ABSTRACT
Contamination with enterobacteria was detectable in 89% of seafood samples from three central seafood markets in Thailand. The average numbers obtained from the same type of seafood were between 1.3 ± 0.9 and 4.5 ± 1.3 log CFU/g per sample. Eighty-one strains and 16 species were distinguished based on ERIC-PCR patterns and TP-RAPD patterns, respectively. The highest prevalence (90% of strains) was resistant to penicillin G whereas none was resistant to gentamycin. In addition, 63% exhibited multidrug resistance. The 16S rDNA sequences of a representative strain from each species exhibited 99% identity to either one of six genera including Citrobacter, Enterobacter, Klebsiella, Providencia, Serratia, and Yersinia. Three β-lactamase genes including blaTEM, ampC, and shv were detected at the frequencies of 43%, 27%, and 24%, respectively. The representative strains possessing β-lactamase genes exhibited β-lactamase activity ranging from 1.96 ± 0.88 to 11.3 ± 0.37 μmol of hydrolyzed nitrocefin/min/mg protein.
RESUMEN
En 80% de las muestras de mariscos tomadas en tres mercados centrales de mariscos de Tailandia fue posible detectar contaminación por enterobacterias. Las mediciones promedio de enterobacterias obtenidas en el mismo tipo de marisco fluctúan entre 1.3 ± 0.9 y 4.5 ± 1.3 log cfu/g por muestra. A partir de patrones ERIC-PCR y TP-RAPD se distinguieron 81 cepas y 16 especies, respectivamente. Un porcentaje más elevado de las cepas (90%) es resistente a la penicilina G y ninguna de ellas es resistente a la gentamicina. Asimismo, 63% de las mismas exhibe resistencia a múltiples drogas. Las secuencias 16S ADNr de una cepa representativa de cada una de las especies exhibieron 99% de identidad con uno de seis géneros, incluyendo Citrobacter, Enterobacter, Klebsiella, Providencia, Serratia y Yersinia. Por otra parte, se detectaron tres genes de β-lactamasa, entre los que se incluyen blaTEM, ampC, y shv, con frecuencias de 43%, 27% y 24%, respectivamente. En las cepas representativas que poseen genes de β-lactamasa la actividad de esta enzimaoscila entre 1.96 ± 0.88 y 11.3 ± 0.37 μmol de proteína de nitrocefina/min/mg hidrolizada.
Introduction
Seafood is a nutrient-rich part of a healthful diet containing a unique dietary source of the marine n-3 fatty acids, eicosapentaenoic acid, docosahexanoic acid, vitamin D, vitamin B12, iodine, and selenium (Dahl, Bjørkkjaer, Graff, Kjellevold, & Klementsen, Citation2006; Iwamoto, Ayers, Mahon, & Swerdlow, Citation2010). Seafood consumption has been shown to be associated with potential health attributes including cognitive development of infant during pregnancy (Oken et al., Citation2005), neurologic development during gestation and infancy (Hibbeln et al., Citation2007), and reduction in risk of heart disease (Mozaffarian & Rimm, Citation2006). Nevertheless, seafood consumption is not risk-free because seafood contributes to an important proportion of food-borne illnesses and outbreaks worldwide. Among the Food and Drug Administration (FDA)-regulated food categories, seafood was responsible for the second most outbreaks and the most relative rate of illness during 2004–2013 [Center for Science in the Public Interest (CSPI), Citation2015]. Bacteria were reported to be a major cause (54%) of food-borne disease outbreaks in the United States in 2015 [Center for Disease Control and Prevention (CDC), Citation2017]. Food poisoning caused by enterobacteria has become a public health concern in Thailand. In 2015, 200.22 food poisoning cases per 100,000 population were reported and Salmonella spp. were the one most frequently found (48%) among pathogenic bacteria identified from 0.57% of all patients (Bureau of Epidemiology, Thailand, Citation2015).
Enterobacteria, which belong to the family Enterobacteriaceae, are known as important seafood-associated pathogens. Up to date, some members have been assigned to novel families: Budviciaceae, Erwiniaceae, Hafniaceae, Morganellaceae, Pectobacteriaceae, and Yersiniaceae which were proposed by Adeolu, Alnajar, Naushad, and Gupta (Citation2016). Enterobacteria have been implicated in the pathogenesis of host diseases such as nonalcoholic steatohepatitis, allergy, and inflammatory bowel disease (Miyata et al., Citation2011). Previous reports demonstrated the presence of enterobacteria in a variety of seafood of various origins (Guo et al., Citation2016; Janecko et al., Citation2016; Rani, Chelladurai, & Jayanthi, Citation2016). Antibiotic resistance is expressed as a common characteristic among enterobacteria. Resistance to antibiotics, especially the β-lactam group, is increasingly dominated by the mobilization of continuously expressed single genes encoding efficient drug modifying enzymes such as β-lactamases (Iredell, Brown, & Tagg, Citation2016). Numbers of antibiotic-resistant enterobacteria have been detected worldwide. For example, extended-spectrum β-lactamases (ESBLs) are commonly found in members of enterobacteria in Latin America (Lincopan et al., Citation2006). Recent studies reported the distribution of enterobacteria in foodstuff in Thailand. The contamination rate of vegetables with ESBL-producing enterobacteria was 18.3%, 94% of which were multiresistant (Zurfluh et al., Citation2015). Coliform bacteria were found to be contaminated in 37% of ready-to-eat food samples sold in middle Thailand (Ananchaipattana, Bari, & Inatsu, Citation2016). Enterobacter and Klebsiella at the frequencies of 37.3% and 1.3%, respectively, were contaminated during puff pastry line production in the commercial bakery industry. Enterobacter asburiae showed the highest abundance of 34.6–37.5% for the first two production lines including dough forming and layer and filling forming (Rumjuankiat, Keawsompong, & Nitisinprasert, Citation2017).
