USA) for different times depending on the experimental procedure applied. The sample was filtered (plastic mesh, 250 μm) and centrifuged (Dynamica Velocity 18R, Metrix®, UK) at 4500×g for 10 min at 4°C. The supernatant was removed, and the remaining starch precipitate was dried for 24 h at 50°C. The starch extraction yield (db) obtained with each experimental design condition was calculated.
Experimental procedures
Two different experimental procedures were carried out: one to select the best process conditions (freezing and sonication) and the other to evaluate the effect of the selected variables on the starch yield extraction and its relationship with the changes in the granule structure.
In the first experimental procedure, a three-factor Box-Behnken design with five central point repetitions and one response (i.e. the yield extraction percentage) was employed. A total of 17 samples were tested. The soaking (0, 15 and 30 min), freezing (0, 24 and 48 h) and sonication (0, 15 and 30 min) times were the factors studied. The conditions were selected based on Ramírez-Miranda (Citation2017) (soaking) and Wang and Wang (Citation2004) (sonication). The freezing times were chosen based on the shortest possible times that could have an effect. The data were analyzed using a multiple regression analysis, and the effect of each variable and the regression analysis were reported.
Based on the results of the first experiment, a single factor was selected for analysis (sonication time), and the other factors (i.e. soaking and freezing) as well as the starch extraction methodology remained constant. Samples taken at the different pretreatment times (0, 10, 20, 30, 45, 60 and 90 min) were evaluated for structural changes by microscopic (CLSM, SEM, light and polarized light) and spectroscopic techniques (XRD), and their thermal properties were measured by DSC.
Confocal laser scanning microscopy
Starch was spread on a slide and stained with a 0.15% Rhodamine B (83,689-1G Sigma) solution in water. The excess dye was removed with deionized water, and the sample was dried (Binder, 53 FD, Germany) at 30ºC for 24 h. To carry out the observations, the dyed starch sample was suspended in a drop of 50% glycerol solution and observed in a confocal-multiphoton microscope (LSM 710 NLO Carl Zeiss, Wurttemberg, Germany) with 63x lenses, using a wavelength of 518 nm for the excitation of Rhodamine B and a maximum emission at 568 nm (Savary, Handschin, Conde-Petit, Cayot, & Doublier, Citation2008). The images were acquired in RGB color.
The jicama tissues were prepared as reported by Ramírez-Miranda et al. (Citation2017).
Scanning electron microscopy
A SEM (Jeol JSM7800F, MA, USA) was used to obtain the micrographs. The starch was spread on double-sided tape and covered with gold film (20 s, 10–1 mTorr, Denton Vacuum Desk II), as proposed by Ulbrich, Natan, and Flӧter (Citation2014), and then different zones were subsequently observed under various magnifications (100, 500 and 1000×) for each sample. The jicama tissues were prepared as reported by Ramírez-Miranda et al. (Citation2017).
Optical and polarized light microscopy
The samples were placed on a slide, and a drop of 50% glycerol solution was added; then, different zones on the slide were observed in a Nikon Eclipse Ti at 60X with a polarized light filter (Acevedo-Agama et al., Citation2004).
Starch crystallinity (XRD)
An X-ray diffractometer (Rigaku Miniflex 600, Tokyo, Japan) was used. The starch samples were placed in the sample holder, and the diffraction patterns were obtained (40 kV, 15 mA, CuK, λ = 0.154 nm, 3°–60° (2θ°)). The degree of crystallinity (Equation 1) was evaluated (Edhirej, Sapuan, Jawaid, & Zahari, Citation2016), and all measurements were performed at least in duplicate.
where:
CR = % relative crystallinity
Ac = crystalline area
Aa = amorphous area
Differential scanning calorimetry
The thermal properties of the extracted starch were evaluated using a differential scanning calorimeter (Q2000, TA Instruments, New Castle, DE, USA) according to Stevenson et al. (Citation2007). Approximately 3 mg of jicama starch with a moisture content of 9% was weighed into an aluminum capsule, and 9 μL of distilled water was added; then, the capsule was hermetically sealed. The samples were equilibrated for 24 h at room temperature and heated at a rate of 10°C/min over a temperature range of 20–120°C. The TA Instruments Universal Analysis 2000 Version 4.5© software was used to analyze the data. The onset (To), peak (Tp) and end (Te) temperatures and the gelatinization enthalpy (ΔHg) were recorded.
