ABSTRACT
Cordyceps militaris is widely cultivated as a functional food and traditional medicine in China and Southeast Asia. In this study, C. militaris hybrid strains were isolated from different parts of hybrid fruiting body cultivated through mating-based sexual reproduction of ascospores. We investigated the effect of strains, solid-state substrates and light condition on production of yield and contents of bioactive compounds (cordycepin and adenosine) using HPLC in fruiting bodies. The results indicated that yield and production of bioactive compounds exist distinct differences on strain separated part, solid-substrates and light conditions. The middle strain obtained the highest yield on wheat substrate. The yield was higher on wheat substrate, the concentration of cordycepin and adenosine in fruiting bodies were higher on rice substrate. The red light is beneficial to high yield, and white light is conducive to the accumulation of cordycepin in fruiting bodies, the light condition has little effect on the adenosine content.
RESUMEN
En China y Asia suroriental se cultiva ampliamente Cordyceps militaris, que sirve como alimento funcional y medicina tradicional. En el presente estudio se aislaron cepas híbridas de C. militaris de diferentes partes de los cuerpos fructíferos híbridos; éstas fueron cultivadas a través de la reproducción sexual, basada en el acoplamiento, de ascosporas. Usando HPLC en los cuerpos fructíferos se investigaron los efectos que las cepas, los sustratos en estado sólido y las condiciones de luminosidad tienen en la producción de rendimientos y contenidos de compuestos bioactivos (cordicepina y adenosina). Los resultados indican que existen diferencias en el rendimiento y la producción de compuestos bioactivos, según se trate de cepas de partes separadas, sustratos en estado sólido, o condiciones de luminosidad. La cepa intermedia registró el rendimiento más alto en el sustrato de trigo. Los rendimientos fueron más altos en los sustratos de trigo, mientras la concentración de cordicepina y adenosina de los cuerpos fructíferos fue más elevada en el sustrato de arroz. La luz roja favorece un rendimiento elevado, mientras que la luz blanca propicia la acumulación de cordicepina en los cuerpos fructíferos. Las condiciones de luminosidad tuvieron poco efecto en el contenido de adenosina.
1. Introduction
Cordyceps militaris (taxonomy information is listed in ) is a model species of Cordyceps sensu lato that comprises more than 600 pathogenic species of arthropods (Sung et al., Citation2007). Many natural species of Cordyceps are used in traditional medicine in China and Southeast Asia for many years. Ophiocordceps sinensis is the most famous and expensive species, but not easy to cultivate; by contrast, C. militaris can be cultivated on cereal substrate and insect larvae or pupae (Adnan, Ashraf, Khan, Alshammari, & Awadelkareem, Citation2017; Paterson, Citation2008; Zhou, Gong, Su, Lin, & Tang, Citation2009), and content of active components are similar to natural ones (Frederiksen, Malling, & Klenow, Citation1965; Hamburger, Citation2007; Kodama, McCaffrey, Yusa, & Mitsuya, Citation2000; Xiong, Xia, Zheng, Shi, & Wang, Citation2010; Yoo, Shin, Cho, Son, & Lee et al., Citation2004). Isolates derived from spores or tissues are used for artificial culturing of fruiting bodies. However, wild C. militaris has reduced gradually by overexploitation and ecologic environment destruction, the wild strains resources cannot meet the development of the industry. Genomic and genetic analyses indicate that C. militaris is heterothallic, with the MAT1-1 locus containing two genes and the MAT1-2 idiomorph comprising one gene (Zheng et al., Citation2011; Zheng, Xia, Zhang, & Wang, Citation2013), which is a typical bipolar mating system. It is an effective way to select strains by cross-mating based on mating types for artificial culturing of C. militaris (Wen, Kang, Li, & Lei, Citation2008).
Table 1. Taxonomy information of the Cordycepes militaris.
Tabla 1. Información taxonómica de Cordycepes militaris.
Cordycepin and adenosine are the major active constituents of C. militaris (Shrestha, Kim, Sung, Spatafora, & Sung, Citation2004; Yokoyama, Yamagishi, & Hara, Citation2005). Cordycepin has demonstrated to have anti-tumor, anti-leukemic, anti-metastatic, anti-bacterial, anti-viral, anti-trypanosomiasis, anti-restenosis, immunomodulatory and anti-inflammatory activities (Paterson, Citation2008). Adenosine can modulate cell proliferation, differentiation, and apoptosis (Szentmiklosi et al., Citation2015; Yang, Wang, Garciaroves, Björnholm, & Fredholm, Citation2010). Due to the high pharmacological activities (Cunningham, Manson, Spring, & Hutchinson, Citation1950), cordycepin and adenosine have recently drawn great attention in natural medicine research. Strain screening (Das, Masuda, Hatashita, Sakurai, & Sakakibara, Citation2008), additives (Fan, Wang, & Zhong, Citation2012; Sari, Suparmin, Kato, & Park, Citation2016), and cultural condition (Tang, Qian, & Zhu, Citation2015) can significantly influence the production of cordycepin.