The purpose of this work was to analyze the prevalence, numbers, and genera of enterobacteria contaminated in seafood sold in central seafood markets in Thailand as well as their antibiotic resistance patterns and the associated genes. The information is valuable for risk assessment, controlling the spread of pathogenic enterobacteria, and planning to use antibiotics in aquaculture.
Materials and methods
Isolation and enumeration of enterobacteria in seafood sold in Thailand
Thirty-five samples of fresh seafood in 15 species were collected from three central seafood markets of Thailand including Talaythai market in Samutsakhon province, Bankhaotakiab market in Prachuapkhirikhun province, and Banphe market in Rayong province in August 2014. All samples were placed in sterile zip-lock plastic bags, kept in ice buckets prior to isolation of enterobacteria. The isolation was done within 6 h after sample collection as described by Da Silva et al. (Citation2012). The quantification of enterobacteria was achieved by the standard pour plate method using violet red bile glucose (VRBG) agar (Titan Biotech. Ltd., Delhi, India). Characteristics of presumptive enterobacteria were pink to violet-red colonies with diameters of 0.5 mm or greater on VRBG agar, Gram-negative bacilli or coccobacilli, and negative oxidase reaction (Walker, Mahon, Lehman, & Manuselis, Citation2015). Enterobacterial isolates were purified, and pure cultures were kept on nutrient agar (NA) slants at 4°C and in 20% glycerol at −80°C.
Enterobacterial repetitive intergenic consensus-polymerase chain reaction (ERIC-PCR) fingerprinting of enterobacterial isolates
ERIC-PCR was performed to analyze genotypic diversity and relatedness among enterobacterial isolates as well as to distinguish individual strains. Genomic DNA of each isolate was used as a template in PCR reactions using a pair of primers ERIC2 and ERIC1R as described previously (Ogutcu, Adiguzel, Gulluce, Karadayi, & Sahin, Citation2009). The presence and size of the amplified fragments were determined by agarose [1% in Tris-borate-EDTA (TBE) buffer] gel electrophoresis and the unweighted pair groups using mathematical averages (UPGMA) dendrogram was constructed using the Phoretix ID Pro. software (TotalLab Ltd., Newcastle upon Tyne, UK). Enterobacterial strains with individual ERIC-PCR patterns were selected for subsequent studies.
Physiological and biochemical characterizations of enterobacterial strains
Enterobacterial strains were examined for their physiological and biochemical characteristics including 1) temperatures, pH values, and NaCl concentrations for growth; 2) production of enzymes (catalase, amylase, urease, caseinase, and protease); 3) fermentation of sugars (glucose, lactose, and sucrose); 4) decarboxylation of amino acids (arginine, lysine, and ornithine); and 5) indole, methyl red (MR), Voges-Proskauer (VP), citrate (IMViC) test.
Examination on antibiotic resistance of enterobacterial strains
Enterobacterial strains were examined for resistance to ten antibiotics as described by European Committee on Antimicrobial Susceptibility Testing (EUCAST, Citation2012).
Detection of antibiotic resistance genes in enterobacterial strains
Genomic DNA of each strain was used as a template in PCR reactions to detect the presence of eight antibiotic resistance genes as previously described. These genes included ampC (Hanson et al., Citation1999), blaCTX (Moghaddam, Beidokhti, Jamehdar, & Ghahraman, Citation2014), blaTEM (Bert, Bramger, & Lambert-Zochovsky, Citation2002), blaZ (Olsen, Christensen, & Aarestrup, Citation2006), mecA (Duran, Ozer, Duran, Onlen, & Demir, Citation2012), oxa1 (Onyango, Ndeda, Wandili, Wawire, & Ochieng, Citation2014), oxa9 (Hanson et al., Citation1999), and shv (Fang, Ataker, Hedin, & Dornbusch, Citation2008).
Two-primers random amplified polymorphic DNA (TP-RAPD) fingerprinting of enterobacterial strains
TP-RAPD was performed to distinguish enterobacterial species. Genomic DNA of each strain was used as a template in PCR reactions using a pair of primers 8F and 1522R as described by Rivas, Velazquez, Valverde, Mateos, and Martinez-Molina (Citation2001). The presence and size of the amplified fragments were determined by agarose (1% in TBE buffer) gel electrophoresis.