Starch damage and total starch
The total starch content and starch damage were respectively determined using the K-TSTA and the K-SDAM kits from Megazyme (Megazyme International Ireland Ltd., Wicklow, Ireland) following the manufacturer’s procedures.
Neutral detergent fiber (NDF)
This analysis was carried out according to the AOAC International (Citation2002)/ISO 16472:2005 method using a Fibertec™ system (Hilleroed, Denmark). Briefly, 1 g of celite, 0.5 g of sample and 0.5 g of sodium sulfite were weighed in a crucible and placed into the apparatus. Then 50 mL of an acid detergent solution at room temperature and 50 µL of heat-stable alpha amylase (Sigma A3306, 20,000–60,000 Un/mL) were added. The mixture was heated for 1 h at 100°C and rinsed with hot water and finally with acetone to remove lipids and pigments. The samples were dried in a forced air oven at 105°C for at least 5 h. The ash content for all samples was determined.
Data analysis
All experiments were carried out at least in triplicate, and the average and standard deviation values reported. The data were statistically analyzed with the SigmaPlot V.12.0 software package (Systat Software Inc, San Jose, CA, USA). Response surface plots and data analysis were obtained using the Design Expert v9.0s software (Stat-Ease Inc., USA). Values of p < 0.05 were considered significant.
Results and discussion
Effect of freezing and sonication pretreatments on the extraction yield of jicama starch
The effect of soaking, freezing and sonication times () are shown in . It can be seen that the starch yield increased as the sonication or freezing times increased, and the largest extraction values were seen at either the longest freezing time (35.6%) without sonication or at the longest sonication time (30.0%) without freezing. It is important to mention that the starch yield values were high and almost constant (27–30%) at any sonication time when not frozen. These results could be related to an increase in temperature that decreased the gas solubility and reduced the number of cavitation bubbles up to a limit at which the extraction yield could not increase (Chemat et al., Citation2017). Similar results have been reported by Sit, Misra, and Deka (Citation2014) for taro (Colocasia esculenta) and by Pinyo et al. (Citation2017) for sago starch.
Figure 1. Effect of soaking, freezing and sonication times on the yield extraction of jicama starch. (a) 0 min; (b) 15 min; (c) 30 min soaking times.
Figura 1. Efecto de los tiempos de remojo, congelación y sonicación en el rendimiento de extracción de almidón de jícama. (a): 0 min; (b): 15 min; (c): 30 min de remojo.

No significant effect for soaking time was obtained (p > 0.05).
It can also be seen from these results that when freezing and ultrasound were simultaneously employed, a decreased starch extraction yield was noticed. It is known that the freezing process causes cell mechanical damage (Edhirej et al., Citation2016; Xin et al., Citation2014) that could result in the breakage of vacuoles filled with mucilaginous contents (Pruyn, Citation2002) that have been related to soluble fiber in jicama (Park, Lee, & Han, Citation2016), thus increasing the viscosity of the sample. In parallel, the sonication process, as previously mentioned, could reduce the number of cavitation bubbles resulting in an antagonistic effect when both pretreatments were combined.
The ANOVA results showed that data could be fitted to a quadratic model, with a determination coefficient (R2) of 0.841, and that freezing and soaking did not have a significant effect on the response (p = 0.8921, p = 0.8202), while sonication, as a model term, did. Regarding process variable interactions, the combined effect of freezing and ultrasound produced a statistically negative effect (p = 0.05), while the soaking alone or in combination with any of the variables did not statistically change the response (p > 0.05). Based on this information, it was decided to continue studying only the effect of ultrasound while increasing the upper limit of the response to 90 min.
Effect of sonication on the extraction yield of jicama starch and on the granule structure
The effect of sonication on the extracted starch yield is shown in . A yield increase in the treated samples compared to the control (p < 0.05) was observed during the first 10 min of sonication, representing an increase of 46.9%, demonstrating that an ultrasound pretreatment helped release the starch from the plant material. Pinyo et al. (Citation2017) reported similar results and established that a 7 min pretreatment was enough to improve the extraction yield of sago starch.
