 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Green tea and bay leaf extracts are natural preservatives for increasing the shelf life of food products. Effects of green tea and bay leaf extract on anchovy marinades were determined by measuring microbiological, sensory, and physical properties. Marinated anchovies were prepared with 4.2% wine vinegar, 9% salt solution and ripened for 24 h at 10°C in a fish processing factory. Green tea extract (1%, 2% w/v), bay leaf extract (0.1%, 0.2 w/v) were added to the marination solution. Following the draining procedure, marinated anchovies were vacuum packaged and stored at 4°C for 240 days. The addition of green tea and bay leaf extract reduced microbial load, TVB-N (total volatile basic nitrogen) level, and TBARS (thiobarbituric acid reactive substances) value. Green tea extract was most effective on lipid peroxidation and yielded a darker color, which is not preferred by customers. Biogenic amines amount in all of the samples were very low due to low acidity level and good manufacturing practices. Plant extracts had no significant effect on biogenic amine accumulation in marinated anchovies.
RESUMEN
Los extractos de té verde y hojas de laurel son conservadores naturales que aumentan la vida útil de los productos alimenticios. En este estudio se determinaron los efectos provocados por extractos de té verde y hojas de laurel en anchoas marinadas, para lo cual se midieron sus propiedades microbiológicas, sensoriales y físicas. Las anchoas marinadas fueron preparadas en una planta pesquera de procesamiento, utilizando una solución de 4.2% de vinagre de vino y 9% de sal, dejándola madurar durante 24 horas a 10°C. Posteriormente, se adicionó el extracto de té verde (1%, 2% p/v) y el extracto de hojas de laurel (0.1%, 0.2 p/v) a la solución de marinado. Una vez escurridas, las anchoas marinadas fueron empacadas al vacío y almacenadas a 4°C durante 240 días. Se constató que la adición de extracto de té verde y hojas de laurel redujo la carga microbiana, el nivel de TVB-N (nitrógeno básico volátil total) y el valor TBARS (sustancias reactivas al ácido tiobarbitúrico). El extracto de té verde demostró ser más efectivo en la peroxidación lipídica, produciendo un color más oscuro que obtuvo menor preferencia entre los consumidores. Debido al bajo nivel de acidez y las buenas prácticas manufactureras, en todas las muestras se produjo una cantidad muy baja de aminas biógenas. Por lo que, los extractos de las plantas utilizadas no provocaron un efecto significativo en la acumulación de aminas biógenas en las anchoas marinadas.
1. Introduction
Marinades are popular semi-preserved ready to eat food, which is formed by the action of acetic acid and salt. The aim of marination process is to extend the shelf life of fish while improving its textural, sensorial, and structural properties (Pons-Sanchez-Cascado, Vidal-Carou, Marine-Font, & Veciana-Nogues, Citation2005). The shelf life of marinated anchovies is limited due to low pH, high water activity, salt, and vinegar content. The main quality problem in marinated anchovies with prolonging shelf life is lipid oxidation.
Tea is brewed from Camellia sinensis and widely consumed around the world in the form of black, green and oolong tea. Tea is known for its anti-carcinogenic, anti-oxidative, and anti-mutagenic properties (Bunkova, Marova, & Nemec, Citation2005). Green tea is processed without fermentation to prevent polyphenols oxidation. Most abundant polyphenols in tea consist of flavanols, flavonols, and epigallocatechin-3-gallate (EGCG) forms 60–70% of flavanols present in tea. Tea polyphenols have been found to be efficient against a variety of foodborne pathogenic bacteria that can be harmful even fatal to human health (Bunkova et al., Citation2005). Polyphenolic compounds of tea have antibacterial effects against a number of such destructive bacteria, namely: Clostridium perfringens, Staphylococcus aureus, Bacillus cereus, Vibrio parahaemolyticus, Vibrio fluvialis, Vibrio metchnikovii, Aeromonas sobria (Ho, Lin, & Shadidi, Citation2009).
Bay (Laurus nobilis) is an evergreen tree of Lauraceae family that is originally from the Mediterranean region. Bay leaves have applications in the culinary and food industry as a spice and aroma substance. It is known in the field of herbal medicine for its antibacterial, antifungal, anti-diabetes and anti-inflammatory properties (Fang et al., Citation2005). Dadalioglu and Evrendilek (Citation2004) studied the antibacterial properties of bay essential oil using four different foodborne pathogens (Listeria monocytogenes, Staphylococcus aureus, S. typhimurium, and E. coli.O157:H7), where strong antibacterial activity was observed in all of the cases.
Many Enterobacteriaceae, and certain lactobacilli (e.g., Lactobacillus buchneri, Lactobacillus curvatus), pediococci and enterococci are particularly active in the formation of biogenic amines. Histamine has one of the highest biological activities of all the amines, and its production of particular interest. Histidine decarboxylase activity is widely distributed in the genera Escherichia, Salmonella, Clostridium, Bacillus, and Lactobacillus (Pons-Sanchez-Cascado, Bover-Cid, Veciana-Nogues, & Vidal-Carou, Citation2005). The European Council of Directive regulated the maximum level of histamine in fish belonging to the Scombridea, Coryphaenidae, Engraulidae, Clupeidae, Pomatomidae and Scrombresosidae families. According to the Commission Regulation (EC) No 2073/2005, the maximum limits are given both in raw (100mg/kg) and salted fish products (200mg/kg). Histidine decarboxylase (HDC) is a specific enzyme (E.C.4.1.1.22) that catalyzes the formation of histamine. Histamine has a significant role in various physiological reactions including edema, rash, migraine headaches, vasodilatation, gastric acid secretion, heart palpitations, neurotransmission and smooth muscle contraction. Several studies showed that EGCG in tea inhibits the HDC enzyme (Melgarejo, Urdiales, Sanchez-Jimenez, & Medina, Citation2010; Ohshita, Okigami, Nitta, & Ueno, Citation2007; Rodriguez-Caso, Rodriguez-Agudo, Sanchez-Jimenez, & Medina, Citation2003). Inhibitory effects of some spice extracts on HDC activity was studied by Rezaei et al. (Citation2007). Bay leaf is very effective with other extracts such as clove, allspice, and cinnamon (Ohshita et al., Citation2007).