Light is an essential environmental factor for C. militaris primordial initiation and fruiting bodies grown (Sato & Shimazu, Citation2002). After exposure to light, the hyphae color changes from white to yellow or orange, and then the primordia begin to develop. Light affects biomass growth (Kho et al., Citation2016) and metabolism, such as cordycepin and carotenoid formation (Dong et al., Citation2013b; Lian, Dong, Yang, & Sun, Citation2014). Fluorescent lamps (Wu, Liang, Tseng, & Hu, Citation2016) and light-emitting diode (LED) (Chiang, Liang, Wang, & Liang, Citation2017; Kho et al., Citation2016) can be used to cultivate C. militaris fruiting bodies. The light intensity that influences the mannitol and polysaccharide contents has been discussed (Wu et al., Citation2016). Fluorescent lamps with longer service life and low cost have been used in plant tissue culture and glasshouse generally.
Although crossing mating through mating-types and effect of light on the growth of C. militaris fruiting bodies has been observed, few studies have examined the growth characteristic of strains which were isolated from hybrid fruiting bodies. In this study, the hybrid strains were isolated from top, middle and base of the fruiting body after crossing through mating types. The yield and bioactive compounds contents in fruiting bodies, which were cultivated on wheat and rice substrate, were investigated. Then comparing the effect of light condition on the yield, cordycepin and adenosine content in fruiting bodies of the middle strain. The contents of cordycepin and adenosine in all strains were analyzed through high-performance liquid chromatography (HPLC). The results will provide guidelines for selecting superior strains and improving yield and quality by optimizing the culture conditions of C. militaris.
2. Materials and methods
2.1. Reference standards and reagents
Standards of cordycepin (purity≥99.6% batch No. CC161213, Hefei Bomei Biological Technology Co.) and adenosine (purity≥99%, batch No. Z23S7J21814, Shanghai source leaf biotechnology Co.) were purchased. Methanol (HPLC-grade) was purchased from Fisher Scientific Co. (Franklin, USA), and all the other chemicals were of analytical grade. LC-grade water was prepared using redistilled water equipment. Fluorescent lamps (Y230 T8W/R/B 30W) which were purchased from Foshan Electrical and Lighting Co., Ltd (Guangdong, China). Incubator (Zxjd-A1270) was purchased from Shanghai Zhicheng Analysis Instrument Co., Ltd (Shanghai, China).
2.2. Fungal specimen material
C. militaris CM1406 and CM1409 was obtained from the Shihezi University of Life Science and Key Laboratory of Agricultural Biotechnology (Xinjiang, China). The mature fruiting bodies were attached with plastic tape to the inner side of a sterilized Petri dish (9-cm diameter), which was then placed over a 1.2% (w/v) agar plate. The moisture accumulated in the Petri dish stimulated the discharge of ascospores from the stromata. After 4–6 h of incubation at room temperature under continuous light, a tiny agar piece with a single ascospore was excised using a dissecting needle with the aid of a stereo-microscope (60× magnification) and transferred to a new Petri dish containing modified PDA agar medium (200 g/L potato, 20 g/L glucose, 5 g/L peptone, 0.5 g/L MgSO4·7H2O, 1.5 g/L K2HPO4, 0.01 g/L vitamin B1 and 12g/L agar in 1000 ml distilled water, pH = 6.0), which was incubated at 25°C under darkness for 2 weeks. The hyphae from well-isolated colonies were transferred to PDA slants that were incubated for 10 days under the same conditions.
2.3. Identification of the mating type of ascospores
The hyphae were scraped from the surface of the medium with a sterilized toothpick; genomic DNA was extracted from C. militaris strains using a modified cetyltrimethylammonium bromide (CTAB) DNA isolation technique.
Two sets of specific primers were used to determine the mating types of ascospores. MAT1-1 and MAT1-2 were identified with the primers listed in based on the partial MAT-α (accession no. AB194982.1) and MAT-HMG (accession no. AB084257.1) sequences in the NCBI database. PCR amplifications were performed using the following program: 94°C for 3 min; 35 cycles of 94°C for 30 s, 56°C for 30 s, 72°C for 30 s, and a final extension of 72°C for 10 min. The PCR products were visualized using 1.2% agarose gel electrophoresis.
Table 2. Specific primers for mating-type genes from Cordyceps militaris.
Tabla 2. Primers específicos para genes del tipo de acoplamiento de Cordyceps militaris.