Figure 2. Effect of sonication on jicama starch yield. Data are the average value ± standard deviation. Bars tagged with a different letter are significantly different (p ˂ 0.05).
Figura 2. Efecto de la sonicación sobre el rendimiento de almidón de jícama. Los datos son el valor promedio ± desviación estándar. Las barras etiquetadas con una letra diferente son significativamente diferentes (p ˂ 0,05).
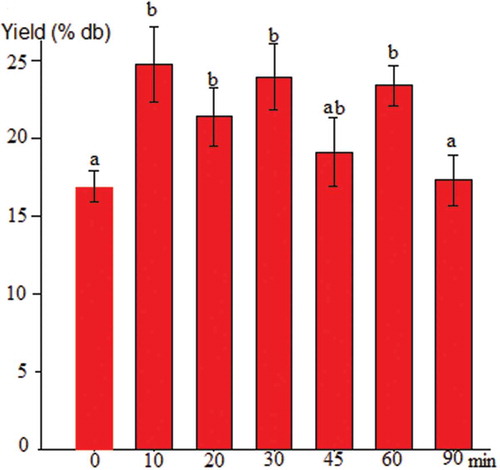
The decrease in the extraction yield observed at 90 min of sonication could be related to the loss of cavitation bubbles (Chemat et al., Citation2017), resulting in a less efficient extraction process or a larger concentration of non-starchy materials extracted from the fibrous matrix, in which the smallest starch granules could become trapped in suspension after release, so they could not be separated. More studies in this area are required.
Structural changes due to sonication evaluated by CLSM
shows the stained jicama starch granules after a sample is processed at different sonication times.
Figure 3. Changes on the starch granules as a result of sonication duration. Laser scanning confocal micrographs. (a) Control sample; (b–g) sonicated samples; (b) 10 min sonication; (c) 20 min; (d) 30 min; (e) 45 min; (f) 60 min; (g) 90 min.
Figura 3. Cambios en los gránulos de almidón como resultado del tiempo de sonicación. Microfotografía de barrido láser confocal. (a): muestra de control; B-G: muestras sonicadas: (b) 10 minutos; (c) 20 min; (d) 30 min; (e) 45 min; (f) 60 minutos; (g) 90 min de sonicación.
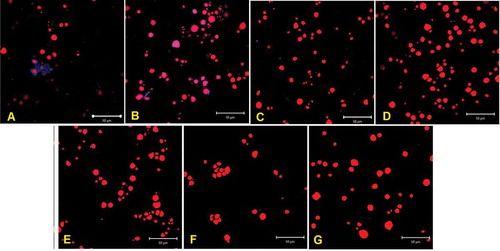
The starch granules (red dots) extracted from the control sample () have a layer (blue dots), probably composed of cellulose or soluble fiber (i.e. pectin or fructan), as expected from the calcofluor white staining. It has been reported that cellulose fiber is part of the cell wall of the parenchyma cells (Ramos-de-la-Peña et al., Citation2013) and is reinforced by a matrix of hemicellulose, pectin and glycoprotein (Gibson, Citation2012) that surrounds either the compound amyloplasts (Ramírez-Miranda et al., Citation2017) or vacuoles filled with mucilaginous contents (Pruyn, Citation2002).
These micrographs also show that as the duration of sonication increased (), the calcofluor signal disappeared, and changes in the concentration of fiber and starch granules were almost negligible after 10 min. This suggests that the main effect of sonication occurs at the beginning of the process (≤10 min), when the outer layers of the parenchyma cells are disrupted, liberating the contents, mainly starch and possibly fructans or pectin, without damaging the granules (). These results are in agreement with the extraction yield data previously shown () and with the assumptions previously mentioned (Chemat et al., Citation2017), related to the loss of cavitation bubbles and to the entrapment of small starch granules in the jicama bagasse.