Once the histidine decarboxylase is present in the fish, it can continue to produce histamine in the fish even if the bacteria are not active. The enzyme can be active at or near refrigeration temperatures. While enzyme remains stable in the frozen state, it may be reactivated very rapidly after thawing (FDA, Citation2011). Natural antimicrobials and plant extracts are a new prospect for the inhibition of biogenic amine producing bacteria.
Therefore, the aim of the study was to determine the effects of green tea and bay leaf extract on the chemical, microbial, sensory, color characteristics, and biogenic amine profile of marinated anchovy products.
2. Materials and methods
2.1. Raw material
Anchovy (Engraulis encrasicolus) was caught in the Black Sea, near Samsun Province in 2015 winter. Following the harvest, anchovies were transferred to the fish processing plant at North Point Black Sea Fish Co., Samsun, Turkey, in polystyrene foam fish boxes containing ice. Fresh anchovies were blast frozen (−35°C) about 24 h in cold storage rooms and kept at −18°C until processing. Frozen fish were brought to the processing area and thawed under running tap water. Following the thawing process, fish in boxes were weighed and brought to the evisceration, gutting area. Gutted, cleaned fish were taken into marination area at the same day. Fishes were marinated about a day in fish baskets with brine (9% salt and 4.2% vinegar). Green tea extract, bay leaf extract was added to the marination brine. Batches consisted from 1% (w/v) green tea extract (GT1), 2% (w/v) green tea extract (GT2), 0.1% (w/v) bay leaf extract (BL0.1) and 0.2% (w/v) bay leaf extract (BL0.2) and control group. The volume and percentage of plant extracts were decided based on literature information. (Gokoglu, Topuz, & Yerlikaya, Citation2009; Ozogul et al., Citation2014, Citation2017; Ucak, Ozogul, & Durmus, Citation2011). Fish were drained and 100 g fish samples were placed on polyethylene packages with vegetable oil (sunflower) and vacuum packaged. Samples were kept at 4°C and analyzed at 0, 30, 150, and 240 days of storage. Photograph of 1% green tea extract added samples are given in .
Figure 1. Image of samples. First line from left to right- Control-BL0.1-BL0.2. Second line from left to right-Control-GT1-GT2. GT1 = Green tea extract 1%, GT2 = Green tea extract 2%, BL0.2 = Bay leaf extract 0.2%, BL0.1 = Bay leaf extract 0.1%
Figura 1. Imágenes de muestras. Primera fila de izquierda a derecha- Control-BL0.1-BL0.2Segunda fila de izquierda a derecha-Control-GT1-GT2GT1 = Extracto de té verde 1%, GT2 = Extracto de té verde 2%, BL0.2 = Extracto de hojas de laurel 0.2%, BL0.1 = Extracto de hojas de laurel 0.1%.

2.2. Extract preparation
Green tea extract was provided by Aromsa, Gebze, Turkey. The dried aerial parts of bay leaf were powdered in a mill. For water extraction, a 25 g sample was boiled with 0.5 L of distilled water for two hours and filtered through Whatman No. 1 filter paper. Then, a clear mixture was obtained. The crude extract was left in the freezer (−20°C) for 24 h and lyophilized in a Christ Alpha 1–2 LD Plus lyophilizer (Christ Model; Martin-Christ, Osterode, Germany) at −50°C. The freeze-dried water extract was kept at −20°C in sterile tubes until usage.
2.3. Proximate analysis
Protein, ash, and moisture content was determined in triplicate for marinated and raw anchovy. Protein content was analyzed according to the Kjeldahl Method (AOAC, Citation2002). Crude protein was calculated by multiplying total Kjeldahl nitrogen by 6.25. Samples were burned at 550°C for 5 h and ash content was determined from the weight of the sample. Moisture content was determined from the weight loss of the sample after being oven-dried at 105°C for 3 h until constant weight.
2.4. Chemical analysis
pH value was measured by homogenizing 10 g of fish sample in 100 mL of distilled water using a pH meter equipped with an electrode (Cyberscan PC 510, Singapore). Total volatile basic nitrogen (TVB-N) was determined with the distillation equipment (Buchi Distillation Unit K-350). Thiobarbituric acid reactive substances (TBARS) was determined in accordance with the procedure proposed by Lemon (Citation1975) and lipid oxidation products were determined as malondialdehyde (MA, mg/kg) equivalents.
Free fatty acid (FFA) analysis, expressed as % of oleic acid, was determined according to the AOCS method (AOAC, Citation1995).