Sequence analysis of partial 16S rDNA of enterobacteria
Partial 16S rDNA of a representative strain from each TP-RAPD pattern was amplified using a pair of universal primers UN16S 926f and UN16S 1392r (Lane, Citation1991). PCR reactions were carried out as described by Pongsilp, Teaumroong, Nuntagij, Boonkerd, and Sadowsky (Citation2002) and the PCR products were purified using QIAquick gel extraction kit (Qiagen, Valencia, CA, USA). The purified PCR products were sequenced by Bio Basic Canada Inc. (Markham, Ontario, Canada). The nucleotide sequences were aligned with reference 16S rDNA sequences using the BLASTN program (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to identify the closest genera.
Sequence analysis of antibiotic resistance genes of enterobacteria
To reinforce the presence of antibiotic resistance genes in seafood-associated enterobacteria, the detected antibiotic resistance genes of a representative strain from each species were amplified. The PCR products were purified using the QIAquick gel extraction kit (Qiagen, Valencia, CA, USA). The purified PCR products were sequenced by Bio Basic Canada Inc. (Markham, Ontario, Canada). The nucleotide sequences were aligned with reference sequences using the BLASTN program (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Measurement of β-lactamase activity of enterobacteria
The β-lactamase activity of a representative strain from each species which possessed β-lactam antibiotic resistance gene(s) was measured. The β-lactamase induction and β-lactamase assay were performed as described by Sharma, Ramnani, and Virdi (Citation2004).
Results and discussion
Prevalence of enterobacteria in seafood sold in Thailand
Enterobacteria were under the detection limit (< 1.2 log CFU/g) in four seafood samples while the remaining 31 samples contained enterobacteria that ranged from 1.3 ± 0.2 to 5.4 ± 0.1 log CFU/g. Average numbers of presumptive enterobacteria per sample of the same type of seafood sold in three central seafood markets of Thailand are shown in . Ninety-six pure isolates of enterobacteria were derived and designated by abbreviations ENTSF followed by the number (1 to 35) that indicates the order of seafood samples. No enterobacterial isolate was derived from the orders 16 and 28. Enterobacteria counts were examined in the previous reports. Unacceptable enterobacteria levels (> 2 log CFU/g) were obtained in 40% of the fresh fish samples sold in Croatia during the summer (Popovic et al., Citation2010). The numbers of enterobacteria in fillets of marine fish sold in Egypt varied from 2.2 to 4.8 log CFU/g (Ghanem, Elshabasy, Ibrahim, & Samaha, Citation2014). Ananchaipattana et al. (Citation2012) reported the presence of coliform bacteria in all investigated seafood samples from markets in Thailand. Members in genera Citrobacter, Cronobacter, Enterobacter, Salmonella, Serratia, Pantoea, Proteus, and Yersinia as well as Escherichia coli and Providencia rettgeri were found in sea and river fishes.
Table 1. Average numbers of presumptive enterobacteria per sample of the same type of seafood sold in Thailand.
Tabla 1. Mediciones promedio de presuntas enterobacterias por muestra del mismo tipo de marisco vendido en Tailandia.
ERIC-PCR fingerprinting of enterobacterial isolates
As shown in , the 96 enterobacterial isolates generated 81 distinct ERIC-PCR patterns, suggesting that 81 strains were obtained. The amplified bands ranged in number from one to eight and in size from approximately 100 to 5,000 bp. ERIC-PCR has been found to be extremely sensitive and can detect minor differences among strains of the same bacterial genus and species (Pongsilp, Citation2012).
Physiological and biochemical characterizations of enterobacterial strains
Eighty-one enterobacterial strains varied in their physiological and biochemical characteristics. All strains grew at a temperature range between 20°C and 40°C. The 18 and 5 strains were able to grow at a minimum temperature of 15°C and maximum temperature of 45°C, respectively. All strains grew at a pH range between 4 and 9. The only one strain was able to grow at a minimum pH of 3. The maximum pH for growth was 11 for 20 strains. The 56 strains tolerated the maximum NaCl concentration of 7%. The 68, 9, and 1 strains produced catalase, urease, and amylase, respectively. None was positive for caseinase production. Protease activity ranging from undetectable to 14.56 ± 1.08 units/ml supernatant was measured by azocasein protease assay (Secades & Guijarro, Citation1999).
The strains exhibited four profiles for sugar fermentation. Sixty-eight strains fermented all tested sugars. Eight strains fermented only glucose. The only one strain fermented both glucose and lactose while four strains fermented both glucose and sucrose. Decarboxylation of arginine, lysine, and ornithine was tested and five profiles were obtained. The 33 and 5 strains decarboxylated only lysine and ornithine, respectively. Two strains decarboxylated both arginine and ornithine. Thirty strains decarboxylated both lysine and ornithine. Eleven strains were unable to decarboxylate either one of these amino acids. The strains exhibited eight IMViC profiles including – + – - (56 strains), + + – + (nine strains), + + – - (five strains), – + – + (five strains), – + + – (three strains), – - + + (one strain), – - + – (one strain) and – + + + (one strain). The 14, 79, 6, and 16 strains were positive for indole production, MR reaction, VP reaction, and citrate utilization, respectively.