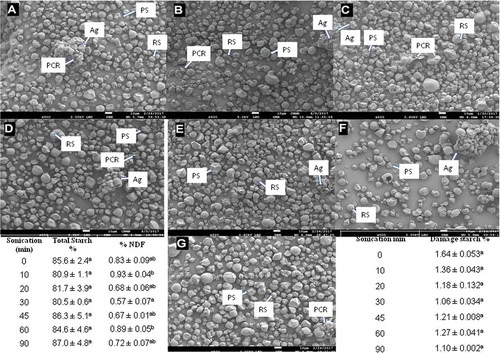
Figure 4. Changes on the morphological structure of jicama starch granules and in their neutral dietary fiber and total starch contents (NDF, TS), and in the damaged starch percentage as a result of sonication duration. Scanning electron micrographs. (a) Control sample; B–G: sonicated samples: (b) 10 min; (c) 20 min; (d) 30 min; (e) 45 min; (f) 60 min; (g) 90 min. RS: round starch granules; PS: polygonal starch granules; Ag: agglomerates starch granules; PCR: parenchyma cell wall residues. Data are the average value ± standard deviation. Values followed by a different letter in the same column are significantly different (p ˂ 0.05).
Figura 4. Cambios en la morfología de los gránulos de almidón de jícama, en el contenido de fibra dietética neutra, almidón total (NDF, TS) y en el porcentaje de almidón dañado como resultado del tiempo de sonicación. Micrografías electrónicas de barrido. (a): muestra control; B-G: muestras sonicadas: (b) 10 min; (c) 20 min; (d) 30 min; (e) 45 min; (f) 60 min; (g) 90 min. RS: gránulos de almidón redondo; PS: gránulos de almidón poligonal; Ag: aglomerado de los gránulos de almidón; PCR: residuos de la pared celular del parénquima. Los datos son el valor promedio ± desviación estándar. Los valores seguidos por una letra diferente en la misma columna son significativamente diferentes (p ˂ 0,05).
Structural changes due to sonication evaluated by microscopy techniques
shows the SEM micrographs of the jicama starch granules obtained with different sonication durations.
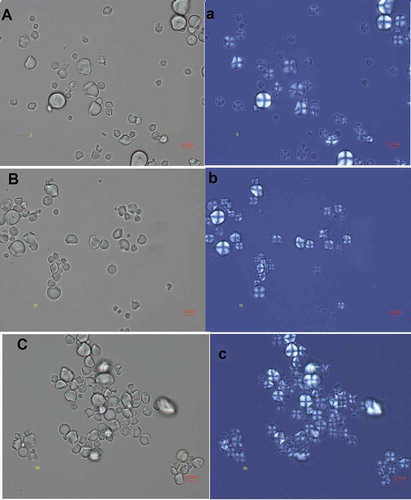
In general, all micrographs show two types of granules, polyhedral (PS) and round (RS), which are in much lower proportions, and some agglomerates (Ag) and parenchyma cell wall residues (PCR). No specific effect due to sonication duration was noted, neither for starch damage nor the presence of insoluble fiber, while the images obtained with optical and polarized light microscopies () show that the crystalline structure and morphology of the starch granule are not apparently changed, regardless of the time of exposure to the treatment. The birefringence is also maintained.
Figure 5. Optical (A–C) and polarized light (a–c) micrographs of sonicated jicama starch during different times. A, (a) control sample; B-C, b-c: sonicated samples; B, (b) 10 min; C, (c) 90 min.
Figura 5. Micrografías ópticas (A-C) y luz polarizada (a-c) de almidón de jícama sonicados a diferentes tiempos. A, (a): muestra de control; B-C, b-c: muestras sonicadas: B, (b) 10 min; C, (c) 90 min.
X-ray diffraction
In , the diffraction bands of all samples showed a characteristic type C pattern, presenting strong peaks at 17° and 23° (2θ) angles and smaller peaks at 5.6° and 15° (2θ) but related to a polymorphism CA type (Cai, Cai, Man, Zhou, & Wei, Citation2014) as indicated by the presence of a shoulder at 18° (2θ). These results are similar to those reported for jicama native starch by Ramírez-Miranda et al. (Citation2017). A statistical analysis showed no significant difference (p > 0.05) between the sonicated samples in the crystallinity percentage, which varied from 26 ± 1.4% to 32 ± 1.7%. These values are in the range of those reported by Stevenson et al. (Citation2007) and Ramírez-Miranda et al. (Citation2017). The differences could be related to small variations in the moisture content of the samples (8–9%), as seen by Cheetham and Tao (Citation1998), who mention that the hydration of the granules can lead to an increase in crystallinity, without changing the original crystal type.