2.5. Microbiological analysis
Marinated anchovy samples (25 g) were transferred aseptically to a Stomacher bag containing 225 mL of 0.85% normal sterile saline and homogenized for 180 s using a Stomacher at highest speed (Stomacher, IUL Instrument, Spain). Total viable bacteria counts, Enterobacteriaceae, and lactic acid bacteria were determined. Plate count agar (PCA) was used for the enumeration of the total viable count and incubated for 2 days at 30°C. For total Enterobacteriaceae and lactic acid bacteria, violet red bile agar (VRBA) and MRS agar were prepared and pour plate method was used. Plates were incubated for 5 days at 30°C for lactic acid bacteria count (MRS agar) and 24 h (VRBA) at 37°C for Enterobacteriaceae count. Microbiological data were expressed as a logarithm of the number of colony forming units (log cfu) per gram of sample.
2.6. Determination of biogenic amines
Biogenic amine content of the samples was determined according to the method of Eerola, Hinkkanen, Lindfors, and Hirvi (Citation1993). Two grams of samples were taken and homogenized with 0.4 M perchloric acid with Ultraturrax blender. Centrifugation at 3000 rpm for 10 min was performed. The supernatant was collected and it was brought to 25 mL with 0.4 M perchloric acid. This solution was filtered through Whatman number 1 filter paper. A 1 mL of supernatant or standard solution was transferred into 10 mL glass tubes and mixed with 20 mL of internal standard (1,7-diaminoheptane), 0.2 mL 2 M NaOH and 0.3 mL saturated aqueous solution of Na2CO3. Derivatization was done with 2 mL of dansyl chloride. The mixture was shaken and heated in a water bath in the dark for 45 min at 40°C. After the reaction, the mixture was cooled and the dansylation reaction was interrupted using 0.1 mL of ammonia (25% v/v).
The 20 of standard and sample derivatives were injected to HPLC (Shimadzu Corporation, Kyoto, Japan). Chromatographic separation was performed using a gradient elution of Ammonium acetate (0.1 M, solvent A) and acetonitrile (100%, solvent B) The gradient-elution program began at 50% solvent B and ended at 90% solvent B in 25 min. Equilibration of the system was done for 10 min before the following analysis. The column temperature was 40°C and the flow rate was 1.0 mL/min. and. A 20 μL sample was injected. Column effluent was monitored at 254 nm with 550 nm as reference using the HPLC system with a column Spherisorb ODS2 150A, 150 × 4.60 mm (Waters, Milford, MA, USA), HPLC (Shimadzu Corporation, Kyoto, Japan) (CBM-20A system control unit, LC-20AT pomp unit, SIL-20A35 autosampler, SPD-M20A PDA detector, and a computer including the Shimadzu package program). The quantitative determinations were carried out with internal standard (1.7-diaminoheptane) method, by using peak areas. Biogenic amine contents were stated as mg/kg. The limit of detection (Quelas et al., Citation2016) was between 0.005 and 0.050 μg/ml and limits of quantification (LOQ) were between 0.010 and 0.100 μg/ml for different biogenic amines.
2.7. Color measurements
Instrumental color analyses were performed using a Hunterlab ColorFlex EZ-45/0 spectrophotometer (Reston, VA, USA). The measurements were done directly on the flesh of marinated anchovies after opening the vacuum packages. Four measurements were performed on each marinated anchovy packages with 3 parallels. Hunter L (lightness; 100 = white, 0 = black), a (redness; +, red; −, green), b (yellowness; +, yellow; −, blue) values were measured.
2.8. Sensory evaluation
Taste panels were formed from ten department members who had been trained in the sensory evaluation of fish and fish products. Marinated anchovy samples in vacuum packaging were opened and served to panelists for evaluation of the sensory attributes (appearance, color, odor, flavor, and texture) by using modified form of Schormüller (Citation1968). The evaluation was made by giving scores between 1 and 9 for appearance, color, odor and flavor, and also sensory properties were indicated as: 9→Very good, 8→Quite Good, 7→Good, 6→Acceptable, 5→No comment, 4→Slightly bad, 3→Bad, 2→Quite bad 1→Very bad.
2.9. Statistical analysis
SPSS Version 21 for Macintosh (SPSS Inc., 119 Chicago, IL, USA, 2012) two-way analysis of variance was used for all of the statistical analyses. P ≤ 0.05 value was used to identify significant differences.
3. Results and discussion
3.1. Proximate composition
The compositional contents mean (±SD) of moisture, protein, salt, and ash (g/100 g fish muscle) in marinated anchovies with various plant extracts (0.1%, 0.2% bay leaf extract, 1%, 2% green tea extracts) analyzed are given in .
Table 1. Proximate composition of marinated anchovies.
Tabla 1. Composición aproximada de anchoas marinadas.
The addition of plant extracts to marination solution had no significant (p < 0.05) effect on moisture, protein, salt content of anchovy samples. Ash content was slightly higher (P < 0.05) in samples treated with 2% green tea extract. Raw material had 72.02% water, 18.12% protein, and 1.54% ash. Upon the marination process, water ratio decreased while protein and ash ratio increased as salt accumulated in fish flesh.
3.2. Chemical analysis and pH
TVB-N values of fresh anchovy and marinated anchovies including the marination brine can be seen in .
