?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The study was to investigate the immunological activities of polysaccharide isolated from Elaeagnus angustifolia L. The extracted Elaeagnus angustifolia L. polysaccharide (EAP) was purified by DEAE cellulose-52 chromatography (DEAE-52) and Sephadex G-100 size-exclusion chromatography (G-100). Then the average molecular weight (Mw) of purified polysaccharide was determined by high-performance gel permeation chromatography (HPGPC). One main fraction (EAP-1a) was obtained through the isolation and purification steps. The results showed that EAP-1a had strong immunological activities. EAP-1a could significantly increase spleen and thymus index, enhance the NK cell activity, stimulate the phagocytosis of peritoneal phagocyte and increase the level of IL-2, IFN-γ and IgG in serum. Thus, EAP-1a isolated from Elaeagnus angustifolia L. could enhance the immunological activities of immunosuppressed mice induced by cyclophosphamide (CY).
RESUMEN
En el presente estudio se propuso investigar las actividades inmunológicas de polisacáridos aislados de Elaeagnus angustifolia L. El polisacárido extraído de Elaeagnus angustifolia L. (EAP) fue purificado utilizando la cromatografía DEAE celulosa-52 y cromatografía por exclusión de tamaño Sephadex G-100. Posteriormente, se determinó el peso molecular (Mw) promedio del polisacárido purificado, empleando para ello cromatografía por permeación de gel de alto rendimiento (HPGPC). Mediante la aplicación de procesos de aislamiento y purificación se obtuvo una de las fracciones principales (EAP-1a). Los resultados demuestran que el EAP-1a tiene fuertes actividades inmunológicas. El EAP-1a puede elevar significativamente los índices del bazo y el timo, mejorar la actividad de células NK, estimular la fagocitosis del fagocito peritoneal y elevar el nivel de IL-2, IFN-γ e IgG en suero. Por lo tanto, el EAP-1a aislado de Elaeagnus angustifolia L. puede mejorar las actividades inmunológicas en ratones inmunosuprimidos, cuya condición fue inducida mediante la administración de ciclofosfamida.
PALABRAS CLAVE:
Introduction
Elaeagnus angustifolia L. (Elaeagnaceae, Elaeagnus), usually known as Russian olive, is a kind of food and medicine plant which is widely cultivated in the western drought areas of China, especially in Xinjiang. Elaeagnus angustifolia L. has strong adaptability. It can against the wind and sand, resistance to the saline-alkali soil and barren soil. Many researches have proved that Elaeagnus angustifolia L. have anti-microbial effects (Khan et al., Citation2016; Okmen & Turkcan, Citation2013a, Citation2013b), antioxidative effects (Farzaei et al., Citation2016; Yalcin & Sogut, Citation2014), anti-inflammatory activity (Beigom et al., Citation2010), wound healing activity (Mehrabani et al., Citation2012), anti-arthritic effect (Nikniaz, Ostadrahimi, Mahdavi, Ebrahimi, & Nikniaz, Citation2014; Talaei-Khozani et al., Citation2011), and cardiovascular effects (Belarbi et al., Citation2011; Wang, Qu, Ma, Sun, & Wang, Citation2015).
Elaeagnus angustifolia L. has multiple phytochemical constituents, such as carbohydrates, flavonoids, β-carboline alkaloids, amino acids, vitamins, phenolic acids, steroids and terpenes (Bendaikha, Gadaut, Harakat, & Magid, Citation2014). Among them, carbohydrates account for about 43–59% in Elaeagnus angustifolia L. polysaccharides are biological macromolecules, and many researches have proved bioactivities of polysaccharide extracted from Elaeagnus angustifolia L., such as antioxidant, anti-fatigue and anti-radiation effects (Chen et al., Citation2014).
At present, the research of Elaeagnus angustifolia L. mainly stays on the phytochemical constituents, nutritional and medicinal value, exploitation and application, while the study of immunological activities of Elaeagnus angustifolia L. polysaccharide (EAP) is still rare. Therefore, the aim of this study is to investigate the biological activities of EAP which can provide a certain theoretical basis for further research.
Materials and methods
Materials and chemicals
Elaeagnus angustifolia L. was collected from Kashi Prefecture, Xinjiang Province, China. The fruit pulp was dried and pulverized into powder for the next experiment. It was authenticated by professor Hui-ping Liu (Tianjin University of Science & Technology, Department of Food Engineering and Biotechnology, China). The plant materials were deposited at Tianjin University of Science & Technology.
DEAE cellulose-52, Sephadex G-100 gel filtration, RPMI-1640 medium, Fetal Bovine Serum (FBS) and cyclophosphamide (CY) were purchased from Solarbio (Beijing Solarbio S&T Co., LTD). Concanavalin A (Con A) and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO) was purchased from Amresco Co. The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltet-razolium bromide (MTT) was purchased from Amresco Co. Mouse IFN-γ, IL-2 and IgG ELISA Kit were obtained from Rapidbio (RB Co., USA). All other reagents were of analytical grade from China.
Extraction, isolation and purification of the polysaccharide
The dry powder (20 g) of Elaeagnus angustifolia L. pulp was used to extract polysaccharide with distilled water (1:20, w/v) for 3 h at 80℃. We repeated this process twice. The extract solution was merged and removed protein by Sevage (chloroform-butanol 4:1, v/v) method. The above solution was concentrated by rotary vacuum evaporator and precipitated by adding 3-fold volume of 95% ethanol for 24 h at 4℃. After centrifugation, the precipitate was dialyzed and lyophilized to get crude polysaccharide (EAP). The polysaccharide content was quantified by the phenol-sulfuric acid method (Masuko et al., Citation2005).