Figure 6. X-ray diffraction patterns of jicama starch sonicated for different times. (a) control sample; b-g: sonicated samples; (b) 10 min; (c) 20 min; (d) 30 min; (e) 45 min; (f) 60 min; (g) 90 min.
Figura 6. Patrones de difracción de rayos X del almidón de jícama sonicados a diferentes tiempos. (a): muestra de control; b-g: muestras sonicadas; (b) 10 min; (c) 20 minutos; (d) 30 min; e) 45 min; (f) 60 minutos; (g) 90 min.
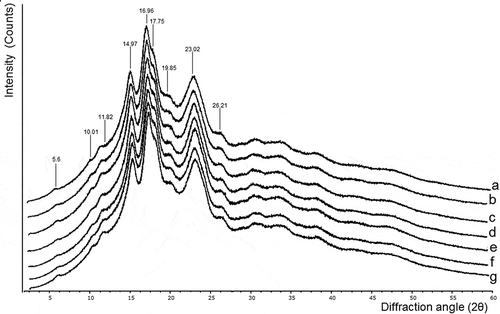
These results also confirmed the micrograph information previously discussed (SEM, confocal laser scanning microscopy [CLSM], optical and polarized), in which no damage of the granules was noticed, corroborating that sonication for the range of durations studied did not modify the crystalline granule structure.
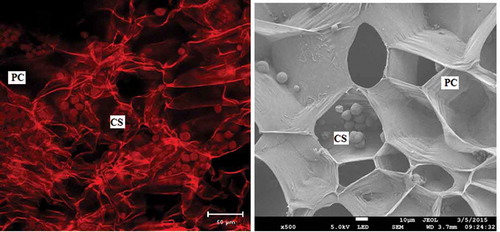
These results could be explained by considering that the jicama is formed by compound starch granules (CS) that are not fused (), as those from rice are, and can be easily separated from the matrix (Matsushima, Maekawa, & Sakamoto, Citation2015) during the first 10 min of the ultrasound pretreatment, at which time maximum extraction occurs, and this value remains constant up to 60 min of sonication. During this process, the air or mucilage solution inside the vacuoles could have been released (Pruyn, Citation2002), and some of the free starch granules could become entrapped, decreasing the extraction yield. At the same time, a protective effect may occur because the damaged starch percentage did not change. This hypothesis is supported by the analysis carried out on the jicama bagasse obtained from the 10 min sonicated sample, for which 16.1% starch was found.
Figure 7. SEM and confocal micrographs of fresh jicama showing the parenchima cells (PC), the native compound starch granules (CS) and some empty vacuoles.
Figura 7. SEM y micrografías confocales de jícama fresca que muestran las células del parenchima (PC), los gránulos de almidón nativo (CS) y algunas vacuolas vacías.
Jicama starch characterization by DSC
The changes in the onset (T0), end (Te) and gelatinization (Tp) temperatures, as well as the enthalpy of gelatinization (ΔHg) as a result of the sonication pretreatment, were minimal, varying from 59.1 to 60.5°C for T0, from 72.9 to 74.2°C for Te, from 65.1 to 67.2°C for Tp and from 1.7 to 2.5 J/g for ΔHg. This last parameter () showed the largest significant differences (p < 0.05), followed by the gelatinization temperature ().
Figure 8. Thermal properties of jicama starch extracted by applying different sonication times. (a) ΔHg: enthalpy of gelatinization (J/g, wb); (b) TG; gelatinization temperature (°C). Data are the average value ± standard deviation. Bars tagged with a different letter are significantly different (p ˂ 0.05).