2.4. Cross-mating with ascospores
Strains with different mating types were simultaneously inoculated in equivalent proportions in the wheat solid-substrates for cross-mating. Seed cultures were prepared by transferring a loopful of colony from PDA slants into a 150-mL Hinton flask containing 50 mL liquid medium (200 g/L potato, 20 g/L glucose, 5 g/L peptone, 0.5 g/L MgSO4·7H2O, 1.5 g/L K2HPO4 and 0.01 g/L vitamin B1 in 1000 ml distilled water, pH = 6.0). The cultures were incubated at 25°C at 120 rpm for 4 days.
A total of 30 g wheat with 45 ml prepared liquid medium plus silkworm pupae (20 g/L) were added to each box, which was then sealed with plastic and sterilized in an autoclave for 20 min at 115°C. The sterilized substrates were inoculated with 5 ml liquid spawn in each bottle, followed by culturing at 20–22°C in darkness until the substrates were covered by white mycelia. These bottles were transferred to a culture room at 22°C under scattered sunlight of 1000 lx for 14 h and darkness at 12°C for 10 h. After 7 days, all bottles were cultivated at 22°C with a 10:14 h light: dark cycle and humidity of 60–80%. The fruiting bodies were observed after 45 days. Three replicates were performed for each treatment.
2.5. Tissue isolation from hybrid and cultivated fruiting bodies
The fresh and complete fruiting bodies were surface sterilized with 75% alcohol (60 s), followed by three washes in sterile water. Tissues (1 cm) were isolated from the top, middle, and base of the same fruiting body and then placed in modified PDA agar medium (as in Section 2.2) and cultured at 25°C in darkness. Mating type was identified as specified in Section 2.3, seed and fruiting bodies cultures of the hybrid strains of the mating types with Mat1-1 and Mat1-2 on solid wheat and rice substrates, as well as the parental strains, is specified in Section 2.4.
2.6. Effect of light condition on yield and quality
The white, red and blue lamps were used for plant culturing in phytotron and selected to evaluate the effect on the yield and bioactive compounds contents, including cordycepin and adenosine. The distance between the lamps and the bottles was adjusted according to the intensity of 1000 lx (2-white, 2-red, and 2-blue), 1500 lx (1-white +1-red and 1-blue +1- red) 2000 lx (1-blue +1-white +1- red). The distance of each lamp was 1 cm, and the bottles were directly beneath the lamps. The strain was cultured in liquid medium for 4 days and then inoculated with 5-ml liquid spawn into each bottle which contained sterile wheat substrates.
After the substrates were covered by hyphae, the bottles were transferred to a culture room at 22°C with a 14:10 h light:dark cycle and darkness at 12°C in incubator for 10 h. After 7 days, all the bottles were cultivated in the culture room at 22°C with a 10:14 h light:dark cycle and 60–80% humidity until fruiting bodies were observed after 45 days. Each treatment was supplied with the same light condition during the cultivation. Three replicates were performed for each treatment.
2.7. Sample treatment
The dry (dried at 60°C for 24 h) weights of the fruiting bodies were recorded for statistical analysis. The samples were pulverized and sifted through a 60-mesh stainless steel sieve. An accurately weighed powder sample (0.2 g) was placed in a 50-ml flask with a stopper, extracted using an ultrasonic method with 25 ml double-distilled water for 30 min and filtered through a 0.22-µm filter membrane prior to injection into the HPLC system.
2.8. Instrumentation and chromatographic conditions
A Waters 2695 Alliance HPLC system with a Waters 2998 PDA detector was used. The analysis was performed on a Scienhome Kromasil C18 column (4.6 mm × 150 mm, 5 µm) at a column temperature of 30°C, using (A) 11% methanol and (B) water as the mobile phase with the gradient elution procedure shown in . The flow rate was 1.0 ml/min, and the detection wavelength was 260 nm. The injection volume was 10 µl, and the bioactive components were well separated under the chromatographic conditions specified above.
Table 3. Time program of the gradient elution.
Tabla 3. Programa de tiempo de elución en gradiente.
2.9. Validation of the method
Accurate amounts of cordycepin and adenosine were dissolved in 90% methanol solution at 2 mg/mL as a stock solution. Linearity was established by injection of 1, 2, 4, 9, 12, 16, and 20 µL of the prepared mixed reference standard solution. Calibration graphs were plotted based on linear regression analysis of the integrated park (Y) versus content (X ng). The mixed standard stock solution was diluted 5-fold by injection of 1, 2, 3, 4, and 5 µL to detect the limit of detection (LOD) and limit of quantification (LOQ). The precision of the chromatographic system was validated by injecting 10 µL of the mixed reference solution six times during one day. A stability study was performed with a sample solution over 12 h (the time-points were 0, 2, 4, 8, 10, and 12 h). Variations were expressed as the relative standard deviation (RSD) of the peak area. The repeatability test was analyzed by injecting six independently prepared samples (the concentration and prepared method specified in section 2.6). The RSD value of the amount was adopted to evaluate repeatability. The recovery tests were studied by adding a mixed reference standard solution to a sample to yield a final concentration. The experiments were repeated six times.
