ABSTRACT
Meatballs are widely consumed ready-to-cook meat products due to their favourable tastes and flavours. They can be contaminated with several saprophyte and pathogenic bacteria due to their ingredients. The aim of this study was to investigate the effect of nisin and EDTA treatments in combination with modified atmosphere packaging (ambient air, 80:20:0/O2:CO2:N2 and 0.4:30:69.60/CO:CO2:N2 gas mixtures) on the survival of Salmonella Enteritidis in Turkish type meatballs stored at 4°C. Shelf-life of meatballs containing nisin and/or EDTA was extended compared to control. The efficient inhibition of Salmonella Enteritidis was enhanced with increasing concentrations of EDTA when applied together with nisin (1000 µg/g nisin and 50 mM EDTA). In conclusion, combined effects of nisin and EDTA incorporation with low level of CO packaging appears to be an important strategy to increase food safety; thus, this application can contribute to minimize the incidence of foodborne diseases originated from Salmonella spp.
RESUMEN
Las albóndigas son productos cárnicos listos para cocinar consumidos ampliamente debido a sus sabores y gustos agradables. Sin embargo, debido a los ingredientes que contienen pueden llegar a contaminarse con varias bacterias saprófitas y patógenas. El objetivo de este estudio fue investigar los efectos que producen tratamientos con nisina y EDTA en combinación con envases en atmósfera modificada (aire ambiente, mezcla de gases 80:20:0/O2:CO2:N2 y 0.4:30:69.60/CO:CO2:N2) en la supervivencia de Salmonella Enteritidis en albóndigas de tipo turco almacenadas a 4 °C. Se constató que, en comparación con las albóndigas de control, la vida útil de las albóndigas que contienen nisina y/o EDTA fue más prolongada. Con concentraciones cada vez mayores de EDTA, aplicado conjuntamente con nisina (1000 µg/g nisina y 50 mM EDTA) se pudo mejorar la eficiente inhibición de la Salmonella Enteritidis. Por lo que se concluye que los efectos combinados de nisina y EDTA utilizados con envases que contienen bajo nivel de CO dan cuenta de una importante estrategia que puede elevar la seguridad de estos alimentos. En este sentido, su aplicación puede ayudar a minimizar la incidencia de enfermedades que tienen su origen en Salmonella spp transmitidas por alimentos.
1. Introduction
Meatballs are widely consumed ready-to-cook meat products due to their favourable tastes and flavours (Anar, Citation2010; Bingol, Colak, Cetin, & Hampikyan, Citation2012). Turkish type meatballs (kofte) are produced mainly using ground meat (beef and/or lamb), fat (beef and/or lamb tallow fat), various spices and/or moistened bread. They are called by different names depending on the ingredients and local areas manufactured in Turkey such as Adana kofte, Tekirdag kofte, Inegol kofte (Colak, Hampikyan, Bingol, & Aksu, Citation2008; Serdaroglu & Degirmencioglu, Citation2004; Ulu, Citation2004).
The microbiological quality of meat and other ingredients, especially spices, the hygienic conditions during manufacturing and the type of packaging and storage conditions affect the shelf-life of meatballs (Anar, Citation2010; Bingol et al., Citation2012). As the products contain ground meat and spices, they can be contaminated with several saprophyte and pathogenic bacteria which results in poor hygienic quality and safety of the product. It has been reported that pathogenic bacteria such as Staphylococcus aureus, Clostridium perfringens, Escherichia coli, Escherichia coli O157:H7, Listeria monocytogenes and Salmonella spp. are frequently present in meatballs (Baskaya et al., Citation2004; Cetin, Bingol, Colak, Ergun, & Demir, Citation2010; Gokmen & Alisarli, Citation2003; Kuplulu, Sarimehmetoglu, & Oral, Citation2003; Ozturk, Yilmaz, & Gunes, Citation2010; Sancak, Boynukara, & Agaoglu, Citation1993; Yilmaz, Yetim, & Ockerman, Citation2002).