Figure 2. Changes in TVB-N value of samples during 240 days of storage at 4°C. n = 3, GT1 = Green tea extract 1%, GT2 = Green tea extract 2%, BL0.2 = Bay leaf extract 0.2%, BL0.1 = Bay leaf extract 0.1%
Figura 2. Cambios en el valor TVB-N de las muestras durante los 240 días de almacenamiento a 4°C. n = 3, GT1 = Extracto de té verde 1%, GT2 = Extracto de té verde 2%, BL0.2 = Extracto de hojas de laurel 0.2%, BL0.1 = Extracto de hojas de laurel 0.1%
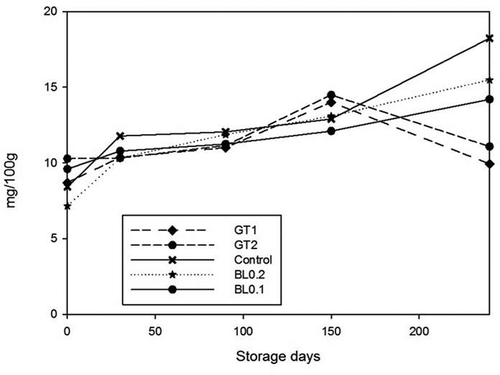
TVB-N value of fresh anchovy samples was found to be 14.75 ± 0.5 mg/100g which was similar to the value reported by Tomac and Yeannes (Citation2015). Topuz, Yerlikaya, Ucak, Gumus, and Buyukbenli (Citation2014) reported 9.82 mg/100g TVB-N value for the initial value of raw anchovy fillets and 8.97 mg/100g following the marination process. The levels of 30–35 mg/100 g muscle are considered the limit of acceptability for processed fish (Clucas, Citation1982).
TVB-N value in raw anchovy fillets decreased right after the marination procedure with wine vinegar, salt, and citric acid. Vinegar and salt leached out the TVB-N components into the marination solution. Marination process acts as a solvent extractor of TVB-N according to our results. Initial mean TVB-N values in marinated anchovies with various plant extracts were 8.70 ± 1.81 mg/100g, 10.28 ± 1.81 mg/100g, 9.60 ± 1.29 mg/100g, 7.15 ± 1.12 mg/100g for GT1, GT2, BL0.1, BL0.2, respectively. These values reached to 9.93 mg/100g, 11.08 mg/100g for GT1 and GT2, 14.20 mg/100g, 15.48 mg/100g for BL0.1 and BL0.2 after 8 months of storage at 4°C. In control samples, TVB-N values increased from 8.40 mg/100g to 18.23 mg/100g in 8 months of storage. Our results were below the maximum permissible value of 25–35 mg/100 g set by the EU regulation (No 1022/2008) for different fish species.
TBARS is a second breakdown product of lipid oxidation and commonly used as an indicator of lipid oxidation. The lipid oxidation is evidenced by measuring malondialdehydes (MA), which are the initial reaction products of polyunsaturated fatty acids with oxygen (Aubourg, Citation1993). The consumable limit value of the TBARS content is between 7 and 8 mg MA/kg. In the present study, the TBARS value of fresh anchovy was 0.76 mg MA/kg. Following the brining processes, sharp increases were observed and the TBARS value of final product reached to the level of 1.17 mg MA/kg ().
Figure 3. Changes in TBARS value of samples during 240 days of storage at 4°C.n = 3, GT1 = Green tea extract 1%, GT2 = Green tea extract 2%, BL0.2 = Bay leaf extract 0.2%, BL0.1 = Bay leaf extract 0.1%Figura 3. Cambios en el valor TBARS presentados en las muestras durante los 240 días de almacenamiento a 4°C. n = 3, GT1 = Extracto de té verde 1%, GT2 = Extracto de té verde 2%, BL0.2 = Extracto de hojas de laurel 0.2%, BL0.1 = Extracto de hojas de laurel 0.1%
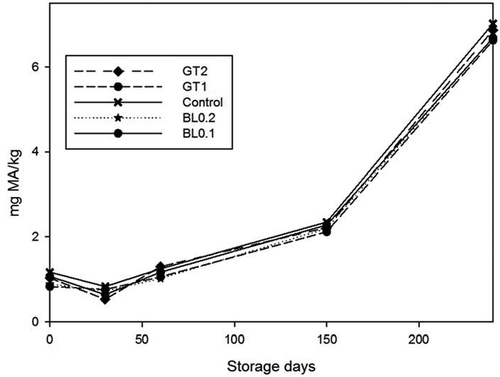
According to statistical variance analysis, significant difference (p < 0.05) was observed between groups starting from the first month of storage. Lowest TBARS level (0.76 mg MA/kg was found in samples treated with 2% green tea extract throughout the storage. Higher TBARS values were found in control samples compared to plant extract treated samples (GT1, GT2, BL0.1, and BL0.2) along the storage. Although the control sample reached the consumable limit of 7 mg MA/kg at the end of the storage, other samples were still below this limit. This indicates that plant extract treatments had a significant effect on the decrease of TBARS content in marinated fish by phenolic properties. GT2 and BL0.2, which containing a higher ratio of green tea and bay leaf extract, showed the highest antioxidant activity, followed by BL0.1 and GT1 showed the lowest antioxidant activity during the storage of samples. Antioxidant activity of bay leaf was previously reported on aqueous and aqueous extracts. Flavon-3-ols (Epicatechin, procyanidin), quercetin, luteolin, apigenin, kaempferol, myricetin are the predominant phenolic compounds in bay leaves. (Dias et al., Citation2014; Skerget et al., Citation2005). Antioxidant activity of green tea is dependent on tea polyphenol content, which increases with the tea concentration. (von Staszewski, Pilosof, & Jagus, Citation2011). A similar pattern of the TBARS value increment during the storage period was reported by several studies (Bensid, Ucar, Bendeddouche, & Ozogul, Citation2014; Gokoglu et al., Citation2009).