Prevalence of antibiotic resistance among enterobacterial strains
Twenty-four antibiotic resistance patterns, as shown in , were observed among 81 enterobacterial strains. The most common resistance pattern (25% of strains) was the ampicillin, erythromycin, penicillin G, and vancomycin co-resistance. The resistance to penicillin G, vancomycin, erythromycin, ampicillin, tetracycline, chloramphenicol, streptomycin, neomycin, and kanamycin was found in 73 (90% of strains), 62 (77%), 56 (69%), 50 (62%), 18 (22%), 3 (4%), 3 (4%), 2 (2%), and 1 (1%) strains, respectively, while none was resistant to gentamycin. The only one strain was susceptible to all ten antibiotics while the remaining strains exhibited resistance to at least one but up to six of the tested antibiotics. These ten antibiotics are sorted into six categories including 1) aminoglycosides (e.g. gentamycin, kanamycin, neomycin, and streptomycin); 2) glycopeptides (e.g. vancomycin); 3) macrolides (e.g. erythromycin); 4) penicillins (e.g. ampicillin and penicillin); 5) phenicols (e.g. chloramphenicol), and 6) tetracyclines (e.g. tetracycline). These antibiotics have been selected to determine the resistance of Enterobacteriaceae members in previous reports. The high prevalence of enterobacterial isolates displayed resistance to ampicillin, erythromycin, penicillin, and vancomycin. In contrast, they were less frequently resistant to chloramphenicol, kanamycin, neomycin, streptomycin, and tetracycline. Both high and low prevalence was reported for gentamycin-resistant isolates (Citron, Tyrrell, Merriam, & Goldstein, Citation2012; Hu, Liu, Zhang, Feng, & Zong, Citation2017; Kilonzo-Nthenge, Rotich, & Nahashon, Citation2013; Kumar, Citation2016). Multidrug resistant (MDR) is defined as nonsusceptibility to at least one agent in three or more antimicrobial categories (Basak, Singh, & Rajurkar, Citation2016). Therefore, 63% of strains were multiresistant. The previous study also noted the incidence of multiresistant enterobacteria present in seafood (Janecko et al., Citation2016; Nawaz et al., Citation2012). Antibiotic resistance may be directly introduced into seafood-associated enterobacteria via terrestrial run-off, in which antibiotic-resistant bacteria or antibiotic compounds were present. Mutidrug resistance of enterobacteria is a challenge for the global public health agenda. Enterobacteria have an intriguing ability to acquire multi-resistance in a single step by capturing several resistance genes from a variety of bacterial species and transferring genes to the same plasmids (Partridge, Citation2015).
Table 2. Antibiotic resistance patterns of seafood-associated enterobacterial strains and numbers of strain(s) belonging to each pattern.
Tabla 2. Patrones de resistencia a los antibióticos de cepas de enterobacterias y mediciones de cepa(s) que pertenece(n) a cada patrón.
Detected antibiotic resistance genes in enterobacterial strains
Positive bands with sizes compatible with the presence of three antibiotic resistance genes including ampC, blaTEM, and shv were detected in PCR reactions. PCR product sizes of the ampC, blaTEM, and shv genes were 395 bp, 867 bp, and 151 bp, respectively. Three antibiotic resistance gene patterns were observed among 35 enterobacterial strains while the remaining 46 strains gave negative results for the presence of all eight antibiotic resistance genes. Antibiotic resistance gene patterns including 1) ampC blaTEM shv; 2) blaTEM; and 3) ampC blaTEM, were present in 19 (24% of strains), 13 (16%), and 3 (4%) strains, respectively. The frequency of the blaTEM gene was highest (43% of strains), followed by the ampC gene (27%), and the shv gene (24%), respectively. These genes encode β-lactamases that confer resistance to the β-lactam group of antibiotics. All strains harboring either one of three antibiotic resistance gene patterns exhibited either the penicillin G resistance or ampicillin and penicillin G co-resistance. The co-occurrence of three β-lactamase genes in single strains was also noticed in this study. The blaTEM, ampC, and shv genes were reported as the most prevalent genes at the frequencies of 54%, 50%, and 25%, respectively, among isolates of ESBL-producing enterobacteria from patients in Rio de Janeiro, Brazil (Vasques, Bello, Da Cruz Lamas, Correa, & Pereira, Citation2011). The blaTEM gene was also the most prevalent (89.2%) among E. coli isolates from animals presenting at a veterinary hospital in Dublin, Ireland. Although the distribution of the shv gene was much lower (6.8%), it was found that the shv gene was always accompanied by the blaTEM gene (Karczmarczyk, Abbott, Walsh, Leonard, & Fanning, Citation2011). While 34.2% of Enterobacter isolates from blood cultures and specimens in Malaysia were found positive for the ampC gene (Khari, Karunakaran, Rosli, & Tay, Citation2016). Other genes conferring resistance to β-lactam antibiotics, including blaCTX, blaZ, mecA, oxa1, and oxa9, were not detected in any strains.