Figura 8. Propiedades térmicas del almidón de jícama extraídas aplicando diferentes tiempos de sonicación. (a): ΔH; entalpía de gelatinización (J/g, bs); (b): TG; temperatura de gelatinización (°C). Los datos son el valor promedio ± desviación estándar. Las barras etiquetadas con una letra diferente son significativamente diferentes (p ˂ 0,05).
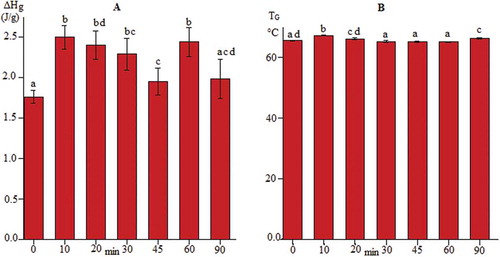
Sriroth, Chollakup, Chotineeranat, Piyachomkwan, and Oates (Citation2000) reported similar results and mentioned that starch granules subjected to sonication had properties very similar to the native starch (Cassava) as measured with a Rapid Visco Analyzer and a Differential Scanning Calorimeter. The differences in the ΔHg values between samples could be ascribed to the presence of other components such as soluble (e.g. fructans or pectin) or insoluble fiber (parenchyma cell wall residue or protein), but in small proportions, as seen in the micrographs ( and ). To support this supposition, the total starch content of the samples was evaluated (), and in all cases, it fell in a range from 85 ± 5.0%, with 15 ± 5.0% coming from other materials. Additionally, the chemical composition of the control and the 10 min sonication samples was determined, and their respective compositions were 1.29% and 2.06% for protein, 1.29% and 2.96% for fiber, and 0.24% and 0.19% for lipids. These differences could change the thermal response. Kohyama and Nishinari (Citation1991) reported that the gelatinization peak temperature (Tp) and the gelatinization enthalpy increased with an increasing sugar concentration, which could be related to the soluble fiber content.
Conclusions
The use of an ultrasound pretreatment in a jicama starch extraction process promoted an increase in the starch yield. The maximum was seen at 10 min of sonication, and the response did not change at longer times. Additionally, the ultrasound pretreatment did not modify the starch granule structure, which could be considered as a protective effect due to the presence of fiber, the loss of cavitation bubbles and a change in the viscosity caused by material released during the sonication process. More studies are required in this area.
Acknowledgments
Lucía B. González-Lemus wishes to express their gratitude to CONACyT and BEIFI-IPN for the scholarship provided.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AOAC International. (2002). Official methods of analysis of AOAC International. 17th ed. Washington, D.C. EUA: Association of Official Analytical Chemists. Inc.
- Acevedo-Agama, E., Ottenhof, M. A., Farhat, I. A., Paredes-López, O., Ortíz Cereceres, J., & Bello Pérez, L. (2004). Efecto de la nixtamalización sobre las características moleculares del almidón de variedades pigmentadas de maíz. Interciencia, 29, 643–649.
- Álvarez-Pérez, O. B., Montañez, J., Aguilar, C. N., & Rojas, R. (2015). Pectin-candelilla wax: An alternative mixture for edible films. Journal of Microbiology, Biotechnology and Food Sciences, 5, 167–171.
- Amaya-Llano, S. L., Martínez-Bustos, F., Martínez-Alegría, A. L., & Zazueta-Morales, J. J. (2011). Comparative studies on some physico-chemical, thermal, morphological, and pasting properties of acid-thinned jicama and maize starches. Food Bioprocess Technology, 4, 48–60.
- Cai, J., Cai, C., Man, J., Zhou, W., & Wei, C. (2014). Structural and functional properties of C-type starches. Carbohydrate Polymers, 101, 289–300.
- Cheetham, N. W. H., & Tao, L. (1998). Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydrate Polymers, 36, 277–284.
- Chemat, F., Rombaut, N., Sicaire, A. G., Meullemiestre, A., Fabiano-Tixier, A.-S., & Abert-Vian, M. (2017). Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry, 34, 540–560.
- Edhirej, A., Sapuan, S. M., Jawaid, M., & Zahari, N. I. (2016). Effect of various plasticizers and concentration on the physical, thermal, mechanical, and structural properties of cassava-starch-based films. Starch/Stärke, 68, 1–11.