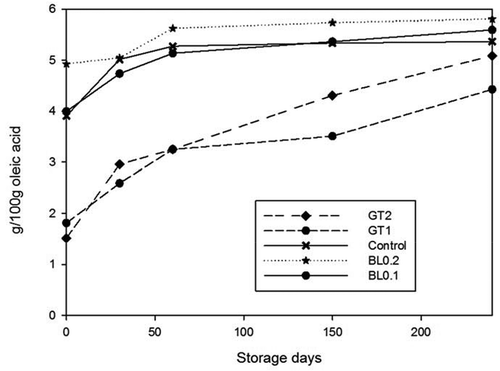
The initial free fatty acid values of marinated anchovies had significant differences (P < 0.05) among the groups. () The lowest values were determined in samples treated with green tea and no statistically significant difference was observed between 1% and 2% green tea and 0.1%- 0.2% bay leaf treated groups. Gokoglu, Yerlikaya, and Topuz (Citation2012) reported that tomato and garlic extract had a retarding effect on free fatty acids amount of marinated anchovies. Free fatty acids in all groups increased significantly (P < 0.05). Higher free fatty acids amount was found in the control sample than those plant extract treated groups for 8 months of storage at 4°C. This result might be related with the inhibitory effect of phenolic compounds of plant extracts on the release of free fatty acids by the enzymes.
Figure 4. Changes in free fatty acids value of samples during 240 days of storage at 4°C. n = 3, GT1 = Green tea extract 1%, GT2 = Green tea extract 2%, BL0.2 = Bay leaf extract 0.2%, BL0.1 = Bay leaf extract 0.1%
Figura 4. Cambios en el valor de ácidos grasos libres presentados en las muestras durante los 240 días de almacenamiento a 4°C. n = 3, GT1 = Extracto de té verde 1%, GT2 = Extracto de té verde 2%, BL0.2 = Extracto de hojas de laurel 0.2%, BL0.1 = Extracto de hojas de laurel 0.1%
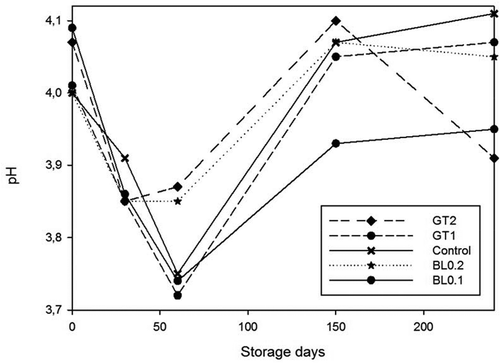
Marinades had lower pH values compared with other fish products due to the addition of vinegar and citric acid during marination process. Initially, the pH of the fresh anchovy fillets was 6.55. It was similar to the value with that reported by Gokoglu et al. (Citation2009). A Significant drop in pH value was seen during the marination procedure and the pH of the marinated flesh and marination solution reached to an equilibrium value of pH 4.00. pH value of the brine prepared with acetic acid and citric acid was 2.09. No significant differences (P < 0.05) were observed in the pH values of brine prepared with green tea extract and bay leaf extract (). Sen and Temelli (Citation2003) reported the mean pH value of frozen anchovies as 6.45 and 3.95–3.98 right after marination process. Varlik, Erkan, Metin, Baygar, and Ozden (Citation2000) stated that pH value must be between 4.1 and 4.5 in marinated products. pH values of marinated products decreased during the first 3 months of storage and started to rise between 3 and 5 months of storage. The result may be linked to the dissolution of carbon dioxide and hydrolysis of ATP to ADP and hydrogen ions in the anchovy samples, resulting in a decrease of pH value, however, the production of protein deterioration components such as trimethylamine and ammonia by fish spoilage bacteria lead to an increase in pH value.
Figure 5. Changes in pH value of samples during 240 days of storage at 4°C.n = 3, GT1 = Green tea extract 1%, GT2 = Green tea extract 2%, BL0.2 = Bay leaf extract 0.2%, BL0.1 = Bay leaf extract 0.1%
Figura 5. Cambios que se presentaron en el valor del pH de las muestras durante los 240 días de almacenamiento a 4°C. n = 3, GT1 = Extracto de té verde 1%, GT2 = Extracto de té verde 2%, BL0.2 = Extracto de hojas de laurel 0.2%, BL0.1 = Extracto de hojas de laurel 0.1%
3.3. Microbiological analysis
Total viable bacteria counts in fresh anchovies and vacuum packaged cold marinated anchovies are given in .
Table 2. Changes in the counts of total viable bacteria, lactic acid bacteria and total coliform bacteria during storage at 4°C.
Tabla 2. Cambios en la medición de bacterias viables totales, bacterias de ácido láctico y bacterias coliformes totales durante el almacenamiento a 4°C.
Total viable count (TVC) of control samples increased significantly during first 5 months of storage at refrigerated temperature. Plant extract treated samples reached the maximum level of 1.60 log cfu/g at the 1st month and dropped to less than 1 log cfu/g till the end of storage. Plant extracts were effective from the beginning of the storage period. Our initial microbial load was lower than (Gunsen, Ozcan, & Aydin, Citation2011). Cui et al. (Citation2012) reported that EGCG in green tea binds directly to peptidoglycans and breaks down the cell wall in gram positive and gram negative bacteria by forming pore-like lesions. Only few studies were conducted on green tea, bay leaf extract and their effect on native microbiota of food matrix. This might be due to the complexity of food systems and the interactions of food matrix with food antimicrobial substances.
The raw material showed good quality properties with the initial total viable bacterial load of 1.40 log cfu/g and less than 1 log cfu/g of lactic acid bacteria (LAB) and Enterobacteriaceae (TVC).
This could be due to good handling procedures at the fishing boat and the blast freezing (−30°C) procedure performed on fresh anchovies right after the arrival to the factory. These anchovies are stored at −18°C till the day of processing. After anchovies were placed in plastic baskets for marination, all these bacteria were inhibited following the marination process. Similar results were reported by Kilinc and Cakli (Citation2004).
