ABSTRACT
Água-mel is a honey-based traditional product typically produced in southern Portugal. The evolution of some parameters during its production using two distinct containers (aluminium and stainless steel with a water cooling system) and two heating sources (gas heating and electricity) were evaluated. All parameters, except moisture, increased over time, reaching a ‘plateau’ after 8 h of heating. Moisture, free acidity, HMF (hydroxymethylfurfural), colour, melanoidins, phenols and glucose depended on the producer and procedure for obtaining água-mel. Kinetic parameters and correlation coefficients were determined. In general, changes in parameters during água-mel production followed zero- and/or first-order kinetics, depending on the producer and heating system.
RESUMEN
El água-mel es un producto tradicional elaborado a base de la miel producida normalmente en el sur de Portugal. El presente estudio evaluó la evolución de algunos parámetros durante su producción utilizando dos contenedores distintos (aluminio y acero inoxidable con un sistema para enfriar el agua) y dos fuentes de calor (gas y electricidad). Excepción hecha de la humedad, todos los parámetros aumentaron con el transcurso del tiempo, alcanzando una ‘meseta’ después de 8 horas de calentamiento. Se constató que los parámetros de humedad, acidez libre, HMF (hidroximetilfurfural), color, melanoidinas, fenoles y glucosa dependen de quién sea el productor y del procedimiento utilizado para obtener água-mel. Se determinaron los parámetros cinéticos y los coeficientes de correlación. En general, los cambios registrados en los parámetros durante la producción de água-mel se ajustan a la cinética de orden cero y/o de primer orden, dependiendo del productor y del sistema de calentamiento.
Introduction
Água-mel is a honey-based product made in the south region of Portugal by beekeepers who start by scalding the honeycomb (still with some honey, pollen and propolis present) with water and squeezing it to recover the remaining honey. The honey/water solution is filtered through a sieve after removal of the honeycomb and left to simmer until a dark brown honey-like product with an appropriate ºBrix is obtained, a procedure that can take several h. This procedure is subjective, not obeying to any rules of industrial production. The procedure with schema was already previously reported (Figueira & Cavaco, Citation2012; Miguel, Antunes, Aazza, Duarte, & Faleiro, Citation2013a). The final product may differ, because the producers use different types of honey and different solid material/water ratios at the beginning of production. Nevertheless, at the end of the procedure, água-mel must be dark coloured. Many times, the procedure for obtaining água-mel is considered finished when the product, after dripped onto the thumbnail, forms a pearl-like drop but of a dark colour.
Água-mel is used not only as sweetener in cakes, tea, on soft cheese, or as a honey-like spread, on bread or sandwiches, but also for alleviating symptoms of simple upper respiratory ailments (Figueira & Cavaco, Citation2012; Miguel et al., Citation2013a, Citation2013b). The chemical characterization and microbiological quality (Miguel et al., Citation2013a), the mineral and volatile composition (Miguel et al., Citation2016) and antimicrobial, antiviral and antioxidant activities (Miguel et al., Citation2013b) of água-mel have been reported. In addition, Figueira and Cavaco (Citation2012) reported the changes in physical parameters (viscosity, total soluble solids and Hunter colour parameters L*, a*, b*, chroma and hue angle) throughout água-mel processing.
The present work aimed at evaluating the changes in the physicochemical parameters including the radical scavenging capacities of água-mel during its production, using two types of heating systems.
Material and methods
Materials
Água-mel samples were prepared by four beekeepers (AMR, FD, CMP, Ce). AMR, FD and CMP used a double jacketed stainless steel container (SSC) with indirect heating by water (electricity) for producing água-mel. CMP also used an aluminium container (AC), that is, the traditional method. Ce only used an aluminium container (AC). When an aluminium container was used, propane gas was used as direct heating source. The temperature was not monitored throughout the process. The production was made according to the normal procedures as previously reported (Miguel et al., Citation2013a). From each container three aliquots were sampled for analysis at the beginning of the assay (time 0), that is, before heating, and 2, 4, 6 and 8 h after ebullition (generally coincided with the end of the heating). Three aliquots were sampled on day after processing (24 h). In all cases, the samples were transported to the University of Algarve and kept at 4ºC for one week, that is, the period during which all analytical determinations were carried out.