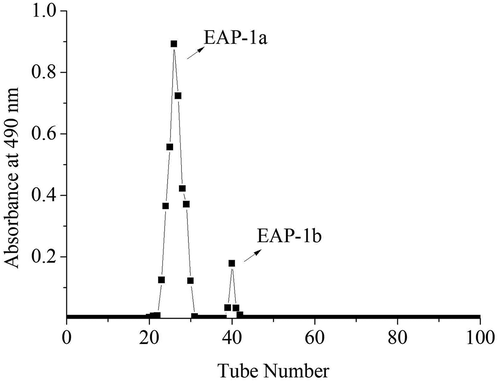
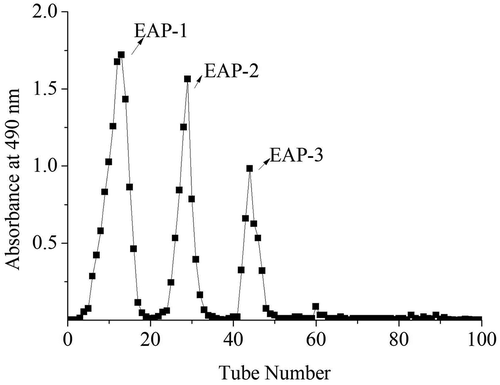
The crude polysaccharide dissolved with distilled water (5 mg/mL) was filtered through 0.45 μm filters and fractionated with a DEAE-52 cellulose column (1.2 × 50 cm) equilibrated with distilled water. The column was eluted with sodium chloride solution (0–0.3 M) at a flow rate of 1.0 mL/min. We obtained three fractions (EAP-1, EAP-2 and EAP-3). The relevant fractions were merged, concentrated, dialyzed and lyophilized. The EAP-1 (5 mg/mL) was further purified by size-exclusion chromatography by a Sephadex G-100 column (2.6 × 50 cm) and eluted with distilled water at a flow rate of 1.0 mL/min. We obtained two fractions (EAP-1a and EAP-1b). The major fraction EAP-1a was merged, concentrated, dialyzed and lyophilized for further experiment.
Determination of molecular weight
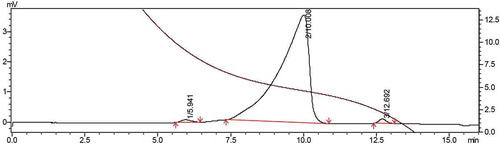
The average molecular weight (Mw) of the purified polysaccharide EAP-1a (1 mg/mL) was determined by high-performance gel permeation chromatography (HPGPC) using a HPLC System (LC-20AT, Japan). Chromatographic column: OHpak SB-803 HQ, detector: RID-10A, mobile phase: ultrapure water, flow rate: 0.6 mL/min, column temperature: 30°C, loading volume: 20 μL.
Experimental animals
50 male KM mice (18–22 g, 6 weeks old) were purchased from Institute of Cancer Research, Beijing, China. The mice were housed under normal laboratory conditions, i.e. a mean fixed temperature of 24 ± 1°C, relative humidity of 60 ± 10%, 12 h light/dark cycle with free access to standard rodent chow and water. All animal experiments were in accordance with the requirement of Animal (Control of Experiments) Ordinance of Laws of China and Tianjin University of Science & Technology.
After adapting for a week, the mice were randomly divided into five groups (ten mice per group): control group (control), model group (model) and three EAP-1a groups (low-dose group, EAP-1a-L; middle-dose group, EAP-1a-M; high-dose group, EAP-1a-H). In order to construct immunosuppression models, cyclophosphamide (CY, 100 mg kg−1 d−1) was administrated to the mice of model group and EAP-1a groups by intraperitoneal injection for three days. After modeling, we intragastrically administered to all the mice once a day in a volume of 0.2 mL d−1 according to the following: control group, saline; model group, saline; EAP-1a-L, 75 mg kg−1 bodyweight EAP-1a; EAP-1a-M, 150 mg kg−1 bodyweight EAP-1a; EAP-1a-H, 300 mg· kg−1 bodyweight EAP-1a. And the five groups mice were continuously gavaged for 10 days.
Immune organ indexes
After 24 h of last gavage, we weighted the body weight of mice and collected blood from retroocular venous plexus. The serum was collected after centrifuged at 4°C, 1000 × g for 15 min. Then, the mice were dislocated to death; then, thymus and spleen were quickly gathered from each mouse. The immune organ index was calculated using the following equation:
Lymphocytes proliferation assay
Spleen of each mouse was washed, ground, collected with 5 mL RPMI-1640 medium and passed through 200 nesh sieve in clean bench. The cell suspension was precipitated for five min in order to remove large tissue. Then, the upper suspension was collected and centrifuged at 4°C, 300 × g for five min. After abandoning the supernatant, the precipitation was added 3 mL erythrocyte lysis buffer for five min, then 3 mL RPMI-1640 medium was added to finish lysis. According to the effect of lysis, repeat above process one or two times. After centrifugation, the precipitation was washed with RPMI-1640 medium for twice. Then, the precipitation was re-suspended in RPMI-1640 medium containing 10% fetal bovine serum (FBS) to obtain splenocyte suspension. The cells were stained with trypan blue and adjusted the cells (survival rate more than 95%) concentration to 2 × 107 cells· mL−1.