Distinguishing of enterobacterial species based on TP-RAPD fingerprinting
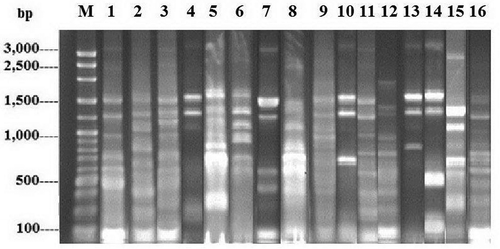
As shown in , the 16 distinct TP-RAPD patterns were generated from 81 strains. The amplified bands ranged in number from four to 11 and in size from approximately 100 to 3,000 bp. TP-RAPD patterns have been developed for taxonomy purpose as the patterns of strains in the same species have been found to be identical (Valverde, Igual, Peix, Cervantes, & Velazquez, Citation2006). Our result reinforces the presence of 16 enterobacterial species in seafood sold in Thailand.
Closest genera of enterobacteria based on partial 16S rDNA sequences
Partial 16S rDNA sequences (approximately 500 bp) of a representative strain from each species were obtained. The closest genera of 16 enterobacterial species were Citrobacter, Enterobacter, Klebsiella, Providencia, Serratia, and Yersinia with 99% identity. These sequences can be retrieved from the GenBank database under accession numbers MF593860 to MF593875. Taken together with TP-RAPD patterns, the data suggest that the seafood-associated enterobacteria included six species closely related to members of Klebsiella (34 strains), four species closely related to members of Enterobacter (30 strains), three species closely related to members of Citrobacter (three strains), one species closely related to members of Providencia (11 strains), one species closely related to members of Yersinia (two strains), and one species closely related to members of Serratia (one strain). The frequencies of the strains closely related to Klebsiella, Enterobacter, Providencia, Citrobacter, Yersinia, and Serratia in 35 seafood samples were 57%, 57%, 23%, 9%, 6%, and 3%, respectively. TP-RAPD patterns of species closely related to members of Klebsiella, Enterobacter, Citrobacter, Providencia, Yersinia, and Serratia correspond to lanes 1 to 6, 7 to 10, 11 to 13, 14, 15, and 16, in , respectively.
In relation to the result of antibiotic resistance, numbers of strain(s) closely related to each genus that exhibited resistance to each antibiotic were obtained and shown in . The common characteristics were the resistance to β-lactam antibiotics and the susceptibility to aminoglycosides. The majority of strains (90%) exhibited the phenotypic resistance to either one or both β-lactam antibiotics including ampicillin and penicillin. All strains were susceptible to gentamycin and only 7% of strains were resistant to either one of three aminoglycosides including kanamycin, neomycin, and streptomycin.
Table 3. Numbers of strain(s) closely related to each genus that exhibited resistance to each antibiotic.
Tabla 3. Mediciones de cepa(s) estrechamente relacionadas con cada género que exhibió resistencia a cada uno de los antibióticos.
In relation to their biochemical characteristics, shared characteristics among six species closely related to Klebsiella included 1) fermentation of glucose, lactose, and sucrose; 2) arginine decarboxylase-negative and lysine decarboxylase-positive reactions; 3) negative indole test; and 4) positive MR test. Similar features among four species closely related to Enterobacter were 1) fermentation of glucose; 2) ornitine decarboxylase-positive reaction; and 3) negative indole test. Three species closely related to Citrobacter shared features including 1) fermentation of glucose and lactose; 2) lysine decarboxylase-negative and ornithine decarboxylase-positive reactions; 3) positive MR test and negative VP test; and 4) negative urease test. A single species closely related to Providencia fermented glucose but did not ferment lactose. They were negative for arginine, lysine, and ornithine decarboxylases, positive for indole and MR tests, negative for VP test as well as displayed variable reactions for sucrose fermentation, citrate utilization, and urease production. A single species closely related to Yersinia fermented glucose, lactose, and sucrose. They were positive for ornithine decarboxylase and MR test but negative for arginine and lysine decarboxylase, indole test, VP test, citrate utilization, and urease production. A strain closely related to Serratia fermented glucose, lactose, and sucrose. It was positive for lysine and ornithine decarboxylases and MR test but negative for arginine decarboxylase, indole test, VP test, citrate utilization, and urease production. The results of carbohydrate fermentation were in accord with the phenotypic features of identified genera described in Walker, Mahon, Lehman, & Manuselis (Citation2015). Glucose fermentation is a common characteristic of enterobacteria which is employed as a basis for their detection. Lactose fermentation is a common characteristic in most members of Citrobacter, Enterobacter, Klebsiella, and Serratia, but it is very rare in Providencia. Sucrose fermentation is common in most members of Klebsiella and Serratia. It is widely variable (0% to 100%) among species of Enterobacter and Yersinia.