Water content or moisture
The water content of the samples was determined by measuring the refractive index at 20ºC according to the International Honey Commission IHC (Bogdanov, Citation2002).
Determination of free acidity
The measurement of pH and determination of free acidity were performed according to the International Honey Commission IHC (Bogdanov, Citation2002). Ten grams of sample was dissolved in 75 mL of carbon dioxide-free water and free acidity was determined by titration with 0.1 M NaOH to pH 8.30.
Hydroxymethylfurfural (HMF) content
The HMF concentration was determined according to the harmonized method for honey (Bogdanov, Citation2002). One gram of sample was diluted to 50 mL with distilled water, filtered through a 0.45 μm filter and immediately analysed by HPLC. The same chromatographic conditions previously reported (Miguel et al., Citation2013a) for água-mel analysis were used.
Estimation of colour and melanoidins’ content
Água-mel colour was estimated spectrophotometrically by measuring net absorbances at (A560–A720) and melanoidin content was estimated based on the browning index (net absorbance at A450–A720), according to Miguel et al. (Citation2013b).
Estimation of total phenols’ content
The total polyphenol content was determined by a modification of the Folin-Ciocalteu method and the results are expressed as mg gallic acid equivalents according to the method described by Miguel et al. (Citation2013a) for água-mel.
Fructose and glucose
The fructose and glucose contents were determined according to the methods reported by Miguel et al. (Citation2013a) for água-mel. About 0.5 g of água-mel was mixed with 10 mL 25% methanol, and 1 mL of the solution was filtered through a 0.45 μm filter prior to HPLC analysis, using a refractive index (RI) detector. The chromatographic conditions were previously reported (Miguel et al., Citation2013a).
DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical-scavenging activity
Fifty microlitres of various concentrations of samples were added to 1.95 mL of a 60 μM methanolic solution of DPPH. Absorbance measurements were read at 517 nm, after 30 min incubation at room temperature (A1). Absorbance of a blank sample (DPPH solution) was the negative control (A0). The percentage of inhibition was calculated as [(A0–A1/A0) x 100]. The percentages calculated according to this formula were plotted against sample concentration, and the IC50 was evaluated (concentration of sample able to scavenge 50% of DPPH free radicals) (Miguel, Nunes, Dandlen, Cavaco, & Antunes, Citation2014).
Calculation of kinetic parameters
Quality changes in food usually follow a zero- (linear change), first- (exponential change), or second-order reaction (van Boekel, Citation2008). The equations for zero- and first-order reactions were applied to data, representing changes in various parameters during água-mel production:
Zero-order reaction: C = C0 ± K0t
First- order reaction: C = C0 exp(± K1t)
where C is the value of the concentration at time t; C0 is the concentration at time zero; k0 is the zero-order kinetic constant (min−1); k1 is the first-order kinetic constant (min−1); t is the processing time (min); (+) and (-) indicate the formation or degradation, respectively.
Statistical analysis
Results are the mean of three determinations and expressed as means ± standard deviation (SD). Paired Student t test was used in some tests to determine differences at 5% and 1% significance. All statistical analysis was performed using the MS Excel 2003.
Results and discussion
shows the evolution of the moisture content during the production of água-mel. As expected, it decreases due to water evaporation. After 8–9 h, the procedure was finished and the product left to cool. Twenty four h after the procedure had begun, the last sample was taken. For the same producer, the moisture content of the initial product was the same, indicating that production started with the same proportions of water and solid material. Independent of initial moisture content, use of SSC resulted in a product after 24 h with more or less the same moisture content, but lower than that of agua-mel produced using AC. After 8–9 h of heating, differences between products as a result of heating system were evident. This may be partly explained by the different heating sources used: stainless steel container with indirect heating and aluminium container with direct heating.
Figure 1. Moisture (%) (A), free acidity (mEq/kg) (B), HMF (mg/kg) (C), Colour (D), and phenol content (mg/g) (E) during the production of água-mel from diverse producers.
Figura 1. Humedad (%) (A), acidez libre (mEq/kg) (B), HMF (mg/kg) (C), color (D), y contenido fenólico (mg/g) (E) durante la producción de água-mel procedente de distintos productores.