The splenocyte suspension (100 μL· well−1) were added to 96-well plates with Con A (final concentration 5 μg· mL−1, 100 μL· well−1) or LPS (final concentration 10 μg· mL−1, 100 μL· well−1). The control wells were added RPMI-1640 solution (100 μL· well−1). After incubating at 5% CO2 incubatorat for 44 h, MTT (5 mg· mL−1, 20 μL· well−1) was added to each well and the plates were incubated for another four hours. After that, DMSO (150 μL· well−1) was added to each well, and the 96-well plates were shaken for 20 min. At last, the absorbance was measured at 570 nm by microplate reader (Bio-Rad, Hercules, CA). The enhancement for lymphocyte proliferation was expressed as a stimulation index (SI).
NK cells activity
The preparation of splenocyte suspension was same with lymphocytes proliferation assay. The spleen cells kept in Ficoll-Hypaque were centrifuged at 700 × g for 20 min to obtain the low-density cells. The low-density cells were centrifuged and washed twice with RPMI-1640 medium to obtain NK cells. Then, the cells concentration of NK (effector cells) and YAC-1 (target cells) were adjusted to 2 × 106 cells· mL−1 and 1 × 105 cells· mL−1 (effector-to-target rate was 20:1).
The test wells were added NK cells and YAC-1 to 96-well plates. The effector control wells were added NK cells and RPMI-1640 medium. The target control wells were added YAC-1 and RPMI-1640 medium. The adding volume of cells was 100 μL·well-1. Then MTT (5 mg mL−1, 10 μL well−1) was added and the 96-well plates were incubated in a incubatorat with 5% CO2 for four hours at 37°C. After centrifugation, 150 µL DMSO was added to each well. The absorbance was measured at 570 nm by microplate reader. The activities of NK cells were calculated using the following equation:
Macrophage phagocytosis assay
Phagocytosis of peritoneal macrophages was measured referred to in references with minor modification (Chen, Zhang, Shen, & Wang, Citation2010; Liu et al., Citation2017). 5 mL saline was injected to abdominal cavity of each mouse. The peritoneal cells were collected and centrifuged at 37°C, 300 × g for five min. After supernatant was removed, the macrophages were washed twice, resuspended in RPMI-1640 medium (containing 10% FBS) and adjusted the cells concentration to 1 × 106 cells· mL−1.
The test wells were added macrophages (100 μL well−1) and the blank wells were added RPMI-1640 medium (100 μL well−1). The 96-well plates were cultured at 37°C in a 5% CO2 incubator for four hours. Non-adherent cells were removed by washing twice with preheated RPMI-1640 medium. Then, 200 μL neutral red solution (0.075%, m/v) was added to each well and the cells were incubated for another four hours. After that, the cultures were washed by medium three times. Then, 100 μL of cell lysis solution (ethanol: acid = 1:1) was added to each well overnight at 4°C. The absorbance was measured at 540 nm using microplate reader. Macrophage phagocytosis activity was calculated according to the following equation:
Determination of IL-2, IFN-γ and IgG in peripheral blood
After 24 h of last gavage, we collected blood from the retroocular venous plexus of mice. The serum was collected after centrifuged at 4°C, 1000 × g for 15 min. The IFN-γ, IL-2 and IgG concentration in serum were measured with an enzyme-linked immunosorbent assay (ELISA kit, Rapidbio) according to the indication of the manufacturer.
Statistical analysis
The results were expressed as mean ± standard deviation and were analyzed using t–test and one-way analysis of variance (ANOVA), using IBM SPSS Statistics 19.0 for meaning differences among the samples. p < 0.05 were considered to be statistically significant.
Results and discussion
Extraction, purification and molecular weight
In the present study, the extracted yield of crude polysaccharide was 5.9% and the polysaccharide content of the crude polysaccharide was 79.25%. Furthermore, the crude polysaccharide was firstly separated through an anion-exchange chromatography of DEAE-52. We obtained three independent elution peaks: EAP-1, EAP-2 and EAP-3 as detected by the phenol-sulfuric acid assay ().
Figure 1. The elution curve of EAP on DEAE-52 column.
Figura 1. Curva de elución del EAP en la columna DEAE-52.
The EAP-1 fraction (relative content 44.56%, polysaccharide content 87.22%, no protein) was collected and purified by gel filtration chromatography Sephadex G-100. In , EAP-1 generated two fractions (EAP-1a and EAP-1b). The big peak named as EAP-1a was collected for further study. The high-performance gel permeation chromatography (HPGPC) was applied to determine the average molecular weight (Mw) of EAP-1a (). The average molecular weight of EAP-1a was calculated to be 10.5 kDa. The structural characterization of EAP-1a was published in our early work (Liu et al., Citation2015).
Immunostimulatory activity in vivo
The mammalian immune system is comprised of two branches: innate and acquired immunity. The innate immune system includes phagocytes (macrophages), NK cells, cytokines (IFN-γ and IL-2) and so on. Acquired immunity is involved in elimination of pathogens in the late phase of infection as well as the generation of immunological memory, which includes humoral immunity mediated by B cells and cell immunity mediated by T cells and NK cells (Akira, Uematsu, & Takeuchi, Citation2006). Exogenous substances, for instance, polysaccharides, could trigger the human immune system to play their biological roles (Bradford, Citation1976; Brown & Gordon, Citation2005; Yang, Zhao, Shi, Yang, & Jiang, Citation2008).