- Escamilla-García, M., Calderón-Domínguez, G., Chanona-Pérez, J. J., Farrera-Rebollo, R. R., Andraca-Adame, J. A., Arzate-Vázquez, I., & Moreno-Ruiz, L. A. (2013). Physical and structural characterisation of zein and chitosan edible films using nanotechnology tools. International Journal of Biological Macromolecules, 61, 196–203.
- Fakhouria, F. M., Martellia, S. M., Caonc, T., Velasco, J. I., & Innocentini Mei, L. H. (2015). Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biology and Technology, (2015(109), 57–64.
- Flores-Farías, S. (2004). Obtención de almidón con tamaño de partícula reducido mediante pulverizado mezclado con alta energía ( Master’s thesis), CICATA-IPN, Cd. de México, México.
- Forsyth, J. L., & Shewry, P. R. (2002). Characterization of the major proteins of tubers of yam bean (Pachyrhizus ahipa). Journal of Agricultural and Food Chemistry, (2002(50), 1939−1944.
- Galván-Mendoza, R. (2002). Estudio para el aprovechamiento de jícama no comercial ( Bachelor degree thesis). Universidad Autónoma de Querétaro. Facultad de Química, Santiago de Querétaro, Querétaro, México.
- Gaona-Sánchez, V. A., Calderón-Domínguez, G., Morales-Sánchez, E., Chanona-Pérez, J. J., Arzate-Vázquez, I., & Terrés-Rojas, E. (2016). Pectin-based films produced by electrospraying. J Journal of Applied Polymer Science, 43779, 1–10.
- Gaona-Sánchez, V. A., Calderón-Domínguez, G., Morales-Sánchez, E., Chanona-Pérez, J. J., Velázquez-De La Cruz, G., Méndez-Méndez, J. V., & Farrera-Rebollo, R. R. (2015). Preparation and characterisation of zein films obtained by electrospraying. Food Hydrocolloids, 49, 1–10.
- Gibson, L. J. (2012). The hierarchical structure and mechanics of plant materials. Journal of the Royal Society, Interface/The Royal Society, 9, 2749–2766.
- Jiménez, A., Fabra, M. F., Talens, P., & Chiralt, A. (2012). Edible and biodegradable starch films: A review. Food and Bioprocess Technology, 5, 2058–2076.
- Kohyama, K., & Nishinari, K. (1991). Effect of soluble sugars on gelatinization and retrogradation of sweet potato starch. Journal of Agricultural and Food Chemistry,39, 1406–1410. doi:10.1021/jf00008a010
- Mali, S., Grossmann, M. V. E., García, M. A., Martino, M. N., & Zaritzky, N. E. (2005). Mechanical and thermal properties of yam starch films. Food Hydrocolloids, (2005(19), 157–164.
- Matsushima, R., Maekawa, M., & Sakamoto, W. (2015). Geometrical formation of compound starch grains in rice implements Voronoi diagram. Plant and Cell Physiology, 56(1), 2150–2157.
- Mélo, E. A., Krieger, N., & Montenegro, T. L. (1994). Physicochemical properties of Jacatupé (Pachyrhizus erosus L. Urban) starch. Starch/Stärke, 7, 245–247.
- Novelo-Cen, L., & Betancur-Ancona, D. (2005). Chemical and functional properties of phaseolus lunatus and manihot esculenta starch blends. Starch/Stärke, 57, 431–441.
- Nursandi, F., Machmudi, M., Santoso, U., & Indratmi, D. (2017). Properties of different aged jicama (Pachyrhizus Erozus) plants. IOP Conference Series: Earth and Environmental Science, Qingdao, China, 26–29, Volume 77.
- Olivato, J. B., Müller, C. M. O., Carvalho, G. M., Yamashita, F., & Grossmann, M. V. E. (2014). Physical and structural characterisation of starch/polyester blends with tartaric acid. Materials Science and Engineering: C, 39, 35–39.