In control samples, TVC continued to rise till 5 months of storage. This could be explained by the bacteria that was not completely inactivated after the marination process and were able to grow by adapting the acid environment. According to Leistner (Citation2000) when microorganisms use their energy to repair mechanisms so that their homeostasis would overcome the hostile environment, they might become exhausted and die. Several researches about marinated fish products show that the marination process done with salt and vinegar inhibited or decreased the number of microorganisms inside the fish (Kilinc & Cakli, Citation2004; Sallam, Citation2008; Sen & Temelli, Citation2003).
LAB are not considered as belonging to aquatic environments, but certain species (i.e. Lactobacillus, Lactococcus Carnobacterium, Vagococcus, Enterococcus) have been found in freshwater fish and their surrounding environment. Less than 1 log cfu/g LAB was determined in fresh anchovies while no existence of LAB was observed in all of the marinated anchovy groups throughout the storage. The same result was also reported by Kilinc and Cakli (Citation2004) for marinated sardines.
Less than 1 log cfu/g Enterobacteriaceae was found in raw anchovy fillets. Marinating had a significant effect on Enterobacteriaceae amount. During the storage period, Enterobacteriaceae could not be detected in all of the sample groups of vacuum packaged marinated anchovy. These results were probably due to both, the adequate vinegar/salt ratio in marination and with reduced pH level. In a study done by Sallam (Citation2008), complete inhibition of Enterobacteriaceae was achieved at 30 and 50 days for fillets marinated in solutions with 3% and 2% acetic acid, respectively.
3.4. Sensory analysis
Sensory analysis results are given in . According to the analysis best odor, color attributes were seen in samples treated with 0.1% bay leaf extract. Two percent Green tea extract was significantly different with having minimal score compared with the rest of the group. Darker color was observed for samples treated with green tea. Penetration of green tea extract into anchovy flesh caused brownish color. I. Ozogul et al. (Citation2014) investigated the effects of laurel and myrtle extracts on the sensory and quality aspects on the refrigerated eel. According to sensory results when the fish was not suitable for consumption sensory scores were 10.67 for control, 8.67 for laurel, 7.17 for myrtle. Bay leaf emulsion had a better color and taste property in cooked rainbow trout stored at 2 ± 2°C for 24 days (Ozogul et al., Citation2017). Color and odor values of marinated sauce crayfish were investigated by (Duman, Coban, & Ozpolat, Citation2015) and thyme, rosemary had a negative effect on the final product. Similar results were reported for sardines in pomegranate sauce by Gokoglu et al. (Citation2009). Sensory analysis result indicated that bay leaf extract addition gave better color results compared with control and green tea group. Customers prefer whitish plain color in marinated fish; therefore, green tea extract cannot be an alternative antioxidant to be used in this type of product.
Table 3. Sensory score of marinated anchovies with different treatment groups.
Tabla 3. Puntaje sensorial de las anchoas marinadas a partir de grupos de tratamiento diferentes.
3.5. Biogenic amines
The contents of biogenic amines in plant extracts treated marinated samples and brine solutions are summarized in and .
Table 4. Biogenic amine content (mg/kg) of brined anchovies during 240 days of storage at 4°C.
Tabla 4. Contenido de aminas biógenas (mg/kg) en anchoas en salmuera durante los 240 días de almacenamiento a 4°C.
Table 5. Biogenic amine content (mg/kg) of marination solutions and fresh anchovy.
Tabla 5. Contenido de amina biógena (mg/kg) en soluciones de anchoas marinadas y anchoas frescas.
In general, the biogenic amount in samples was very low or not detected (fish and vinegar + salt). This biogenic amine profile was also observed in several studies (Dehaut et al., Citation2014; Pons-Sanchez-Cascado et al., Citation2005). Putrescine was not detected in all of the marination solutions used. Initial contents of spermine and spermidine in ungutted anchovy and gutted anchovy were (0.58 ± 0.05 mg/kg, 0.36 ± 0.02 mg/kg) and (0.50 ± 0.07 mg/kg, 0.31 ± 0.06 mg/kg) respectively. These natural polyamines remained constant throughout the storage period during the study. Both polyamines are usually present in fresh fish because they are important for cellular growth and not responsible for bacterial spoilage. Although histamine levels showed fluctuations during the storage of marinated anchovies (up to 3.77 ± 0.65 mg/kg), they were still much lower than the maximum values permitted by the EU (200 mg/kg) for fresh and marinated anchovies. Low amount of histamine can be associated with salt level during marination process and quality of raw material. Lower histamine levels could be linked to the freshness of anchovies used in the manufacture of the product. Flick and Ankenman Granata (Citation2005) reported that 3.5–5.5% salt could inhibit some of histamine forming bacteria resulting in less histamine formation in food matrix. Microorganism in food and storage conditions determines the type and amount of biogenic amines formed. Enterobacteriaceae and lactic acid bacteria are responsible for biogenic amine formation in food matrix (Halasz, Barath, Simonsarkadi, & Holzapfel, Citation1994). In our study marination procedure inhibited LAB and TCB, therefore limited amount of biogenic amines were found in the samples we analyzed. No significant difference (P < 0.05) was observed for biogenic amines amount between plant extract applications. The initial microorganism load, processing conditions, hygiene applications, handling procedure are the main factors that may influence degredation of anchovies. Our results indicated that the process conditions the factory anchovies produced are in accordance with the food control systems like HACCP, BRC, IFS.