In order to control or eliminate the growth of spoilage and pathogenic microorganisms, several preservation approaches (Gould, Citation1995; Rahman, Citation2007; Zeuthen & Bøgh-Sørensen, Citation2003) have been investigated including different packaging techniques, such as vacuum or modified atmosphere packaging (MAP), alone or in combination with other procedures including the use of natural antimicrobial preservatives (Economou, Pournis, Ntzimani, & Savvaidis, Citation2009). MAP is a widely used modern meat packaging technique which is intended to maintain the microbial and sensory quality of the product by inhibiting or retarding the growth of undesirable microflora through manipulating the meat microenvironment (Farber, Citation1991; Hotchkiss, Citation1988). Packaging atmosphere is designed by altering the gas mixtures of the packages. Although elevated CO2 and reduced O2 levels in MAP can inhibit the growth of various spoilage and pathogenic microorganisms (Jeremiah, Citation2001), oxidative degradation and colour changes may limit the shelf-life of the product. Low levels (0.4%) of CO appear to be an alternative packaging atmosphere to control the growth of spoilage organisms and pathogenic bacteria, prevent the surface discolouration, bone darkening and premature browning, and also enhance the flavour acceptability, desirable red colour stability and tenderness (AMSA, Citation2008).
Nisin, a bacteriocin produced by Lactococcus lactis subsp. Lactis, is a generally recognized as safe (GRAS) substance. It is active against Gram-positive organisms including bacterial spores (Colak et al., Citation2008; Delves-Broughton, Citation1993). The antimicrobial activity of nisin is primarily based on pore formation in the cytoplasmic membrane of target organisms. It alters the cell membrane of sensitive organisms resulting in leakage of low molecular weight cytoplasmic components and the destruction of the proton motive force (PMF) (Bruno, Kaiser, & Montville, Citation1992). However, nisin is not generally active against Gram-negative bacteria, yeasts and fungi. Gram-negative bacteria are resistant to nisin due to the difference in the structure of the cell wall envelope. The inhibitory activity of nisin on Gram-negative bacteria could be improved by combining it with other antimicrobial factors (Fang & Tsai, Citation2003). Treatment with food grade chelators such as ethylenediaminetetraacetic acid (EDTA) can alter the outer membrane permeability of Gram-negative bacteria which enhances the antimicrobial effect of nisin (Colak et al., Citation2008; Delves-Broughton, Citation1993). EDTA has an antimicrobial effect by limiting the availability of cations and acts to destabilize the cell membranes of bacteria by complexing divalent cations which play a bridge role between macromolecules, such as lipopolysaccharides preservatives (Economou et al., Citation2009).
Consequently, Gram-negative bacteria such as Salmonella generally exhibit resistance to the lantibiotics, such as nisin (Arqués, Rodríguez, Nuñez, & Medina, Citation2011; Dischinger, Chipalu, & Bierbaum, Citation2014) which can become susceptible to the action of these antimicrobial peptides with the use of chelator EDTA (Galvao, Prudencio, & Vanetti, Citation2015; Govaris, Solomakos, Pexara, & Chatzopoulou, Citation2010; Prudêncio, Vanetti, & Prieto, Citation2015; Wan Norhana, Poole, Deeth, & Dykes, Citation2012).
Thus, the aim of the present study was to investigate the effect of nisin and/or EDTA treatments in combination with modified atmosphere packaging on the survival of Salmonella Enteritidis in Turkish type meatballs stored at 4°C.
2. Materials and methods
2.1. Preparation of treatment solutions
Pure nisin from Lactococcus lactis subsp. lactis was obtained from Sigma–Aldrich (N5764, Darmstadt, Germany) and 2.5 g of nisin was solubilized in 200 mL 0.02 M HCl with heating to 60–70°C. EDTA was used as chelator (Sigma–Aldrich Company Ltd, Darmstadt, Germany) and prepared to the concentrations of 10 mM and 50 mM. Both nisin and EDTA solutions were sterilized by filtration through a 0.22 µm membrane filters (Millex, Millipore, Maidstone, UK). For each experimental production, the nisin and EDTA solutions were prepared daily. For each nisin–EDTA treatment combination, the pH was adjusted to 6.5 with concentrated HCl.