- Park, C. J., Lee, H. A., & Han, J. S. (2016). Jicama (Pachyrhizus erosus) extract increases insulin sensitivity and regulates hepatic glucose in C57BL/Ksj-db/db mice. Journal of Clinical Biochemistry and Nutrition, 58(1), 56–63.
- Park, S. H., Bean, S. R., Wilson, J. D., & Schober, T. J. (2006). Rapid isolation of sorghum and other cereal starches using sonication. Cereal Chemistry Journal, 83, 611–616.
- Pinyo, J., Luangpituksa, P., Suphantharika, M., Hansawasdi, C., & Wongsagonsup, R. (2017). Improvement of sago starch extraction process using various pretreatment techniques and their pretreatment combination. Starch/Stärke, 1700005, 1–10.
- Pruyn, M. L. (2002). Parenchyma. In J. Wiley & L. T. D. Sons (eds), Encyclopedia of life sciences. New York, USA.
- Ramírez-Miranda, M. (2017). Caracterización estructural térmica y funcional de almidón de jícama nativo y procesado térmicamente y su posible utilización en productos libres de gluten (Doctoral dissertation). Escuela Nacional de Ciencias Biológicas. Instituto Politécnico Nacional. Cd. de México, México.
- Ramírez-Miranda, M., Ribotta, P. D., Silva-González, A. Z. Z., Salgado-Cruz, M. P., Andraca-Adame, J. A., Chanona-Pérez, J. J., & Calderón-Domínguez, G. (2017). Morphometric and crystallinity changes on jicama starch (Pachyrizus erosus) during gelatinization and their relation with in vitro glycemic index. Starch/Stärke, 1600281, 1–11.
- Ramos-de-la-Peña, A. M., Renard, C. M. G. C., Wicker, L., & Contreras-Esquivel, J. C. (2013). Advances and perspectives of Pachyrhizus spp. in food science and biotechnology. Trends in Food Science & Technology, 29, 44–54.
- Santana, Á. L., & Meireles, M. A. A. (2014). New starches are the trend for industry applications: A review. Food and Public Health, 4, 229–241.
- Santayana, M., Rossel, G., Núñez, J., Sørensen, M., Delêtre, M., Robles, R., … Heider, B. (2014). Molecular characterization of cultivated species of the genus Pachyrhizus Rich. ex DC. by AFLP markers: Calling for more data. Tropical Plant Biology, 7, 121–132.
- Savary, G., Handschin, S., Conde-Petit, B., Cayot, N., & Doublier, J. L. (2008). Structure of polysaccharide-starch composite gels by rheology and confocal laser scanning microscopy: Effect of the composition and of the preparation procedure. Food Hydrocolloids, 22(1), 520–530.
- Sit, N., Deka, S. C., & Misra, S. (2014). Combined effect of ultrasound and enzymatic pretreatment on yield and functional properties of taro (Colocasia esculenta) starch. Starch/Stärke, 66, 959–967.
- Sit, N., Misra, S., & Deka, S. C. (2014). Yield and functional properties of taro starch as affected by ultrasound. Food and Bioprocess Technology, 7, 1950–1958.
- Sriroth, K., Chollakup, R., Chotineeranat, S., Piyachomkwan, K., & Oates, C. G. (2000). Processing of cassava waste for improved biomass utilization. Bioresource Technology, 71(1), 63–69.
- Stevenson, D. G., Jane, J., & Inglett, G. E. (2007). Characterisation of Jícama (Mexican Potato) (Pachyrhizus erosus L. Urban) starch from taproots grown in USA and Mexico. Starch/ Stärke, 59, 132–140.
- Ulbrich, M., Natan, C., & Flӧter, E. (2014). Acid modification of wheat, potato, and pea starch applying gentle conditions-impacts on starch properties. Starch/Stärke, 66, 903–913.
- Wang, L., & Wang, Y. J. (2004). Application of high-intensity ultrasound and surfactants in rice starch isolation. Cereal Chemistry, 81, 140–144.
- Xin, Y., Zhang, M., & Adhikari, B. (2014). The effects of ultrasound-assisted freezing on the freezing time and quality of broccoli (Brassica oleracea L. var. botrytis L.) during immersion freezing. International Journal of Refrigeration, 41, 82–91.