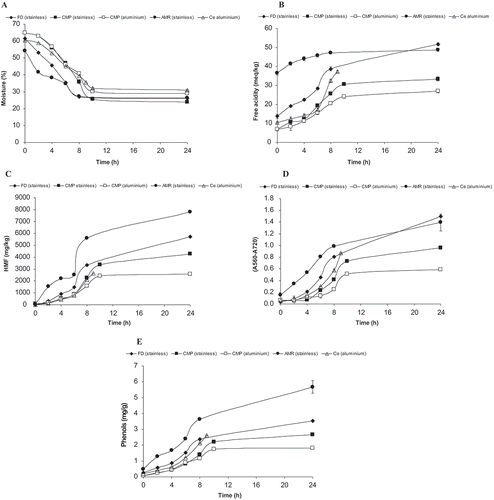
The free acidity increased during the heating process (). At the beginning, this parameter was substantially higher in the AMR sample than the other samples. In spite of the relative higher free acidity of this sample at the beginning, after 24 h, the level was lower than that of the FD sample, which was the second most acidic at the beginning, but significantly less acidic than the AMR sample. The lowest free acidity was observed for CMP samples, either at time 0 or after 24 h.
The HMF concentration increased during the production of água-mel, as expected (). Água-mel produced in SSC had a higher HMF content than when AC was used, as demonstrated for producer CMP.
In spite of similar HMF concentrations at the beginning, after 24 h higher HMF concentrations were noted for products having lower moisture contents and higher acidity at the beginning of the process. The results reveal a negative correlation (r = -0.93; p < 0.001) between the initial moisture and final HMF content, indicating the importance of water added to the system at the beginning of the process for the HMF content of the final product. In those cases where higher moisture was found, lower amount of honey should be present, and therefore, for the same time of heating and energy provided, the levels of HMF were less than those in which the initial quantity of honey was higher. The results show that the quantity of honey used in the production of água-mel as well as the heating source used are important factors determining the levels of HMF at the end of the process. These results partly agree with those reported by Vázquez, Verdú, Miquel, Burló, and Carbonell-Barrachina (Citation2007) for the production of Spanish turron, because they concluded that the HMF level was only dependent on the initial concentration in honey. In our case, we observe the importance of this factor, along with the heating source, particularly for the producer that used both sources of heating for producing água-mel (). When using SSC, the water heating jacket permitted heating the bottom and side walls of the container, as opposed to the AC containers, which were only heated from the bottom of the container. The heat distribution supplied to the systems was, therefore, different.
In addition, the significant highest HMF levels observed in AMR sample throughout the heating processing coincided with the highest free acidity. In heated honey, Fallico, Zappalà, Arena, and Verzera (Citation2004) reported that HMF correlated, among other factors, with acidity. A positive correlation (r = 0.76; p < 0.01) was observed between free acidity and HMF in the samples having the highest free acidity and HMF (AMR and FD).
The colour in honey is attributed to several factors such as the presence and concentration of compounds possessing conjugated double bonds that absorb light in visible range (400–700 nm): polyphenols, flavonoids, terpenes, carotenoids and Maillard reaction products (Brudzynski and Kim (Citation2011). This attribute was also measured throughout água-mel processing and the results are presented in . At the beginning the colour of the mixture was higher in the AMR product, nevertheless, at the end, it was not significantly different to that of FD. The darkening of the samples can be attributed not only to the concentration of the metabolites (phenols and other compounds), due to evaporation of water, but also to the formation of Maillard reaction products (Pita-Calvo & Vázquez, Citation2017, Citation2018). Miguel et al. (Citation2013b) reported this kind of products in different amounts in samples of água-mel from different producers. The authors suggested several factors possibly responsible for such differences: type of honey used by beekeepers and processing, time and temperature used during the production. For the same producer (CMP), use of the SSC resulted in a darker colour of água-mel, as observed for HMF production (). The two CMP samples representing the same initial product and same heating period, but produced by two heating systems, differed in colour at the end of the procedure (). These results may suggest that for the same type of honey and heating time, other factors regulate the final colour of água-mel, such as the type of container in which the heating distribution is different.
During the production of água-mel, the phenol concentration increased (), expected due to a concentration effect as a result of water evaporation. The possible interference between the reducing sugars and the Folin-Ciocalteu reagent (Miguel et al., Citation2013b; Pita-Calvo & Vázquez, Citation2018), however, cannot be ruled out as the reducing sugar content also increased during the heating process.