2.2. Preparation of inoculum
Salmonella enterica subsp. enterica serovar Enteritidis (ATCC 13076) strain was obtained from Microbiologics® (Minnesota, USA) and stored in glycerol (30%) at −80°C. The sample was streaked on Tryptone Soya Agar (Oxoid CM131, Basingstoke, UK) plates and was incubated at 35°C overnight. After 24 h, S. Enteritidis was grown aerobically in Tryptone Soya Broth (TSB; Oxoid CM129) at 37°C for 18 h to obtain cells in early stationary growth phase. Before use, the optical density of the suspension was measured using a spectrophotometer (Shimadzu UV-1202 UV-VIS, Japan) at 620 nm and the suspensions were diluted in TSB to the bacterial density required. This population was also verified by subsequent plating on standard plate count agar (Oxoid CM0463) incubated at 35°C for 24 h.
2.3. Manufacturing of meatballs
Well-matured and finely grounded veal (M. Longissimus dorsi) meat was purchased from a local market in Avcilar-Istanbul and was used in experimental meatball production and the samples were produced according to the following traditional recipe (Colak et al., Citation2008). The ground veal (84%), which contains 10% fat, was mixed with ground black pepper (0.1%), cumin (0.4%), red pepper (2.0%), onion rind (3.0%), garlic clove rind (0.5%), salt (2.0%) and toasted bread (made of wheat flour) crumbs (8.0%). The mix was kneaded for 30 min by hand (with a sterile glove) to obtain a homogeneous dough; then divided into two batches, one for inoculation and one for non-inoculation (control) treatment.
The experimental meatball samples were manufactured at room temperature (20°C ± 2) in triplicate for each group on different dates.
2.4. Inoculation of meatballs and treatment with nisin-EDTA solutions
One batch of meatball dough was used for the inoculation with Salmonella enterica subsp. enterica serovar Enteritidis (ATCC 13076) strain, while another batch (non-inoculated) was kept for physico-chemical and total viable bacteria counts (TVBC) analyses. For inoculated groups, an aliquot of 10 mL of bacterial suspension was added in meatball dough to obtain 106 CFU/mL per kg and mixed thoroughly for approximately 10 min and kept for 30 min at 4°C to allow the attachment of Salmonella Enteritidis cells. Afterwards, each batch was divided into six equal sub-groups, and each of the groups was treated separately with different nisin and/or EDTA concentrations as described in and homogenized carefully. Finally, the mixture was shaped by hand into 5-cm-diameter meatballs with a weight of 40 ± 5 g.
Table 1. Nisin-EDTA concentrations and gas compositions of experimental meatball samples.
Tabla 1. Concentraciones de nisina-EDTA y composiciones de gases en muestras experimentales de albóndigas.
2.5. Packaging of meatballs
Both inoculated and non-inoculated nisin and/or EDTA added meatballs were placed in low O2 permeable (8–12 cm3/m2/24 h at STP) polystyrene/ethyl vinyl alcohol (EVOH)/polyethylene (PE) trays and were heat-sealed with a Multivac packaging unit (Multivac A 300/16, Sepp Haggenmüller, D 87787 Wolfertschwenden, Germany) using a low O2 permeable (3 cm3/m2/24 h) lidding film (20 mm of a laminate orientated polypropylene (OPP) and a co-extrusion layer (50 mm) of PE/EVOH/PE) (Wrap Film Systems Ltd., Shropshire, UK) for aerobic and modified atmosphere packaging (). Packages were stored at refrigerator temperature (4 ± 1°C) for 12 days and examined at intervals of 0 (3 h after packaging), 2, 4, 6, 9 and 12 days of storage.