Antibiotic resistance gene sequences of enterobacteria
As positive bands of three antibiotic resistance genes including ampC, blaTEM, and shv were detected by PCR, sequence analysis reinforced the presence of these antibiotic resistance genes with 99% identity to the published sequences. The sequences of the ampC and shv genes were obtained from one species closely related to members of Enterobacter and one species closely related to members of Providencia. The distribution of the blaTEM gene was found in two species closely related to members of Klebsiella, two species closely related to members of Enterobacter, one species closely related to members of Providencia, and one species closely related to members of Serratia. These sequences can be retrieved from the GenBank database under accession numbers MF593876 to MF593885.
β-lactamase activity of the selected enterobacterial strains harboring different β-lactamase genes
A representative strain from each of six species that possessed at least one β-lactamase gene was selected to examine the β-lactamase activity. Among the ten strains examined, the strain ENTSF 22–1 displayed the highest β-lactamase activity. The β-lactamase activity of the selected enterobacterial strains is listed in . However, it is possible that β-lactamase activity was partly resulted from other β-lactamase genes that co-occur in the same strains.
Table 4. β-lactamase activity of the selected enterobacterial strains.
Tabla 4. Actividad de β-lactamasa de las cepas de enterobacterias seleccionadas.
Conclusion
The results of this study provide information on the prevalence of enterobacteria, a major group of food-borne pathogenic bacteria, in seafood sold in Thailand that would be valuable for hygienic and sanitary management. Most of the seafood samples (89%) were positive for contamination with enterobacteria, in which 63% were multiresistant. The resistance to antibiotics in five categories was exhibited by 7% of all strains. The multi-drug resistance in nonclinical strains emphasizes the risk of spread via food and environment. This issue of concern should be involved in surveillance programs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adeolu, M., Alnajar, S., Naushad, S., & Gupta, R. S. (2016). Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. International Journal of Systematic and Evolutionary Microbiology, 66(12), 5575–5599.
- Ananchaipattana, C., Bari, M. D. L., & Inatsu, Y. (2016). Bacterial contamination into ready-to-eat foods sold in Middle Thailand. Biocontrol Science, 21(4), 225–230.
- Ananchaipattana, C., Hosotani, Y., Kawasaki, S., Pongsawat, S., Bari, M. D. L., Isobe, S., & Inatsu, Y. (2012). Bacterial contamination in retailed foods purchased in Thailand. Food Science and Technology Research, 18(5), 705–712.
- Basak, S., Singh, P., & Rajurkar, M. (2016). Multidrug resistant and extensively drug resistant bacteria: A study. Journal of Pathogens, 2016, 4065603.
- Bert, F., Bramger, C., & Lambert-Zochovsky, N. (2002). Identification of PSE and OXA β-lactamase genes in Pseudomonas aeruginosa using PCR–Restriction fragment length polymorphism. Journal of Antimicrobial Chemotherapy, 50(1), 11–18.
- Bureau of Epidemiology, Thailand. (2015). Annual epidemiological surveillance report, 2015. Retrieved from http://www.boe.moph.go.th/Annual/AESR2015/aesr2558/Part%201/ 07/food_poisoning.pdf
- Center for Disease Control and Prevention (CDC). (2017). Surveillance for foodborne disease outbreaks, United States, 2015, Annual Report. Retrieved from https://www.cdc.gov/foodsafety/pdfs/2015FoodBorneOutbreaks_508.pdf
- Center for Science in the Public Interest (CSPI). (2015, November). Outbreak alert 2015!: a review of foodborne illness in the U.S. from 2004-2013. Retrieved from https://cspinet.org/sites/default/files/attachment/outbreak-alert-2015.pdf
- Citron, D. M., Tyrrell, K. L., Merriam, C. V., & Goldstein, E. J. C. (2012). In vitro activities of CB-183,315, vancomycin, and metronidazole against 556 strains of Clostridium difficile, 445 other intestinal anaerobes, and 56 Enterobacteriaceae species. Antimicrobial Agents and Chemotherapy, 56(3), 1613–1615.
- Da Silva, N., Taniwaki, M. H., Junqueira, V. C. A., Silveira, N., Da Silva Do Nascimento, M., & Gomes, R. A. R. (2012). Microbiology examination methods of food and water: A laboratory manual. Boca Raton, FL: CRC Press.
- Dahl, L., Bjørkkjaer, T., Graff, I. E., Kjellevold, M., & Klementsen, B. (2006). Fish - more than just omega-3. Tidsskrift for Den Norske Laegeforening, 126(3), 309–311.
- Duran, N., Ozer, B., Duran, G. G., Onlen, Y., & Demir, C. (2012). Antibiotic resistance genes & susceptibility patterns in staphylococci. Indian Journal of Medical Research, 135(3), 389–396.