3. Results
3.1. Mating type of single-ascospore and hybrid strains
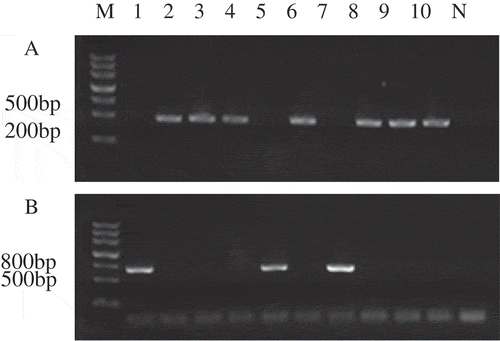
DNA extracted from different single-ascospore strain was used as a template for PCR amplification, which generated the expected 427-bp amplicon for MAT1-1 and 719-bp amplicon for MAT1-2. The single ascospores from the CM1406 and CM1409 strains showed different nuclear compositions (). Strains CM1406-2, CM1406-3,CM1406-4, CM1409-1,XM1409-3, CM1409-4, CM1409-5 were MAT1-1 mating type, and strains CM1406-1, CM1406-5, and CM1409-2 were MAT1-2 mating type.
Figure 1. PCR assay for mating type of ascospore M: molecular Ⅲ, 1–5: ascospore of CM1406; 6–10: Ascospore of CM1409; N: Negative control; (a) PCR products with MAT-α primers; (b) PCR products with MAT-HMG primers.
Figura 1. Ensayo PCR para el tipo de acoplamiento de la ascospora M: molecular Ⅲ, 1–5: ascospora de CM1406; 6–10: ascospora de CM1409; N: Control negativo; (a) Productos PCR con primers MAT-α; (b) Productos PCR con primers MAT-HMG.
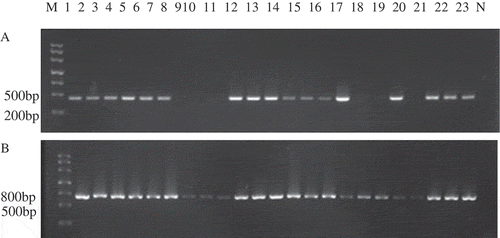
After verifying the mating type genes of single ascospore strain, different ascospores were selected for crossing on wheat substrates. The results indicated that combinations CM1406-1 (MAT1-2) and CM1409-5 (MAT1-1) produced more abundant fruiting bodies (); however, all of the single ascospore strain produced few or did not produce fruiting bodies. The strains isolated from different fruiting bodies possessed one (MAT1-1 or MAT1-2) or two (MAT1-1 and MAT1-2) of mating type genes (), but different parts of the same fruiting body had the same mating type. It is clear that despite obvious heterothallism, homothallism was occasionally observed in C. militaris.
Figure 2. Fruiting bodies produced of combinations CM1406-1×CM1409-5.
Figura 2. Cuerpos fructíferos producidos por combinaciones de CM1406-1×CM1409-5
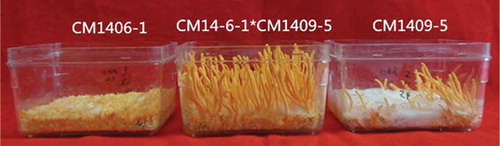
Figure 3. PCR assay for mating types of hybrid strains M: molecular Ⅲ, 1–23: different Strains; N: Negative control (a) PCR products with MAT-α primers; (b) PCR products with MAT-HMG primers.
Figura 3. Ensayo PCR para los tipos de acoplamiento de cepas híbridas M: molecular Ⅲ, 1–23: distintas cepas; N: Control negativo (a) Productos PCR con primers MAT-α; (b) Productos PCR con primers MAT-HMG.
3.2. Effect of strain separated parts and solid-state substrate on yield and quality
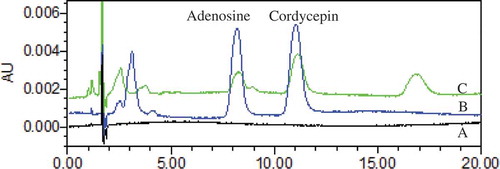
The strains with MAT1-1 and MAT1-2 mating-type isolated from the top, middle and base of the same fruiting body and parental strains were cultured on wheat and rice solid-substrate (n = 3). The production of certain strains isolated from the hybrid fruiting body was higher than the parental strains (). There was no significant difference of top strain on rice and wheat substrate. The middle strain produced the highest dry weight (3.39 ± 0.16 g) on the wheat substrate. As for the rice substrate, the top strain obtained the higher dry weight (2.36 ± 0.28), which showed no significant difference with the base strain. Under the chromatographic conditions adopted in the study, all calibration curves exhibited good linearity (r2 ≥0.9945) across a relatively wide linear range as shown in . The precision, stability, recovery, and repeatability of cordycepin and adenosine are shown in . The RSD of the precision variations were less than 1.6%. Validation studies of the method proved that this assay had good reproducibility with RSD less than 1.89% and the sample solutions were stable during 12 h at room temperature with RSD less than 1.9%. The recovery results showed that this method had good accuracy with the overall recovery of 100.1% and 99.87%. Under the chromatographic condition, the cordycepin and adenosine had a good separation ().