At the beginning and during the heating process, sample AMR had always higher levels of phenols than the other samples. For the same producer (CMP), using the same initial product but two distinct heating systems, produced two batches of product having different concentrations of total phenols, with the SSC batch having the higher phenol concentration () A possible higher water lost during heating () does not explain the results, because the difference between the moisture content of the two products (AC vs SSC) is 20% (28.92–24.01%), whereas the respective differences in phenol concentration is about 40% (1.81–2.67 mg/g). Moreover, the SSC product had a higher fructose concentration () than the AC product (3%; 354.6–343.02 g/kg). Such differences in the fructose concentration and moisture content are not enough to explain the relative higher phenol content in the SSC sample (CMP). Some Maillard products are formed during the heating process (Pita-Calvo, Guerra-Rodríguez, & Vázquez, Citation2017). Higher formation of these products may have occurred in the SSC (at least higher HMF accumulation was found in this type of heating), and such compounds may interfere with the Folin reagent, as for reducing sugars. The heating (SSC) and acidic conditions may also have contributed to the hydrolysis of flavonoid glycosides and other phenolic compounds (Nuutila, Kammiovirta, & Oksman-Caldentey, Citation2002). The resulting aglycones with the free hydroxyl groups are able to react with Folin reagent. However, Escriche, Kadar, Juan-Borras, & Domenech (Citation2014) concluded that industrial thermal treatments of honey did not affect the phenol profile of several monofloral honey.
Figure 2. Fructose content (g/kg) (A), glucose content (g/kg) (B), and melanoidins (C) during the production of água-mel from diverse producers.
Figura 2. Contenido de fructosa (g/kg) (A), contenido de glucosa (g/kg) (B), y melanoidinas (C) durante la producción de água-mel procedente de distintos productores.
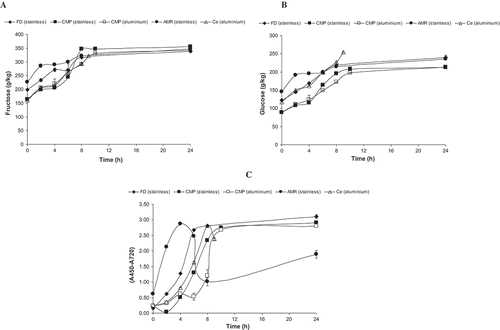
Fructose and glucose levels increased during the heating process ( and ). After 24 h, the amounts of these monosaccharides were similar to those that can be found in honey, as already reported for abbamele and água-mel from Italy and Portugal, respectively (Miguel et al., Citation2013b; Spano et al., Citation2008). The glucose content of the CMP product, independent of the heating system used was less than that of the other samples (). The glucose content of the starting material was already less than that of the other starting materials. In contrast to the other parameters reported above for CMP samples differently heated, the glucose content for água-mel produced, using different heating systems, was similar. The fructose content of the SSC sample was higher (3%) than that of the AC sample ().
Melanoidins are brown coloured, carbohydrate-based, nitrogen-containing polymeric macromolecules found in thermally processed foods, including honey (Brudzynski & Miotto, Citation2011). The quantification of these compounds is generally monitored spectrophotometrically at wavelengths between 420 and 450 nm, whereas honey colour is based on the net absorbance at A560–A720 nm (Brudzynski & Miotto, Citation2011). The melanoidins in the present work were quantified by measuring the absorbance of samples at 450 nm. An increase in absorbance was observed in all samples taken during the heating process of three products, resulting in their darkening. For products produced by AMP and Ce, the increase was observed up to 6–8 h of heating, thereafter decreasing. Such decrease in melanoidin concentration was particularly drastic in the AMP sample (), nevertheless getting even darker. Brudzynski and Miotto (Citation2011) also found a decrease of the net absorbance (A450-A720 nm) of some heated honey, despite a remarkable darkening of honey. The authors suggested that the heat-treatment had caused precipitation of some brown coloured compounds (high molecular weight melanoidins). Therefore, the darker colour of heated honey may also be attributed to other coloured substances formed at high temperatures, including the thermal degradation products of melanoidins (Brudzynski & Miotto, Citation2011). Such observation had been more accentuated in dark honey. Hofmann (Citation1998) hypothesised that coloured low molecular weight substances contributed more to the colour formation of foods than high molecular weight melanoidins which are detected at 450 nm.