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). (2012, February). Antimicrobial susceptibility testing EUCAST disk diffusion method version 2.1. Retrieved from https://asmsig.files.wordpress.com/2013/08/manual_v_2-1_eucast_disk_test.pdf
- Fang, H., Ataker, F., Hedin, G., & Dornbusch, K. (2008). Molecular epidemiology of extended-spectrum β-lactamases among Escherichia coli isolates collected in a Swedish hospital and its associated health care facilities from 2001 to 2006. Journal of Clinical Microbiology, 46(2), 707–712.
- Ghanem, N. A., Elshabasy, N. A., Ibrahim, H. A., & Samaha, I. A. (2014). Enterobacteria in some marine fish fillet. Alexandria Journal of Veterinary Sciences, 40, 124–131.
- Guo, Y., Zhou, H., Qin, L., Pang, Z., Qin, T., Ren, H., … Zhou, J. (2016). Frequency, antimicrobial resistance and genetic diversity of Klebsiella pneumoniae in food samples. Public Library of Science One, 11(4), e0153561.
- Hanson, N. D., Thomson, K. S., Moland, E. S., Sanders, C. C., Berthold, G., & Penn, R. G. (1999). Molecular characterization of a multiply resistant Klebsiella pneumoniae encoding ESBLs and a plasmid-mediated AmpC. Journal of Antimicrobial Chemotherapy, 44(3), 377–380.
- Hibbeln, J. R., Davis, J. M., Steer, C., Emmett, P., Rogers, I., Williams, C., & Golding, J. (2007). Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. The Lancet, 369(9561), 578–585.
- Hu, Y., Liu, L., Zhang, X., Feng, Y., & Zong, Z. (2017). In vitro activity of neomycin, streptomycin, paromomycin and apramycin against carbapenem-resistant Enterobacteriaceae clinical strains. Frontiers in Microbiology, 8, 2275.
- Iredell, J., Brown, J., & Tagg, K. (2016). Antibiotic resistance in Enterobacteriaceae: Mechanisms and clinical implications. British Medical Journal, 352, h6420.
- Iwamoto, M., Ayers, T., Mahon, B. E., & Swerdlow, D. L. (2010). Epidemiology of seafood-associated infections in the United States. Clinical Microbiology Reviews, 23(2), 399–411.
- Janecko, N., Martz, S., Avery, B. P., Daignault, D., Desruisseau, A., Boyd, D., … Reid-Smith, R. J. (2016). Carbapenem-resistant Enterobacter spp. in retail imported from Southeast Asia to Canada. Emerging Infectious Diseases, 22(9), 1675–1677.
- Karczmarczyk, M., Abbott, Y., Walsh, C., Leonard, N., & Fanning, S. (2011). Characterization of multidrug-resistant Escherichia coli isolates from animals presenting at a university veterinary hospital. Applied and Environmental Microbiology, 77(20), 7104–7112.
- Khari, F. I. M., Karunakaran, R., Rosli, R., & Tay, S. T. (2016). Genotypic and phenotypic detection of AmpC β-lactamases in Enterobacter spp. isolated from a Teaching Hospital in Malaysia. PLos One, 11(3), e0150643.
- Kilonzo-Nthenge, A., Rotich, E., & Nahashon, S. N. (2013). Evaluation of drug-resistant Enterobacteriaceae in retail poultry and beef. Poultry Science, 92(4), 1098–1107.
- Kumar, H. (2016). Multiple antibiotic resistance patterns of the Enterobacteriaceae in the untreated municipal sewage. Journal of Clinical and Diagnostic Research, 10(9), DL01–DL02.
- Lane, D. J. (1991). 16S/23S rRNA sequencing. In E. Stackebrandt & M. Goodfellow (Eds.), Nucleic acid techniques in bacterial systematic (pp. 115–175). New York, NY: Wiley.
- Lincopan, N., Leis, R., Vianello, M. A., De Araujo, M. R. E., Ruiz, A. S., & Mamizuka, E. M. (2006). Enterobacteria producing extended-spectrum β-lactamases and IMP-1 metallo-β-lactamases isolated from Brazilian hospitals. Journal of Medical Microbiology, 55(Pt 11), 1611–1613.
- Miyata, M., Yamakawa, H., Hamatsu, M., Kuribayashi, H., Takamatsu, Y., & Yamazoe, Y. (2011). Enterobacteria modulate intestinal bile acid transport and homeostasis through apical sodium dependent bile acid transporter (SLC10A2) expression. Journal of Pharmacology and Experimental Therapeutics, 336(1), 188–196.
- Moghaddam, M. N., Beidokhti, M. H., Jamehdar, S. A., & Ghahraman, M. (2014). Genetic properties of blaCTX−M and blaPER β-lactamase genes in clinical isolates of Enterobacteriaceae by polymerase chain reaction. Iran Journal of Basic Medical Sciences, 17(5), 378–383.
- Mozaffarian, D., & Rimm, E. B. (2006). Fish intake, contaminants, and human health. Evaluating the risks and the benefits. JAMA, 296(15), 1885–1899.