3.6. Color analysis
The color of fresh meat product is also reflected by another aspect of major importance from a consumer viewpoint. Hence, color stability during storage and the retail display is important to meat processing and the retailers. Color values of our samples are given in . Color attributes were measured by analyzing the interior part of anchovy fillets shaped as a butterfly. Significant differences (P < 0.05) were observed in L values of samples. GT2 had the lowest L score, following GT1. Green tea polyphenols were the main reason for this darkening effect. The highest L value was seen in BL0.1 samples. Regarding a value, negative values were determined in all of the groups except green tea applied group. B values of BL0.1, BL0.2, and control group decreased probably due to loss of water and lipid oxidation.
Table 6. Color changes of marinated anchovies during 240 days of storage at 4°C.
Tabla 6. Cambios de color presentados en las anchoas marinadas durante los 240 días de almacenamiento a 4°C.
4. Conclusion
Anchovy marinades have limited shelf life, and the main problem is lipid oxidation, increment of hydrolytic-oxidative rancidity because of the high proportion of long chain fatty acids present in anchovy. Green tea and bay leaf have reducing effect on TVB-N and TBARS levels. Green tea extract had a reducing effect on lipid oxidation parameters while giving an undesirable brownish color to the marinades. Both extracts did not have a negative effect on sensory properties. Biogenic amine amounts determined in fresh fish, marination solution, and plant extract treated samples were very low. No significant differences were observed between treatment groups. As a result, bay leaf extract with higher concentration could extend the shelf life of anchovy marinades as well as the sensorial properties of the product.
Acknowledgments
The authors thank North Point Black Sea-SASTAŞ Cooling Inc. for the production of the samples. We would like to thank AROMSA for providing the green tea extracts. The authors would like to thank the Ondokuz Mayis University Research Foundation (PYO.MUH.1901.13.010) for financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AOAC. (1995). Official methods of analysis of the association of official analytical chemists free fatty acids analysis (16 ed.). Washington, DC: Author.
- AOAC. (2002). Official methods of analysis (17th ed.) 981.10 Protein synthesis in meat and meat products. Washington, DC: Author.
- Aubourg, S. P. (1993). Interaction of malondialdehyde with biological molecules - new trends about reactivity and significance - review. International Journal of Food Science and Technology, 28(4), 323–335.
- Bensid, A., Ucar, Y., Bendeddouche, B., & Ozogul, F. (2014). Effect of the icing with thyme, oregano and clove extracts on quality parameters of gutted and beheaded anchovy (Engraulis encrasicholus) during chilled storage. Food Chemistry, 145, 681–686.
- Bunkova, R., Marova, I., & Nemec, M. (2005). Antimutagenic properties of green tea. Plant Foods for Human Nutrition, 60(1), 25–29.
- Clucas, I. J. (1982). Fish handling, preservation and processing in the tropics. London: Tropical Development and Research Institute.
- Cui, Y., Oh, Y. J., Lim, J., Youn, M., Lee, I., Pak, H. K., … Park, S. (2012). AFM study of the differential inhibitory effects of the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiology, 29(1), 80–87.
- Dadalioglu, I., & Evrendilek, G. A. (2004). Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. Journal of Agricultural and Food Chemistry, 52(26), 8255–8260.
- Dehaut, A., Himber, C., Mulak, V., Grard, T., Krzewinski, F., Le Fur, B., & Duflos, G. (2014). Evolution of volatile compounds and biogenic amines throughout the shelf life of marinated and salted anchovies (Engraulis encrasicolus). Journal of Agricultural and Food Chemistry, 62(32), 8014–8022.
- Dias, M. I., Barros, L., Duenas, M., Alves, R. C., Oliveira, M. B. P. P., Santos-Buelga, C., & Ferreira, I. C. F. R. (2014). Nutritional and antioxidant contributions of Laurus nobilis L. leaves: Would be more suitable a wild or a cultivated sample? Food Chemistry, 156, 339–346.
- Duman, M., Coban, O. E., & Ozpolat, E. (2015). Effects of rosemary and thyme oils on shelf life of marinated sauce crayfish. Journal of Animal and Plant Sciences, 25(6), 1771–1778.
- Eerola, S., Hinkkanen, R., Lindfors, E., & Hirvi, T. (1993). Liquid-chromatographic determination of biogenic-amines in dry sausages. Journal of Aoac International, 76(3), 575–577.
- Fang, F., Sang, S. M., Chen, K. Y., Gosslau, A., Ho, C. T., & Rosen, R. T. (2005). Isolation and identification of cytotoxic compounds from bay leaf (Laurus nobilis). Food Chemistry, 93(3), 497–501.
- FDA. (2011). Fish and fishery products hazards and controls guidance (4th ed.). Gainesville, FL: Diane publishing.
- Flick, G. J., & Ankenman Granata, L. (2005). Toxins in food. Boca Raton, FL: CRC Press.
- Gokoglu, N., Topuz, O. K., & Yerlikaya, P. (2009). Effects of pomegranate sauce on quality of marinated anchovy during refrigerated storage. Lwt-Food Science and Technology, 42(1), 113–118.
- Gokoglu, N., Yerlikaya, P., & Topuz, O. K. (2012). Effects of tomato and garlic extracts on oxidative stability in marinated anchovy. Journal of Food Processing and Preservation, 36(3), 191–197.
- Gunsen, U., Ozcan, A., & Aydin, A. (2011). Determination of some quality criteria of cold storaged marinated anchovy under vacuum and modified atmosphere conditions. Turkish Journal of Fisheries and Aquatic Sciences, 11(2), 233–242.