Effect of immune organ index in mice
The spleen and thymus are major organs of the immune system, determination of thymus and spleen weight can reflect the effects of drugs on the immune organs, viscera index is one of the indicators to measure the immune function (Fu et al., Citation2017; Zalys, Zagon, Bonneau, Lang, & Mclaughlin, Citation2000).
In the study, we used cyclophosphamide (CY) to establish the model of immunosuppression in mice. No mortality and any adverse reactions happened. After 10 days of gavage treatment, the effects of EAP-1a on the spleen and thymus index were measured. shows that the spleen index and thymus index of model group decreased significantly when compared to control group (p < 0.05). The spleen index of EAP-1a-L, EAP-1a-M and EAP-1a-H had significant increased when compared to model group (p < 0.05). While the thymus index, only EAP-1a-M and EAP-1a-H had significant increased when compared to model group (p < 0.05). Besides, EAP-1a-H had the highest spleen index and thymus index compared with other groups except the control group.
Figure 4. Effects of EAP-1a on the spleen index (a) and thymus index (b). Note: ap < 0.05 when compared with control; bp < 0.05 when compared with model.
Figura 4. Efectos del EAP-1a en los índices esplénico (a) y del timo (b). Nota: ap < 0.05 comparado con el control; bp < 0.05 comparado con el modelo.
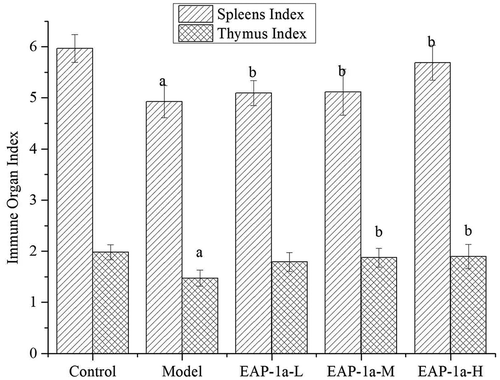
As a cytotoxic drug, CY can damage the structure of DNA, cut off its copy and result in cell death in vivo (Deng et al., Citation2018; Martello, David, & Matuo et al., Citation2016). It is widely used in the treatment of autoimmune diseases and cancer (Manente, Quinello, & Ferreira et al., Citation2017; Yin, Zhou, & Wang et al., Citation2016). Previous research revealed that high doses of CY could lead to suppression of immune system (Sun, Yang, & Pan et al., Citation2018). Because of immunosuppression, the major immune organs (spleen and thymus) were destroyed (Cheng et al., Citation2017; Duggina, Kalla, Varikasuvu, Bukke, & Tartte, Citation2015), resulting in the decrease of spleen index and thymus index, while EAP-1a could effectively protect these immune organs.
Effect of splenic lymphocyte proliferation
Proliferation of splenic cells is an important part in the activation pathway of cellular or humoral immunity (Zhao, Li, & Luo et al., Citation2006). The ability of lymphocytes to respond to mitogen reflects the immune potential of the organism (Singh, Haldar, & Rai, Citation2006). The mitogen ConA and LPS were frequently used to stimulate T and B lymphocyte proliferation, respectively (Cerqueira, Cordeirodasilva, & Gasparmarques et al., Citation2004). Proliferative capacity of splenic cells has been widely used in screening novel immune stimulants (Lee, Lee, & Park et al., Citation2007). The effects of EAP-1a on the splenic lymphocyte proliferation were investigated by comparing the stimulation indexes of T lymphocytes and B lymphocytes stimulated by ConA and LPS.
shows that the lymphocyte proliferation of model group decreased significantly when compared with the control group (p < 0.05). The study demonstrated that cyclophosphamide (CY) could suppress splenic lymphocyte proliferation. This result was in accordance with previous reports (Cho et al., Citation2014; Moynihan & Cohen, Citation1989) that mitogen-stimulated T and B lymphocytes proliferation could be suppressed by CY administration. Moreover, treatment with EAP-1a could significantly increase ConA or LPS stimulated splenic lymphocyte proliferation compared with the model group (p < 0.05). In the three EAP-1a groups, high-dose EAP-1a group presented the strongest promoting effect, while low-dose EAP-1a group showed the weakest stimulating activity whether stimulated by ConA or LPS. In the other words, as the dose of EAP-1a increase, the capacity of the proliferation of T and B lymphocytes was increased.
Table 1. Effects of EAP-1a on proliferation of splenic lymphocyte induced by Con A and LPS.
Tabla 1. Efectos del EAP-1a en la proliferación del linfocito esplénico inducida por Con A y LPS.