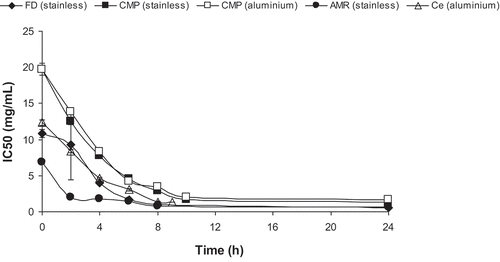
All samples had the highest DPPH radical scavenging capacity at the end of the heating process (8–9 h) reaching a constant after this period (). At the starting of the heating process, samples had different activities, with the initial AMR product having the highest activity (i.e. lowest IC50 value), followed by the FD and Ce products. The lowest activity was observed for the initial CMP product, nevertheless after 24 h, the differences in activity between products were less pronounced. The capacity for scavenging this type of free radicals was already reported by Miguel et al. (Citation2013b) for água-mel samples.
Figure 3. Capacity for scavenging DPPH free radicals, expressed as IC50 (mg/mL).
Figure 3. Capacidad del método DPPH para eliminar radicales libres, expresada como IC50 (mg/mL).
Kinetic measurements
The relationships between some physicochemical parameters and água-mel processing time were investigated. Kinetic parameters and correlation coefficients are presented in . In general, changes in parameters could be described of zero- or first-order models, although in some cases, the correlation coefficients of the two models were practically similar (). Correlation coefficients were used to select the better model (Figueira & Cavaco, Citation2012), studying the changes in viscosity, total soluble solids and Hunter colour parameters L*, a*, b*, chroma and hue angle of água-mel during its processing also fitted the same models.
Table 1. Results of some parameters from zero- and first-order reaction kinetics of água-mel.
Tabla 1. Resultados de algunos parámetros de las reacciones cinéticas de orden cero y de primer orden del água-mel.
According to Huidobro and Simal (Citation1984), a linear relationship exists between the absorbance of caramel-glycerine solutions (colour of these mixtures similar to those of honey) at 560 nm and the colour determined as Pfund, with the advantage that abnormal absorbance readings at lower wavelengths are prevented for some honey. The absorbance readings at 720 nm measure turbidity. The difference in absorbance at 560 and 720 nm gives a measure of the colour of the samples. Melanoidin content was estimated based on the browning index (net absorbance at A450–A720) (Brudzynski & Miotto, Citation2011; Ramonaityte, Keršiene, Adams, Tehrani, & de Kimpe, Citation2009).
For practically all producers (FD SSC, CMP SSC, CMP AC, and Ce AC), the first-order reaction model described the change in colour (net absorbance at A560–A720) throughout água-mel processing better than the zero-order model, with the exception of AMR SSC. Figueira and Cavaco (Citation2012) reported that the changes in Hunter colour L* and b* parameters of água-mel followed zero-order kinetics, whereas the changes in a* and C* parameters followed first-order kinetics.
Zero- or first-order kinetic models have been reported to evaluate the non-enzymatic browning of products during storage or submitted to heating processes (Kwok, MacDougall, & Niranjan, Citation1999), although zero-order kinetics prevail in most cases. Some authors proposed that two steps which follow different kinetics may always be considered: the first step that includes the formation of brown polymeric compounds, which follows zero-order kinetics, and the second step, which comprises the decomposition of these brown components giving colourless compounds, that follows a first-order kinetic model (Carabasa-Giribet & Ibarz-Ribas, Citation2000; Garza, Ibarz, Pagán, & Giner, Citation1999). In the present work, only two samples fitted the zero-order kinetic model (AMR and FD). The formation of brown pigments and HMF depends on several factors (pH; temperature, water activity, time, compounds present in the systems (Bozkurt, Göğüš, & Eren, Citation1999; Fallico et al., Citation2004). In the present work, such parameters were not determined; only free acidity, moisture and HMF. For those samples with higher free acidity, zero-order kinetics explained the formation of melanoidins and HMF (precursor of coloured products in the Maillard reactions) (, ), whereas in the remaining samples, their formation was better described by a first-order kinetic model ().