Table 4. Linear regression equation of investigated compounds.
Tabla 4. Ecuación de regresión lineal de los compuestos investigados
Table 5. Precision, stability, recovery, and repeatability data.
Tabla 5. Datos sobre precisión, estabilidad, recuperación y repetibilidad.
Table 6. Yield of stromata and bioactive compound production of different strains.
Tabla 6. Rendimiento de estromas y compuestos bioactivos de distintas cepas.
Figure 4. Stacked view of HPLC chromatograms (a) solvent (b) mixed reference standards; (c) sample.
Figura 4. Vista simultánea de cromatogramas HPLC (a) solvente (b) estándares de referencia mixta; (c) muestra
The cordycepin and adenosine contents in fruiting bodies were determined using the calibrated curve of investigated compounds (). The cordycepin content was ranked as follows on wheat substrates: middle strain (0.050%) >CM1409 (0.046%) > top strain (0.045%) > base strain (0.019%) > CM1406. However, on rice substrates, the cordycepin content was ranked as follows: top strain (0.082%) > base strain (0.078%) > CM1409 (0.062%) > middle strain (0.035%) > CM1406 (0.026%).
On wheat substrates, the adenosine content was ranked as follows: base strain (0.246%) > CM1406 (0.185%) > top strain (0.14%) > CM1409 (0.135%) > middle strain (0.123%). By contrast, on rice substrates, the adenosine content was ranked as follows: middle strain (0.239%) > top strain (0.193%) > CM1409 (0.174%) > CM1406 (0.150) > base strain (0.032%). Cordycepin could not be detected in CM1406 on wheat substrates, but the adenosine content was 0.185% higher than on rice substrates. The hybrid strain had advantages in yield and some bioactive compounds. The yield of middle and base strain was significantly higher on the wheat substrate than the rice substrate. The rice substrate was conducive to the accumulation of cordycepin for top and base strains; adenosine content was higher on rice substrate for the top and middle strain.
3.3. Effect of light condition on yield and quality
The middle strain was inoculated on wheat substrates and cultured under different light conditions. As shown in , the red light and red + white light condition dramatically improved yield, which was similar to studies by Dong (Dong, Liu, Lei, Zheng, & Wang, Citation2012) and Chiang (Chiang et al., Citation2017), but the cordycepin content was significantly lower than other light conditions. The highest content of cordycepin was observed under the white light condition which is 5.4 fold compared to red light, i.e., 1.38 g ± 0.15 dry weight. The light condition had a significant influence on production of fruiting bodies and bioactive compounds. Red light of 1000 lx can significantly improve the number of fruiting bodies, but was not conducive to accumulate cordycepin. The content of adensine remained relatively stable under different light conditions.
Table 7. Yield of stromata and bioactive compound production under different light conditions.
Tabla 7. Rendimiento de la producción de estromas y compuestos bioactivos bajo distintas condiciones de luminosidad.
4. Discussion
C. militaris has been used to cure diseases for the pharmacological values. For higher yield and bioactive compounds, superior strains were urgently needed for industrialized cultivation (Avin et al., Citation2016). Mating type genes have been reviewed for various homothallic and heterothallic species of filamentous ascomycetes (Turgeon & Yoder, Citation2000), and crossing mating through single ascospore (Bhushan, Han, Jae-Mo, & Gi-Ho, Citation2012) can provide abundant superior strains. In this study, we used 7 single ascospores with MAT1-1 and 3 single ascospores with MAT1-2 by cross-mating on the wheat substrate. The difference of fruiting bodies production between different cross combination and hybrid strains (figures not shown) showed that ascospores are genetically diverse. Although the mechanism of fruiting bodies formation is not entirely clear, cross-mating with ascospore provide an effective method to select strains for industrial-scale culturing of C. militaris and other edible mushrooms (Sung et al., Citation2006; Zhang & Yue, Citation2013). In this study, self-mating produced fruiting bodies () and some fruiting bodies contained only one mating gene locus () showed that C. militaris have heterothallic and homothallism. The characteristics of homokaryon strains deserve further research.