CY can inhibit T lymphocyte and B lymphocyte proliferation through alkylating the DNA of the splenic cell (Artym, Zimecki, & Kruzel, Citation2004), while the study indicated that EAP-1a could promote the mitogen-stimulated proliferation of splenic lymphocyte. Previous studies showed that polysaccharides had the ability of activating lymphocytes and promoting their proliferation. Chen, Tan & Chan (Citation2008) indicated that polysaccharides isolated from lycium barbarum L. significantly promoted the proliferation of T lymphocytes, and the signaling pathways that activated T lymphocytes might be achieved by activating NFAT, ap-1, and CD25. Hu and Zheng (Citation2004) showed that sophora subprosrate polysaccharides enhanced the activity of PKC on T lymphocyte membrane and the concentration of free Ca2+ in cells, and Ca2+ was a key factor to activate T lymphocytes. Han et al. (Citation2001) indicated that polysaccharides isolated from the radix of platycodon grandiflorum significantly enhanced the proliferation of B lymphocytes in mice, and the pathway of activated B lymphocytes was related to CD19 and CD79 receptors. Shao et al. (Citation2004) showed that astragalus membranaceus polysaccharides stimulated B lymphocyte proliferation and regulated B lymphocyte activity in BALB/c mice by binding to mIg receptor. Han et al. (Citation2003) indicated that polysaccharides isolated from acanthopanax senticosus could bind to the TLR2/4 receptor on the surface of B lymphocytes to promote the proliferation of B lymphocytes.
Effect of NK cells activity
Natural killer (NK) cells are non-specific immune killer cells which belong to the innate immune system (Zhou et al., Citation2017). It can kill target cells directly without antibodies participation (Pellicci et al., Citation2002). NK cells have been recognized as the most ideal effect cells in immune surveillance cells that can regulate the function of T cells, B cells and bone marrow stem cells (Subleski, Wiltrout, & Weiss, Citation2009).
As shown in , the NK cells activity of mice in model was significantly lower than that of control group (p < 0.05) and EAP-1a group (p < 0.05). In addition, the NK cells activity of EAP-1a-L, EAP-1a-M and EAP-1a-H groups were dose dependent. Besides, the NK cells activity of the high EAP-1a group was considerable compared with control group.
Figure 5. Effects of EAP-1a on NK cell activity. Note: ap < 0.05 when compared with control; bp < 0.05 when compared with model.
Figura 5. Efectos del EAP-1a en la actividad de las células NK. Nota: ap < 0.05 comparado con el control; bp < 0.05 comparado con el modelo.
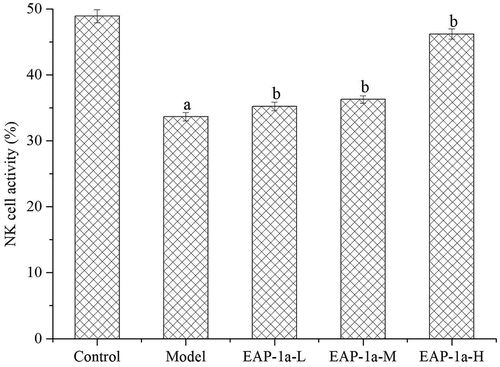
In our study, EAP-1a could enhance the immunological activity of mice by improving the activity of NK cells. The reason why NK cell activity increased might be that EAP-1a activated NK cells and promoted their proliferation. Tsai et al. (Citation2012) drew the similar results that oligosaccharide and peptidoglycan of ganoderma lucidum could activate the immune response of NK cells and promote their proliferation (Tsai et al., Citation2012).
Effect of macrophage phagocytosis
Macrophage is the most important profession phagocyte and it plays an essential and pivotal role in host defense against any type of invading cells including tumor cells (Katsiari, Liossis, & Sfikakis, Citation2010). As shown in , the activity of peritoneal macrophages in the model was significantly lower than that of the control group (p < 0.05). Besides, it indicated that EAP-1a group had the activity of promoting peritoneal macrophages to devour neutral red and caused a significant increase compared with the model group (p < 0.05). The administration of EAP-1a-H group presented the strongest phagocytic activity compared with the other two EAP-1a groups.
Figure 6. Effects of EAP-1a on phagocytosis of macrophage. Note: ap < 0.05 when compared with control; bp < 0.05 when compared with model.
Figura 6. Efectos de la EAP-1a en la fagocitosis del macrófago. Nota: ap < 0.05 comparado con el control; bp < 0.05 comparado con el modelo.
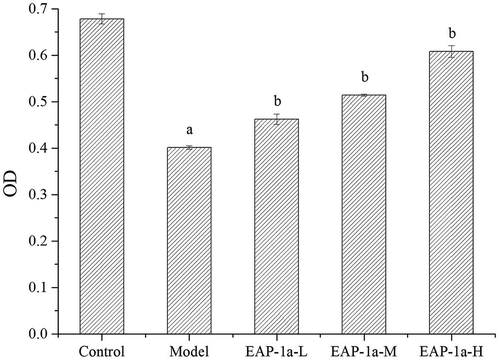
Recent studies have shown that the main function of polysaccharides is to activate the immune response of macrophages (Schepetkin et al., Citation2008). Lee et al. (Citation2004) indicated that polysaccharides isolated from poria cocos activated macrophages through the TLR4-mediated p38 signaling pathway. Li and Xu (Citation2011) showed that polyporus umbellatus (pers.) fries polysaccharides activated macrophages in mice through the NF-κB signaling pathway mediated by TLR4 receptor. Han et al. (Citation2001) indicated that polysaccharides isolated from the radix of platycodon grandiflorum activated macrophages through CD14 and CR3. Nakamura, Suzuki, Wada, Kodama, and Doi (Citation2006) showed that fucosan polysaccharides activated macrophages to release NO through SR receptors. Once macrophages are activated, they can release a significant amount of NO and IL-2, which contributes to devour pathogens (Cheng, Wan, Wang, Jin, & Xu, Citation2008; Park et al., Citation2017).