Variations in total phenol content during água-mel processing followed a first-order kinetic model for practically all samples with the exception of AMR. Such kinetic models are coincident with those of colour (net absorbance A560–A720). Considering that the colour is due to the presence of several types of phenols, among other compounds, the colour followed the same kinetic behaviour than of the phenol concentration. Variations in the free acidity followed the same trend than that of the net absorbance (absorbance A560–A720) and phenols: first-order kinetic model for all samples with the exception of AMR ().
Changes in glucose and fructose during heat processing of água-mel fitted zero- and first-order kinetic models (). For AMR samples, the changes in monosacharide were better described by the zero-order kinetic model, whereas for the CMR (SSC) product, the change was better described by the first-order kinetic model. For both FD and CMP (AC) samples, it was difficult to distinguish between zero-, and first-order kinetics, considering the changes in glucose concentrations (). The same could be observed for fructose in the Ce samples. In short, the kinetic models obtained for glucose and fructose did not follow a trend. According to Garza et al. (Citation1999), the kinetic reactions followed by these two monosaccharides during a thermal treatment is very complex. In contrast, sucrose generally follows a first-order kinetic model (Garza et al., Citation1999).
Changes in the moisture content during the heating of the AMR and Ce products are described by first-order kinetics, whereas in remaining samples, the zero- order kinetic model prevailed ().
Changes in the capacity for scavenging DPPH free radicals, that is, the antioxidant activity of all samples were fitted to first-order reaction kinetics. Turkmen, Sari, Poyrazoglu, and Valioglu (Citation2006) also measured the capacity of heated honey at different temperatures (50, 60 and 70 ºC) for scavenging DPPH free radicals. The authors found that changes in antioxidant activity of heated honey could be fitted to second-, first, and zero-order kinetics at 50, 60 and 70ºC, respectively, depending, therefore, on the temperature of the honey.
The kinetic models obtained in the present work depended on the producers of água-mel even if the same heating process was applied. For example, changes in água-mel produced by CMP, using SSC, mostly followed first-order kinetics, while zero-order kinetics described changes in the AMR (SSC) product. For the Ce (AC) product, changes followed mostly first-order kinetics, but for the CMP (AC) product, the number of parameters that varied according to zero- or first-order kinetics was almost the same.
Although sugars predominated in água-mel, many other compounds constitute this product and therefore can be considered as a complex system, in which many factors cannot be controlled. In addition, the honey used by beekeepers are mixtures of different floral origin honey, and they used different proportions of water/honey for producing água-mel, as evident from the initial moisture content of the mixture. The unknown factors, due to the system complexity, along with these factors may be responsible for the differences in the kinetics of the parameters among samples from diverse beekeepers.
Conclusions
The physicochemical parameters of água-mel were dependent on the producer and for the same producer they were dependent on the type of heating and/or type of container, particularly moisture content, HMF content, colour, and phenol content. The kinetic models were also dependent on the água-mel producers. The complex composition of honey together with differences in the production process of água-mel (ratio of honey to water; type of heating) may altogether be responsible for the differences of kinetic parameters among samples from diverse beekeepers.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bogdanov, S. (2002). Harmonized Methods of the International Honey Commission. http://www.apiculturacluj.com/ApiculturaCluj/italiano/Documents/IHCmethods_e.pdf
- Bozkurt, H., Göğüš, F., & Eren, S. (1999). Nonenzymic browning reactions in boiled grape juice and its models during storage. Food Chemistry, 64, 89–93.
- Brudzynski, K., & Kim, L. (2011). Storage-induced chemical changes in active components of honey de-regulate its antibacterial activity. Food Chemistry, 126, 1155–1163.
- Brudzynski, K., & Miotto, D. (2011). The recognition of high molecular weight melanoidins as the main components responsible for radical-scavenging capacity of unheated and heat-treated Canadian honeys. Food Chemistry, 125, 570–575.
- Carabasa-Giribet, M., & Ibarz-Ribas, A. (2000). Kinetics of colour development in aqueous glucose systems at high temperatures. Journal of Food Engineering, 44, 181–189.