The spores or tissues from wild and cultured C. militaris can be used in the industrial cultivation (Bhushan et al., Citation2005). This is the first study to isolate stains from different parts of the fruiting body after ascospore crossing mating by mating types. However, the strains isolated from the same crossing combination or same fruiting body have different production of fruiting bodies and bioactive compounds, it is necessary to prior identify the mating-type and whether can produce fruiting bodies. The superiority of hybrid strains in production or bioactive compounds contents compared with parental strains which is similar with current works (Chen, Wang, Shao, & Huang, Citation2017). The cultural characteristics of ascospore showed diversity (Liang, Zhang, An, & Cai, Citation2005) and the superior strains can be selected from cross-mating by ascospores. Also the low production of parental strain caused by subculturing induced degeneration (Yin, Xin, Weng, & Gui, Citation2017). We also found that certain hybrid strains with MAT1-1 and MAT1-2 mating type genes cannot change color or form fruiting bodies showed that the fruiting bodies formation are controlled by the mating type system and other factors such as DNA methylation, genetic mutations, and virus infections (Zhang & Yue, Citation2013).
The C. militaris fruiting body yield and bioactive compounds production are effected by the strain and culture conditions. Different solid-state substrates have been studied to culture C. militaris fruiting bodies (Gu, Wang, Li, & Yuan, Citation2007; Huang, Li, Chen, Wang, & Zhou, Citation2009). As widely cultivated cereal crop, wheat and rice have the advantages of high yield and low price. The dry weight of fruiting bodies was higher on wheat substrates, but with lower concentration of cordycepin and adenosine for top and base strain. Although the contents of cordycepin and adenosine were ranked as top > middle > base of fruiting bodies (Dong et al., Citation2013a), the middle strain and base strain can still have advantages in yield or contents of bioactive compounds. It is interesting to note that the base strain exhibited the highest production of adenosine but the lowest production of cordycepin on wheat substrates. It is possible that adenosine made a contribution to cordycepin biosynthesis (Masuda, Das, Fujihara, Hatashita, & Sakurai, Citation2011). The adenosine level decreased and cordycepin increased from day 40 of culture to day 50 generally (Lim, Lee, & Chang, Citation2012) showed that the cordecepin accumulates extensively at the later fruiting stage. The carbon/nitrogen ratios affected the cordycepin and adenosine content clearly (Lim et al., Citation2012; Shih, Tsai, & Hsieh, Citation2006). The cordycepin also affected by the nutrients (Chen, Liu, & Chang, Citation2011). The quality differences of fruiting bodies harvested from different substrates maybe because the fruiting bodies growth period and nutrients composition of wheat and rice.
Light plays an essential role in fruiting bodies development and metabolite production in C. militaris (Chiang et al., Citation2017; Lian et al., Citation2014). Red light resulted in the highest yield but the lowest content of cordycepin on the wheat substrate, red + white light combination showed that the white light can reduce the number of fruiting bodies. The blue light can obtain better yield and quality of fruiting bodies. The light conditions, including wavelengths, intensity, and light/dark cycle had the greatest impact on the bioactive compound production (Chen et al., Citation2011; Chiang et al., Citation2017). Based on these results, during the period of the primodium formation with red light and fruiting bodies growth with white light maybe can improve the yield and quality of fruiting bodies. The number of fruiting bodies are directly linked to the yield and may be related to the bioactive compounds.
5. Conclusions
The effect of strains, solid-state substrates and light condition on the production of C. militaris fruiting bodies yield and contents of bioactive compounds were investigated. The superior strain can be selected through cross-mating of single ascospore with opposite mating type. Isolating strains from different parts of the fruiting body had a significant influence on the yield and bioactive compounds contents in different substrates. The top and base strain obtained higher production of fruiting bodies and cordycepin on rice substrate. For higher production of fruiting bodies, the middle and base strain are more suitable to cultivate on the wheat substrate. Red light can improve the number and production of fruiting bodies and white light was conducive to accumulate cordycepin. This paper provides a reference for strain breeding as well as the extraction and quantification of cordycepin and adenosine in fruiting bodies of C. militaris and other Cordyceps fungi.
Acknowledgments
The work was funded by the Special Project of the Science and Technology Program of the Regiment (2014CC005) and also thanks to the Key Laboratory of Phytomedicine Resources of Shihezi University of PR China. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adnan, M., Ashraf, S. A., Khan, S., Alshammari, E., & Awadelkareem, A. M. (2017). Effect of ph, temperature and incubation time on cordycepin production from Cordyceps militaris using solid-state fermentation on various substrates. Cyta Journal of Food, 15, 617–621.
- Avin, F. A., Bhassu, S., Rameeh, V., Tan, Y. S., & Vikineswary, S. (2016). Genetics and hybrid breeding of pleurotus pulmonarius: Heterosis, heritability and combining ability. Euphytica, 209(1), 85–102.
- Bhushan, S., Han, S. K., Jae-Mo, S., & Gi-Ho, S. (2012). Fruiting body formation of Cordyceps militaris from multi-ascospore isolates and their single ascospore progeny strains. Mycobiology, 40, 100–106.