Levels of IL-2, IFN-γ and IgG in mice serum
Previous reports showed that immune cells (T cells, B cells and NK cells) can synthesize and secret cytokines and cytokines can regulate immunological activities (Schoenaker et al., Citation2018). IL-2 could promote the killing activity of T cells, stimulate differentiation and activation of B lymphocytes and NK cells. INF-γ is an efficient antiviral cytokine, as well as has broad-spectrum immune regulative effects (Zhou et al., Citation2018).
As shown in , the result showed that cyclophosphamide (CY) could decrease the immunological activity of mice by reducing the serum cytokine levels. While the levels of IL-2, IFN-γ and IgG in serum treated with different doses of EAP-1a significantly increased compared with the model group (p < 0.05). And the high-dose EAP-1a group had the highest cytokines level and low-dose EAP-1a group had the lowest cytokines level.
Table 2. Levels of IFN-γ, IL-10 and IgG in serum.
Tabla 2. Niveles de IFN-γ, IL-10 e IgG en suero.
Conclusions
In this study, the results showed that EAP-1a had beneficial effect on immunological activity of cyclophosphamide-induced immunosuppression mice. Therefore, EAP-1a may be explored as an immunomodulator in the field of pharmaceutical. The clinical significance of these findings should be taken more attention in further investigation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akira, S., Uematsu, S., & Takeuchi, O. (2006). Pathogen recognition and innate immunity. Cell, 124, 783–801.
- Artym, J., Zimecki, M., & Kruzel, M. (2004). Normalization of peripheral blood cell composition by lactoferrin in cyclophosphamide-treated mice. Medical Science Monitor International Medical Journal of Experimental & Clinical Research, 10, 84–89. PMID:14976460.
- Beigom, T. J., Anbari, F., Maleki, Z., Boostani, S., Zarghi, A., & Pouralibaba, F. (2010). Efficacy of Elaeagnus angustifolia topical gel in the treatment of symptomatic oral lichen planus. Journal of Dental Research Dental Clinics Dental Prospects, 4, 29–32.
- Belarbi, M., Bendimerad, S., Sour, S., Soualem, Z., Baghdad, C., Hmimed, S., & Visioli, F. (2011). Oleaster oil positively modulates plasma lipids in humans. Journal of Agricultural & Food Chemistry, 59, 8667–8669.
- Bendaikha, S., Gadaut, M., Harakat, D., & Magid, A. (2014). Acylated flavonol glycosides from the flower of Elaeagnus angustifolia L. Phytochemistry, 103, 129–136.
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
- Brown, G. D., & Gordon, S. (2005). Immune recognition of fungal β-glucans. Cellular Microbiology, 7, 471–479.
- Cerqueira, F., Cordeirodasilva, A., Gasparmarques, C., Simões, F., Pinto, M. M., & Nascimento, M. S. (2004). Effect of abietane diterpenes from Plectranthus grandidentatus on T- and B-lymphocyte proliferation. Bioorganic & Medicinal Chemistry, 12, 217–223, PMID:14697786.
- Chen, Q., Chen, J., Du, H., Li, Q., Chen, J., Zhang, G., & Wang, J. (2014). Structural characterization and antioxidant activities of polysaccharides extracted from the pulp of Elaeagnus angustifolia L. International Journal of Molecular Sciences, 15, 11446–11455.
- Chen, W. X., Zhang, W. Y., Shen, W. B., & Wang, K. C. (2010). Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cellular Immunology, 262, 69–74.
- Chen, Z. S, Tan, B. K. H, & Chan, S. H. (2008). Activation of t lymphocytes by polysaccharide-protein complex from lycium barbarum l. International Immunopharmacology, 8, 1663–1671. doi: 10.1016/j.intimp.2008.07.019
- Cheng, A., Wan, F., Wang, J., Jin, Z., & Xu, X. (2008). Macrophage immunomodulatory activity of polysaccharides isolated from Glycyrrhiza uralensis fish. International Immunopharmacology, 8, 43.
- Cheng, K., Song, Z. H., Zheng, X. C., Zhang, H., Zhang, J. F., Zhang, L. L., Zhou, Y. M., & Wang, T. (2017). Effects of dietary vitamin e type on the growth performance and antioxidant capacity in cyclophosphamide immunosuppressed broilers. Poultry Science, 96, 1159–1166.
- Cho, C. W., Han, C. J., Rhee, Y. K., Lee, Y. C., Shin, K. S., & Hong, H. D. (2014). Immunostimulatory effects of polysaccharides isolated from Makgeolli (traditional Korean rice wine). Molecules, 19, 5266–5277.
- Deng, J., Zhong, Y., Wu, Y., Luo, Z., Sun, Y., Wang, G., … He, R. (2018). Carnosine attenuates cyclophosphamide-induced bone marrow suppression by reducing oxidative DNA damage. Redox Biology, 14, 1–6.
- Duggina, P., Kalla, C. M., Varikasuvu, S. R., Bukke, S., & Tartte, V. 2015. Protective effect of centella triterpene saponins against cyclophosphamide-induced immune and hepatic system dysfunction in rats: Its possible mechanisms of action. Journal of Physiology & Biochemistry, 71, 435–454 PMID:26168711.