- Nawaz, M., Khan, S. A., Tran, Q., Sung, K., Khan, A. A., Adamu, I., & Steele, R. S. (2012). Isolation and characterization of multidrug-resistant Klebsiella spp. isolated from shrimp imported from Thailand. International Journal of Food Microbiology, 155(3), 179–184.
- Ogutcu, H., Adiguzel, A., Gulluce, M., Karadayi, M., & Sahin, F. (2009). Molecular characterization of Rhizobium strains isolated from wild chickpeas collected from high altitudes in Erzurum-Turkey. Romanian Biotechnological Letters, 14(2), 4294–4300.
- Oken, E., Wright, R. O., Kleinman, K. P., Bellinger, D., Amarasiriwardena, C. J., Hu, H., … Gillman, M. W. (2005). Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environmental Health Perspectives, 113(10), 1376–1380.
- Olsen, J. E., Christensen, H., & Aarestrup, F. M. (2006). Diversity and evolution of blaZ from Staphylococcus aureus and coagulase-negative staphylococci. Journal of Antimicrobial Chemotherapy¸, 57(3), 450–460.
- Onyango, D. M., Ndeda, V. M., Wandili, S. A., Wawire, S. A., & Ochieng, P. (2014). Antimicrobial profile of Salmonella enterica serotype Choleraesuis from free-range swine in Kakamega fish market, western Kenya. The Journal of Infection in Developing Countries, 8(11), 1381–1390.
- Partridge, S. R. (2015). Resistance mechanisms in. Enterobacteriaceae. Pathology, 47(3), 270–284.
- Pongsilp, N. (2012). Phenotypic and genotypic diversity of rhizobia. Sharjah, UAE: Bentham Science Publishers.
- Pongsilp, N., Teaumroong, N., Nuntagij, A., Boonkerd, N., & Sadowsky, M. J. (2002). Genetic structure of indigenous non-nodulating and nodulating populations of Bradyrhizobium in soils from Thailand. Symbiosis, 33, 39–58.
- Popovic, N. T., Skukan, A. B., Dzidara, P., Coz-Rakovac, R., Strunjak-Perovic, I., Kozacinski, L., … Brlek-Gorski, D. (2010). Microbiological quality of marketed fresh and frozen seafood caught off the Adriatic coast of Croatia. Veterinarni Medicina, 55(5), 233–241.
- Rani, M. K., Chelladurai, G., & Jayanthi, G. (2016). Isolation and identification of bacteria from marine market fish Scomberomorus guttatus (Bloch and Schneider, 1801) from Madurai district, Tamil Nadu, India. Journal of Parasitic Diseases, 40(3), 1062–1065.
- Rivas, R., Velazquez, E., Valverde, A., Mateos, P. F., & Martinez-Molina, E. (2001). A two primers random amplified polymorphic DNA procedure to obtain polymerase chain reaction fingerprints of bacterial species. Electrophoresis, 22(6), 1086–1089.
- Rumjuankiat, R., Keawsompong, S., & Nitisinprasert, S. (2017). Bacterial contaminants from frozen puff pastry production process and their growth inhibition by antimicrobial substances from lactic acid bacteria. Food Science And Nutrition, 5(3), 454–465.
- Secades, P., & Guijarro, J. A. (1999). Purification and characterization of an extracellular protease from fish pathogen Yesinia ruckeri and effect of culture conditions on production. Applied and Environmental Microbiology, 65(9), 3969–3975.
- Sharma, S., Ramnani, P., & Virdi, J. S. (2004). Detection and assay of β-lactamases in clinical and non-clinical strains of Yersinia enterocolitica biovar 1A. The Journal of Antimicrobial Chemotherapy, 54(2), 401–405.
- Valverde, A., Igual, J. M., Peix, A., Cervantes, E., & Velazquez, E. (2006). Rhizobium lusitanum sp. nov. a bacterium that nodulates Phaseolus vulgaris. International Journal of Systematic Evolutionary Microbiology, 56(Pt 11), 2631–2637.
- Vasques, M. R. G., Bello, A. R., Da Cruz Lamas, C., Correa, J., & Pereira, J. A. A. (2011). β-lactamase producing enterobacteria isolated from surveillance swabs of patients in a Cardiac Intensive Care Unit in Rio de Janeiro, Brazil. The Brazilian Journal of Infectious Diseases, 15(1), 28–33.
- Walker, K. E., Mahon, C. R., Lehman, D. C., & Manuselis, G. (2015). Enterobacteriaceae. In C. R. Mahon, D. C. Lehman, & G. Manuselis (Eds.), Textbook of diagnostic microbiology (pp. 420–454). St. Louis, MO: Saunders.
- Zurfluh, K., Nuesch-Inderbinen, M., Morach, M., Berner, A. Z., Hachler, H., & Stephan, R. (2015). Extended-spectrum-?-lactamase-producing enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. Applied and Environmental Microbiology, 81(9), 3115–3120.