- Bhushan, S., Han, S. K., Won-Ho, L., Seong-Keun, C., Je-O, L., & Jae-Mo, S. (2005). Distribution and in vitro fruiting of Cordyceps militaris in korea. Mycobiology, 33(4), 178–181.
- Chen, A., Wang, Y., Shao, Y., & Huang, B. (2017). A novel technique for rejuvenation of degenerated caterpillar medicinal mushroom, Cordyceps militaris (ascomycetes), a valued traditional Chinese medicine. International Journal of Medicinal Mushrooms, 19(1), 87–91.
- Chen, Y. S., Liu, B. L., & Chang, Y. N. (2011). Effects of light and heavy metals on Cordyceps militaris, fruit body growth in rice grain-based cultivation. Korean Journal of Chemical Engineering, 28(3), 875–879.
- Chiang, S. S., Liang, Z. C., Wang, Y. C., & Liang, C. H. (2017). Effect of light-emitting diodes on the production of cordycepin, mannitol and adenosine in solid-state fermented rice by Cordyceps militaris. Journal of Food Composition & Analysis, 60, 51–56.
- Cunningham, K. G., Manson, W., Spring, F. S., & Hutchinson, S. A. (1950). Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature, 166, 949.
- Das, S. K., Masuda, M., Hatashita, M., Sakurai, A., & Sakakibara, M. (2008). A new approach for improving cordycepin productivity in surface liquid culture of Cordyceps militaris using high-energy ion beam irradiation. Letters App Microbiologic, 47, 534–538.
- Dong, J. Z., Ding, J., Yu, P. Z., Lei, C., Zheng, X. J., & Wang, Y. (2013a). Composition and distribution of the main active components in selenium-enriched fruit bodies of Cordyceps militaris link. Food Chemistry, 137, 164–167.
- Dong, J. Z., Lei, C., Zheng, X. J., Ai, X. R., Wang, Y., & Wang, Q. (2013b). Light wavelengths regulate growth and active components of Cordyceps militaris fruit bodies. Journal of Food Biochemistry, 37, 578–584.
- Dong, J. Z., Liu, M. R., Lei, C., Zheng, X. J., & Wang, Y. (2012). Effects of selenium and light wavelengths on liquid culture of Cordyceps militaris, link. Applied Biochemistry and Biotechnology, 166, 2030–2036.
- Fan, D. D., Wang, W., & Zhong, J. J. (2012). Enhancement of cordycepin production in submerged cultures of Cordyceps militaris, by addition of ferrous sulfate. Biochemical Engineering Journal, 60, 30–35.
- Frederiksen, S., Malling, H., & Klenow, H. (1965). Isolation of 3ʹ-deoxyadenosine (cordycepin) from the liquid medium of Cordyceps militaris (L. ex Fr.) Link. Biochimica Et Biophysica Acta, 95, 189–193.
- Gu, Y. X., Wang, Z. S., Li, S. X., & Yuan, Q. S. (2007). Effect of multiple factors on accumulation of nucleosides and bases in Cordyceps militaris. Food Chemistry, 102(4), 1304–1309.
- Hamburger, M. (2007). Comment on Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. Journal Agricultural Food Chemical. 55, 7213–7214;. author reply 7215–7216.
- Huang, L., Li, Q., Chen, Y., Wang, X., & Zhou, X. (2009). Determination and analysis of cordycepin and adenosine in the products of cordyceps spp. African Journal of Microbiology Research, 3(12), 957–961.
- Kho, C. H., Kan, S. C., Chang, C. Y., Cheng, H. Y., Lin, C. C., Chiou, P. C., Shieh C. J., & Liu Y. C. (2016). Analysis of exopolysaccharide production patterns of Cordyceps militaris, under various light-emitting diodes. Biochemical Engineering Journal, 112, 226–232. doi:10.1016/j.bej.2016.04.028
- Kodama, E. N., McCaffrey, R. P., Yusa, K., & Mitsuya, H. (2000). Antileukemic activity and mechanism of action of cordycepin against terminal deoxynucleotidyl transferase-positive (TdT+) leukemic cells. Biochemical Pharmacology, 59, 273–281.
- Lian, T. T., Dong, C. H., Yang, T., & Sun, J. D. (2014). Effects of blue light on the growth and bioactive compound production of Cordyceps militaris. Mycosystema, 33, 838–846.
- Liang, Y., Zhang, G., An, M., & Cai, Z. (2005). Cordyceps Militaris: Ascospore germination and cultural characteristics of progeny population. Mycosystema, 24, 525–532.
- Lim, L., Lee, C., & Chang, E. (2012). Optimization of solid state culture conditions for the production of adenosine, cordycepin, and d-mannitol in fruiting bodies of medicinal caterpillar fungus Cordyceps militaris (l.: Fr.)link (ascomycetes). International Journal of Medicinal Mushrooms, 14(2), 181–187.