2.6. Microbiological and physico-chemical analyses
Meatball samples were analysed before inoculation to identify the present microbiota. For this purpose, 25 g of meatball sample was added in 225 mL of saline peptone water and homogenized in a stomacher (Lab Blender 400, Model BA6021, Steward Lab., London, UK). Then, an aliquot of 0.1 mL of decimal dilution was transferred to plate count agar (Oxoid CM0463, Basingstoke, UK) and incubated at 30°C for 72 h. (ISO 4833, 05/2003).
A pre-detection step was applied to determine if there were any Salmonella Enteritidis in the samples before inoculation. For this purpose, 25 g of meatball was added into 225 mL of buffered peptone water (Oxoid CM0509, Basingstoke, UK) and incubated at 35°C for 24 h. After the pre-enrichment step, 0.1 mL of aliquot was transferred to 10 mL Rappaport Vassiliadis Broth (Oxoid CM0669, Basingstoke, UK) and another 0.1 mL of aliquot to 10 mL MKTTn Broth (Oxoid CM0343, Basingstoke, UK) and incubated for 24 h, at 42 ± 1°C and 37 ± 1°C, respectively. The test cultures from RV and MKTTn broths were separately streaked in Xylose Lysine Desoxycholate Agar (Oxoid CM0469, Basingstoke, UK) and Hektoen Enteric Agar (Oxoid CM0419, Basingstoke, UK), and incubated for 24 h at 37°C (ISO 6579/A1, Citation2006). Randomly selected typical colonies from the agar plates were confirmed to be Salmonella spp. using typical biochemical and serological tests. For this purpose, typical colonies were checked and selected for growing on nutrient agar (NA – Oxoid, CM0003) at 35°C for 18–24 h and identified by triple sugar iron agar (TSI – Oxoid, CM0277), lysine iron agar (LIA – Oxoid, M0381) fermentation tests, urease test (urea broth – Oxoid, CM0071) and Voges-Proskauer, indol, O-, Vi and H-antigen tests (Murex Salmonella Polyvalent Agglutinating Sera).
To enumerate the level of Salmonella Enteritidis of inoculated meatball samples, 25 g of meatball was added in 225 mL of saline peptone water and homogenized in a stomacher. Then, an aliquot of 0.1 mL of decimal dilution was transferred to Xylose Lysine Desoxycholate Agar and Hektoen Enteric Agar, and incubated for 24 h at 37°C. Typical colonies were enumerated as Salmonella Enteritidis and defined as log CFU/g.
To determine the TVBC of non-inoculated meatball samples, a 0.1 mL inoculum of appropriate dilutions of non-inoculated meatballs was spread on Plate Count Agar (Oxoid, CM0325) and incubated for 72 h at 30°C (ISO 4833, 05/2003).
The pH was also determined using a pH meter (Hanna HI-9321, Woonsocket, RI) by calculating the mean of three measures in each non-inoculated meatball sample at room temperature (AOAC, Citation1990). Water activity (aw) measurement was carried out using a hygrometer (Lufft, Fellbach, Germany) at room temperature (AOAC, Citation1990).
2.7. Statistical analyses
General Linear Model procedure (PROC GLM) of SPSS 13.0 program was conducted to investigate the effect of nisin and/or EDTA on different packaging conditions and storage times of meatballs (SPSS, Citation2001). Univariate Analysis of Variance was performed to analyse data values including the fixed effects of treatment, packaging and storage time. Mean separations were obtained using Duncan’s multiple range tests (P < 0.05), and significant two-way interactions between the main effects were also evaluated.
3. Results and discussion
The initial bacterial population of non-inoculated meatballs was determined as approximately 4.5 log CFU/g and no Salmonella Enteritidis or other Salmonella species were detected. The bacterial counts of nisin and/or EDTA treated meatball samples stored in refrigerator condition differed to control samples during the storage period. The significance of analyzed parameters of meatball samples are presented in .
Table 2. Mean values, standard errors (SE) and significant interaction of microbiological parameters in experimental meatball samples during storage at refrigerator temperature (log CFU/g).
Tabla 2. Valores medios, errores estándar (SE) e interacción significativa de los parámetros microbiológicos en muestras experimentales de albóndigas durante su almacenamiento a temperaturas de refrigerador (log CFU/g).