- Farzaei, M. H., Khanavi, M., Moghaddam, G., Dolatshahi, F., Rahimi, R., Shamsardekani, M. R., & Hajimahmoodi, M. (2016). Standardization of tragopogon graminifolius DC. extract based on phenolic compounds and antioxidant activity. Journal of Chemistry, 2014, 1–7.
- Fu, Y., Jiang, L., Zhao, W., Meng, X., Huang, S., Yang, J., & Chen, H. (2017). Immunomodulatory and antioxidant effects of total flavonoids of Spatholobus suberectus Dunn on PCV2 infected mice. Scientific Reports, 7, 8676.
- Han, S. B., Park, S. H., Lee, K. H., Lee, C. W., Lee, S. H., Kim, H. C., Yim, Y. S., Lee, H. S., & Kim, H.M. (2001). Polysaccharide isolated from the radix of platycodon grandiflorum selectively activates B cells and macrophages but not T cells. International Immunopharmacology, 1, 1969–1978, PMID:11606028
- Han, S. B., Yoon, Y. D., Ahn, H. J., Lee, H. S., Lee, C. W., Yoon, W. K., Park, S. K., & Kim, H. M. (2003). Toll-like receptor-mediated activation of b cells and macrophages by polysaccharide isolated from cell culture of acanthopanax senticosus. International Immunopharmacology, 3, 1301–1312.
- Hu, T., & Zheng, R. 2004. Promotion of sophora subprosrate, polysaccharide on nitric oxide and interleukin-2 production in murine t lymphocytes: Implicated Ca2+, and protein kinase C. International Immunopharmacology, 4, 109–118 PMID:14975365.
- Katsiari, C. G., Liossis, S. N., & Sfikakis, P. P. (2010). The pathophysiologic role of monocytes and macrophages in systemic lupus erythematosus: A reappraisal. Seminars in Arthritis & Rheumatism, 39, 491–503.
- Khan, S. U., Khan, A. U., Shah, A. U., Shah, S. M., Hussain, S., Ayaz, M., & Ayaz, S. (2016). Heavy metals content, phytochemical composition, antimicrobial and insecticidal evaluation of Elaeagnus angustifolia. Toxicology & Industrial Health, 32, 154–161.
- Lee, K. Y., You, H. J., Jeong, H. G., Kang, J. S., Kim, H. M., Rhee, S. D., & Jeon, Y. J. (2004). Polysaccharide isolated from poria cocos sclerotium induces nf-kappab/rel activation and inos expression through the activation of p38 kinase in murine macrophages. International Immunopharmacology, 4, 1029–1038.
- Lee, Y. S., Lee, G. H., Park, J. H., Kwon, Y. K., & Sang, W. S. (2007). Water extracted evodiae fructus possesses immunomodulatory activities on cyclophosphamide induced immunesuppression. Journal of Physiology & Pathology in Korean Medicine, 21, 1450–1455.
- Li, X., & Xu, W. (2011). Tlr4-mediated activation of macrophages by the polysaccharide fraction from polyporus umbellatus (pers.) fries. Journal of Ethnopharmacology, 135, 1–6.
- Liu, A. J., Yu, J., Ji, H. Y., Zhang, H. C., Zhang, Y., & Liu, H. P. (2017). Extraction of a novel cold-water-soluble polysaccharide from astragalus membranaceus and its antitumor and immunological activities. Molecules, 23, 62–74.
- Liu, X. Q., Liu, H. P., Zhao, F., Zhang, C. P., Wang, Y., & Jiao-Jiao, X. U. (2015). Purification and preliminary analysis of Elaeagnusangustifolia L.polysaccharide-1a. Science & Technology of Food Industry, 36, 138–143.
- Manente, F. A., Quinello, C., Ferreira, L. S., De, C. A., Jellmayer, J. A., Portuondo, D. L., Batista, D. A., & Carlos, I. Z. (2017). Experimental sporotrichosis in a cyclophosphamide-induced immunosuppressed mice model. Medical Mycology, 1–12. 10.1093/mmy/myx098
- Martello, M. D., David, N., Matuo, R., et al. (2016). Campomanesia adamantium extract induces DNA damage, apoptosis, and affects cyclophosphamide metabolism. Genetics & Molecular Research Gmr, 15, 1–11.
- Masuko, T., Minami, A., Iwasaki, N., Majima, T., Nishimura, S., & Lee, Y. C. (2005). Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Analytical Biochemistry, 339, 69–72.
- Mehrabani, N. M., Pasalar, P., Kamalinejad, M., Dehpour, A. R., Tavangar, S. M., Sharifi, R., & Gerayeshnejad, S. (2012). Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta Medica Iranica, 50, 589. PMID:23165807.
- Moynihan, J., & Cohen, N. (1989). The kinetics of recovery of leukocyte number and lymphocyte function following an injection of a single high dose of cyclophosphamide in C3H/HeJ mice. International Journal of Immunopharmacology, 11, 517–527.
- Nakamura, T., Suzuki, H., Wada, Y., Kodama, T., & Doi, T. (2006). Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and nf-κb-dependent signaling pathways through macrophage scavenger receptors. Biochemical & Biophysical Research Communications, 343, 286–294.
- Nikniaz, Z., Ostadrahimi, A., Mahdavi, R., Ebrahimi, A. A., & Nikniaz, L. (2014). Effects of Elaeagnus angustifolia L. supplementation on serum levels of inflammatory cytokines and matrix metalloproteinases in females with knee osteoarthritis. Complementary Therapies in Medicine, 22, 864–869.