- Masuda, M., Das, S. K., Fujihara, S., Hatashita, M., & Sakurai, A. (2011). Production of cordycepin by a repeated batch culture of a Cordyceps militaris mutant obtained by proton beam irradiation. Journal of Bioscience & Bioengineering, 111(1), 55-60.
- Paterson, R. R. (2008). Cordyceps: A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry, 69, 1469–1495.
- Sari, N., Suparmin, A., Kato, T., & Park, E. Y. (2016). Improved cordycepin production in a liquid surface culture of Cordyceps militaris, isolated from wild strain. Biotechnology Bioproc E, 21, 595–600.
- Sato, H., & Shimazu, M. (2002). Stromata production for Cordyceps militaris (clavicipitales: Clavicipitaceae) by injection of hyphal bodies to alternative host insects. Applied Entomology & Zoology, 37, 85–92.
- Shih, I. L., Tsai, K. L., & Hsieh, C. (2006). Effects of culture conditions on the mycelial growth and bioactive metabolite production in submerged culture of Ccordyceps militaris. Biochemical Engineering Journal, 33(3), 193–201.
- Shrestha, B., Kim, H. K., Sung, G. H., Spatafora, J. W., & Sung, J. M. (2004). Bipolar heterothallism, a principal mating system of Cordyceps militaris in vitro. Biotechnology Bioprocess Engineering, 9, 440–446.
- Sung, G. H., Hywel-Jones, N. L., Sung, J. M., Luangsa-Ard, J. J., Shrestha, B., & Spatafora, J. W. (2007). Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology, 57, 50–59.
- Sung, J. M., Park, Y. J., Lee, J., Han, S. K., Lee, W. H., Choi, S. K., & Shrestha B. (2006). Selection of superior strains of Cordyceps militaris with enhanced fruiting body productivity. Mycobiology, 34(3), 131–137.
- Szentmiklosi, A. J., Galajda, Z., Cseppento, A., Gesztelyi, R., Susan, Z., Hegyi, B., & Nanasi P. P. (2015). The janus face of adenosine: Antiarrhythmic and proarrhythmic actions. Current Pharmaceutical Design, 21(8), 965–976.
- Tang, J. P., Qian, Z. Q., & Zhu, L. (2015). Two-step shake-static fermentation to enhance cordycepin production by Cordyceps militaris. Chem Engineering Trans, 46, 19–24.
- Turgeon, B. G., & Yoder, O. C. (2000). Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genetics & Biology Fg & B, 31, 1–5.
- Wen, T. C., Kang, J. C., Li, G. R., & Lei, B. X. (2008). Effect of culture condition on the fruit body and cordycepin production in Cordyceps militaris in solid-state fermentation. Guizhou Agricultural Sciences, 36, 92–94.
- Wu, C. Y., Liang, Z. C., Tseng, C. Y., & Hu, S. H. (2016). Effects of illumination pattern during cultivation of fruiting body and bioactive compound production by the caterpillar medicinal mushroom, Cordyceps militaris (ascomycetes). International Journal of Medicinal Mushrooms, 18(7), 589.
- Xiong, C., Xia, Y., Zheng, P., Shi, S., & Wang, C. (2010). Developmental stage-specific gene expression profiling for a medicinal fungus Cordyceps militaris. Mycology, 1, 25–66.
- Yang, J., Wang, Y., Garciaroves, P., Björnholm, M., & Fredholm, B. B. (2010). Adenosine a3 receptors regulate heart rate, motor activity and body temperature. Acta Physiologica, 199(2), 221.
- Yin, J., Xin, X., Weng, Y., & Gui, Z. (2017). Transcriptome-wide analysis reveals the progress of Cordyceps militaris subculture degeneration. PLoS One, 12(10), e0186279.
- Yokoyama, E., Yamagishi, K., & Hara, A. (2005). Structures of the mating-type loci of Cordyceps takaomontana. Applications Environment Microbiologic, 69, 5019–5022.
- Yoo, H. S., Shin, J. W., Cho, J. H., Son, C. G., Lee, Y. W., Park S. Y., & Cho C. K. (2004). Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacologica Sinica, 25, 657–665.
- Zhang, G., & Yue, L. (2013). Improvement of fruiting body production in Cordyceps militaris, by molecular assessment. Archives of Microbiology, 195, 579–585.
- Zheng, P., Xia, Y., Xiao, G., Xiong, C., Hu, X., Zhang, S., … Wang, C. (2011). Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biology, 12, R116.
- Zheng, P., Xia, Y., Zhang, S., & Wang, C. (2013). Genetics of Cordyceps and related fungi. Applications Microbio Biotechnology, 97, 2797–2804.
- Zhou, X., Gong, Z., Su, Y., Lin, J., & Tang, K. (2009). Cordyceps fungi: Natural products, pharmacological functions and developmental products. The Journal of Pharmacy and Pharmacology, 61, 279–291.