- Escriche, I., Kadar, M., Juan-Borrás, M., & Domenech, E. (2014). Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chemistry, 142, 135–143.
- Fallico, B., Zappalà, M., Arena, E., & Verzera, A. (2004). Effects of conditioning on HMF content in unifloral honeys. Food Chemistry, 85, 305–313.
- Figueira, A. C., & Cavaco, T. (2012). Changes in physical and chemical parameters of the traditional Portuguese product água-mel during the production process. Journal of Food Processing and Preservation, 36, 285–290.
- Garza, S., Ibarz, A., Pagán, J., & Giner, J. (1999). Non-enzymatic browning in peach puree during heating. Food Research International, 32, 335–343.
- Hofmann, T. (1998). Characterization of the chemical structure of novel colored Maillard reaction products from furan-2-carboxaldehyde and amino acids. Journal of Agricultural and Food Chemistry, 46, 932–940.
- Huidobro, J. F., & Simal, J. (1984). Determinación del color y de la turbidez en las mieles. Anales De Bromatologia, 36, 225–245.
- Kwok, K. C., MacDougall, D. B., & Niranjan, K. (1999). Reaction kinetics of heat-induced colour changes in soymilk. Journal of Food Engineering, 40, 15–20.
- Miguel, M. G., Aazza, S., Antunes, M. D., Faleiro, M. L., Barroso, J. G., Pedro, L. G., & Figueiredo, A. C. (2016). Mineral and volatile composition of água-mel from Portugal. European Food Research and Technology, 242, 171–178.
- Miguel, M. G., Antunes, M. D., Aazza, S., Duarte, J., & Faleiro, M. L. (2013a). Honey-based “água-mel” chemical characterization and microbiological quality. Italian Journal of Food Science, 25, 275–282.
- Miguel, M. G., Faleiro, L., Antunes, M. D., Aazza, S., Duarte, J., & Silvério, A. R. (2013b). Antimicrobial, antiviral and antioxidant activities of “água-mel” from Portugal. Food and Chemical Toxicology, 56, 136–144.
- Miguel, M. G., Nunes, S., Dandlen, S. A., Cavaco, A. M., & Antunes, M. D. (2014). Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Science and Technology (Campinas), 34, 16–23.
- Nuutila, A. M., Kammiovirta, K., & Oksman-Caldentey, K.-M. (2002). Comparison of methods for the hydrolysis of flavonoids and phenolic acids from onion and spinach for HPLC for HPLC analysis. Food Chemistry, 76, 519–525.
- Pita-Calvo, C., Guerra-Rodríguez, M. E., & Vázquez, M. (2017). Analytical methods used in the quality control of honey. Of Agricultural and Food Chemistry, 65, 690–703.
- Pita-Calvo, C., & Vázquez, M. (2017). Differences between honeydew and blossom honeys: A review. Trends in Food Science and Technology, 59, 79–87.
- Pita-Calvo, C., & Vázquez, M. (2018). Honeydew honeys: A review on the characterization and authentication of botanical and geographical origins. Journal of Agricultural and Food Chemistry, 66, 2523–2537.
- Ramonaityte, D. T., Keršiene, M., Adams, A., Tehrani, K. A., & de Kimpe, N. (2009). The interaction of metal ions with Maillard reaction products in a lactose-glycine model system. Food Research International, 42, 331–336.
- Spano, N., Ciulu, M., Floris, I., Panzanelli, A., Pilo, M. I., Piu, P. C., … Sanna, G. (2008). Chemical characterization of a traditional honey-based Sardinian product: Abbamele. Food Chemistry, 108, 81–85.
- Turkmen, N., Sari, F., Poyrazoglu, E. S., & Valioglu, Y. S. (2006). Effects of prolonged heating on antioxidant activity and colour of honey. Food Chemistry, 95, 653–657.
- van Boekel, M. A. J. S. (2008). Kinetic modeling of food quality: A critical review. Comprehensive Reviews in Food Science and Food Safety, 7, 144–158.
- Vázquez, L., Verdú, A., Miquel, A., Burló, F., & Carbonell-Barrachina, A. A. (2007). Changes in physico-chemical properties, hydroxymethylfurfural and volatile compounds during concentration of honey and sugars in Alicante and Jijona turrón. European Food Research and Technology, 225, 757–767.