- Okmen, G., & Turkcan, O. (2013a). A study on antimicrobial, antioxidant and antimutagenic activities of Elaeagnus angustifolia L. leaves. African Journal of Traditional Complementary & Alternative Medicines Ajtcam, 11, 116–120.
- Okmen, G., & Turkcan, O. (2013b). The antibacterial activity of elaeagnus angustifolia l. Against mastitis pathogens and antioxidant capacity of the leaf methanolic extracts. Journal of Animal & Veterinary Advances, 12, 491–496.
- Park, H. R., Hwang, D., Hong, H. D., Shin, K. S., Park, H. R., Hwang, D., & Shin, K. S. (2017). Antitumor and antimetastatic activities of pectic polysaccharides isolated from persimmon leaves mediated by enhanced natural killer cell activity. Journal of Functional Foods, 37, 460–466.
- Pellicci, D. G., Hammond, K. J. L., Uldrich, A. P., Baxter, A. G., Smyth, M. J., & Godfrey, D. I. (2002). A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1(−)CD4(+)CD1d-dependent precursor stage. Journal of Experimental Medicine, 195, 835–844. PMID: 11927628.
- Schepetkin, I. A., Xie, G., Kirpotina, L. N., Klein, R. A., Jutila, M. A., & Quinn, M. T. (2008). Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. International Immunopharmacology, 8, 1455–1466.
- Schoenaker, M. H. D., Henriet, S. S., Zonderland, J., Deuren, M. V., Qiang, P. H., Sluijs, S. J. P., & Ijspeert, H. (2018). Immunodeficiency in Bloom’s syndrome. Journal of Clinical Immunology, 38, 35–44.
- Shao, B. M., Xu, W., Dai, H., Tu, P., Li, Z., & Gao, X. M. 2004. A study on the immune receptors for polysaccharides from the roots of astragalus membranaceus, a chinese medicinal herb. Biochem Biophys Res Commun, 320, 1103–1111 PMID:15249203.
- Singh, S. S., Haldar, C., & Rai, S. (2006). Melatonin and differential effect of l -thyroxine on immune system of Indian tropical bird Perdicula asiatica. Gen Comp Endocrinol, 145, 215–221.
- Subleski, J. J., Wiltrout, R. H., & Weiss, J. M. (2009). Application of tissue-specific NK and NKT cell activity for tumor immunotherapy. Journal of Autoimmunity, 33, 275.
- Sun, C., Yang, J., Pan, L., et al. (2018). Improvement of icaritin on hematopoietic function in cyclophosphamide-induced myelosuppression mice. Immunopharmacology & Immunotoxicology, 40, 1–10.
- Talaei-Khozani, T., Vojdani, Z., Dehghani, F., Heidari, E., Kharazinejad, E., & Panjehshahin, M. R. (2011). Toxic effects of Elaeagnus angustifolia fruit extract on chondrogenesis and osteogenesis in mouse limb buds. Tokai Journal of Experimental and Clinical Medicine, PMID:21932186. 36(3), 63–70.
- Tsai, C. C., Yang, F. L., Huang, Z. Y., Chen, C. S., Yang, Y. L., Hua, K. F., Li, J. J., Chen, S. T., & Wu, S. H. (2012). Oligosaccharide and peptidoglycan of ganoderma lucidum activate the immune response in human mononuclear cells. Journal of Agricultural Food Chemistry, 60, 112830–2837 PMID:22364151.
- Wang, B., Qu, H., Ma, J., Sun, X., & Wang, D. (2015). Protective effects of Elaeagnus angustifolia leaf extract against myocardial ischemia/reperfusion injury in isolated rat heart. Journal of Chemistry, 2014, 6797–6802.
- Yalcin, G., & Sogut, O. (2014). Influence of extraction solvent on antioxidant capacity value of oleaster measured by ORAC method. Natural Product Research, 28, 1513–1517.
- Yang, B., Zhao, M., Shi, J., Yang, N., & Jiang, Y. (2008). Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chemistry, 106, 685–690.
- Yin, J. J., Zhou, Q., Wang, L., et al. (2016). Protective effect of extract of Mauremys mutica against cyclophosphamide (CY)-induced suppression of immune function in mice. Food & Agricultural Immunology, 27, 577–588.
- Zalys, R., Zagon, I. S., Bonneau, R. H., Lang, C. M., & Mclaughlin, P. J. (2000). In vivo effects of chronic treatment with [Met5]-enkephalin on hematological values and natural killer cell activity in athymic mice. Life Sciences, 66, 829–834.
- Zhao, C., Li, M., Luo, Y. F., & Wu, W. K. (2006). Isolation and structural characterization of an immunostimulating polysaccharide from fuzi, Aconitum carmichaeli. Carbohydrate Research, 341, 485–491, PMID:16406277.
- Zhou, S., Zhang, T., Peng, B., Luo, X., Liu, X., Hu, L., & Deng, Y. (2017). Targeted delivery of epirubicin to tumor-associated macrophages by sialic acid-cholesterol conjugate modified liposomes with improved antitumor activity. International Journal of Pharmaceutics, 523, 203–216.
- Zhou, Y., Chen, X., Yi, R., Li, G., Sun, P., Qian, Y., & Zhao, X. (2018). Immunomodulatory effect of tremella polysaccharides against cyclophosphamide-induced immunosuppression in mice. Molecules, 23, 239.