- Halasz, A., Barath, A., Simonsarkadi, L., & Holzapfel, W. (1994). Biogenic-amines and their production by microorganisms in food. Trends in Food Science & Technology, 5(2), 42–49.
- Ho, C. T., Lin, J., & Shadidi, F. (2009). Tea and tea products chemistry and health-promoting properties. Boca Raton, FL: CRC Press.
- Kilinc, B., & Cakli, S. (2004). Chemical, microbiological and sensory changes in thawed frozen fillets of sardine (Sardina pilchardus) during marination. Food Chemistry, 88(2), 275–280.
- Leistner, L. (2000). Basic aspects of food preservation by hurdle technology. International Journal of Food Microbiology, 55(1–3), 181–186.
- Lemon, D. W. (1975). An improved TBA test for rancidity. New Series Circular No. 51. Halifax: Environment Canada Fisheries and Marine Service, Halifax Laboratory, Canada.
- Melgarejo, E., Urdiales, J. L., Sanchez-Jimenez, F., & Medina, M. A. (2010). Targeting polyamines and biogenic amines by green tea epigallocatechin-3-gallate. Amino Acids, 38(2), 519–523.
- Ohshita, J., Okigami, H., Nitta, Y., & Ueno, H. (2007). Evaluation of inhibitory efffects of spice-derived natural extracts on histidine decarboxylase. Japan Society of Home Economics, 58(1), 17–22.
- Ozogul, I., Polat, A., Ozogul, Y., Boga, E. K., Ozogul, F., & Ayas, D. (2014). Effects of laurel and myrtle extracts on the sensory, chemical and microbiological properties of vacuum-packed and refrigerated European eel (Anguilla anguilla) fillets. International Journal of Food Science and Technology, 49(3), 847–853.
- Ozogul, Y., Yuvka, I., Ucar, Y., Durmus, M., Kosker, A. R., Oz, M., & Ozogul, F. (2017). Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. Lwt-Food Science and Technology, 75, 677–684.
- Pons-Sanchez-Cascado, S., Bover-Cid, S., Veciana-Nogues, M. T., & Vidal-Carou, M. C. (2005). Amino acid-decarboxylase activity of bacteria isolated from ice-preserved anchovies. European Food Research and Technology, 220(3–4), 312–315.
- Pons-Sanchez-Cascado, S., Vidal-Carou, M. C., Marine-Font, A., & Veciana-Nogues, M. T. (2005). Influence of the freshness grade of raw fish on the formation of volatile and biogenic amines during the manufacture and storage of vinegar-marinated anchovies. Journal of Agricultural and Food Chemistry, 53(22), 8586–8592.
- Quelas, J. I., Althabegoitiu, M. J., Jimenez-Sanchez, C., Melgarejo, A. A., Marconi, V. I., Mongiardini, E. J., . . . Lodeiro, A. R. (2016). Swimming performance of Bradyrhizobium diazoefficiens is an emergent property of its two flagellar systems. Scientific Reports, 6ARTN 23841. doi:10.1038/srep23841.
- Rezaei, M., Montazeri, N., Langrudi, H. E., Mokhayer, B., Parviz, M., & Nazarinia, A. (2007). The biogenic amines and bacterial changes of farmed rainbow trout (Oncorhynchus mykiss) stored in ice. Food Chemistry, 103(1), 150–154.
- Rodriguez-Caso, C., Rodriguez-Agudo, D., Sanchez-Jimenez, E., & Medina, M. A. (2003). Green tea epigallocatechin-3-gallate is an inhibitor of mammalian histidine decarboxylase. Cellular and Molecular Life Sciences, 60(8), 1760–1763.
- Sallam, K. I. (2008). Effect of marinating process on the microbiological quality of Pacific saury (Cololabis saira) during vacuum-packaged storage at 4 degrees C. International Journal of Food Science and Technology, 43(2), 220–228.
- Schormüller, J. (1968). Handbuch der lebensmittelchemie. Berlin: Springer-Verlag.
- Sen, M. K. C., & Temelli, S. (2003). Microbiological and chemical qualities of marinated anchovy prepared with different vegetable additives and sauce. Revue De Medecine Veterinaire, 154(11), 703–707.
- Skerget, M., Kotnik, P., Hadolin, M., Hras, H. R., Simonic, M., & Knez, Z. (2005). Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chemistry, 89(2), 191–198.
- Tomac, A., & Yeannes, M. I. (2015). Quality Changes in Gamma Irradiated Marinades of Anchovy (Engraulis anchoita) During Refrigerated Storage. Journal of Aquatic Food Product Technology, 24(7), 686–697.
- Topuz, O. K., Yerlikaya, P., Ucak, I., Gumus, B., & Buyukbenli, H. A. (2014). Effects of olive oil and olive oil-pomegranate juice sauces on chemical, oxidative and sensorial quality of marinated anchovy. Food Chemistry, 154, 63–70.
- Ucak, I., Ozogul, Y., & Durmus, M. (2011). The effects of rosemary extract combination with vacuum packing on the quality changes of Atlantic mackerel fish burgers. International Journal of Food Science and Technology, 46(6), 1157–1163.
- Varlik, C., Erkan, N., Metin, S., Baygar, T., & Ozden, O. (2000). Determination of the shelf-life of marinated fish balls. Turkish Journal of Veterinary & Animal Sciences, 24(6), 593–597.
- von Staszewski, M., Pilosof, A. M. R., & Jagus, R. J. (2011). Antioxidant and antimicrobial performance of different Argentinean green tea varieties as affected by whey proteins. Food Chemistry, 125(1), 186–192.