There were significant differences among the microbial counts according to treatments, packaging type and storage time (P < 0.05). In all meatball samples, the addition of nisin and EDTA together in the recipe delayed the microbial growth significantly compared to individual usage of these antimicrobials. The most efficient inhibition of Salmonella Enteritidis and TVBC was observed in EDTA treatment; especially in NE50 (). Packaging conditions affected the growth of microorganisms during the whole storage period (P < 0.05). MAP and CO packaging of meatballs differed significantly from air packaging. Especially, meatballs which were packaged with CO showed a 1 to 2.5 log CFU/g inhibition in every examined day of the storage in terms of Salmonella Enteritidis and TVBC ().
The total microbial population of control (C-Air) sample reached to 6.760 log CFU/g and varied from 5.687 to 6.063 log CFU/g for treated meatballs at the end of the storage under air packaging. Likewise, samples packaged with MAP and CO reached to 6.047 log CFU/g for C-MAP and 5.777 log CFU/g for C-CO and varied from 4.897 to 5.740 log CFU/g and 3.950 to 5.273 log CFU/g, respectively, for treated meatballs at the end of the storage.
For Salmonella Enteritidis, the inoculated initial bacteria count was 6.5 log CFU/g and reached to 8.243, 7.937 and 7.023 log CFU/g for control samples of each packaging condition, while the counts for treated samples varied from 7.037 to 7.920 log CFU/g for air packaging, 6.827 to 7.710 log CFU/g for MAP and 5.877 to 6.987 log CFU/g for CO packaging on day12th.
Gänzle, Hertel, and Hammes (Citation1999) indicated that smooth strains of Salmonella enterica serovar Typhimurium were highly resistant towards nisin, whereas mutants that possess the core of the lipopolysaccharide (LPS) were sensitive. In addition to this, Govaris et al. (Citation2010) declared that treatment of minced sheep meat only with nisin at 500 or 1000 µg/g, proved insufficient bactericidal influence against Salmonella Enteritidis. In a study conducted by Lappe, Motaa, Sant’anna, and Brandelli (Citation2009), it was demonstrated that EDTA alone in concentrations of 20, 50 and 100 mM caused a significant reduction of viable counts of Salmonella Enteritidis with a 1.77, 2.27 and 2.47 log CFU/mL inhibition, respectively. They stated that the greater the concentration of EDTA, the greater the inhibitory effect was. These findings are in agreement with the results of the present study.
Prudêncio et al. (Citation2015) emphasized that the EDTA treatment alone reduced the growth of Salmonella Typhimurium cells and highlighted that in the presence of only nisin, the growth was similar to the control group. Similar to the present study, smaller reduction in viability was observed during the individual treatments; however, the combined action of nisin and EDTA resulted in significant reductions with increasing concentrations of EDTA against Salmonella Enteritidis and TVBC. Additionally, Prudêncio, Mantovani, Cecon, Prieto, and Vanetti (Citation2016) demonstrated that the reduction in the number of viable cells of Salmonella Typhimurium varied according to the temperature and pH, and noted that major reduction in logarithmic cycles of viable cells occurred with a temperature increase. Regarding to this knowledge, breakdown in the storage temperature of the product or non-hygienic prior/during and post-processing may shorten the shelf-life of meatballs (Koutsoumanis, Stamatiou, Drosinos, & Nychas, Citation2008; Temelli, Sen, & Anar, Citation2011) by increasing the growth potential of saprophytic and/or pathogenic bacteria.
Khan, Vu, Riedl, and Lacroix (Citation2015) reported that nisin concentration of 125–150 mg/mL with a Na-EDTA concentration of 20–30 mM and a pH value of 5–6 inhibited the Salmonella Typhimurium count. Likewise, Stevens, Sheldon, Klapes, and Klaenhammer (Citation1991), stated that the inhibitory activity of nisin appears when used in combination with the chelating agent EDTA, against a wide variety of Salmonella species. A 30-min exposure to 20 mM EDTA and 50 µg of nisin/mL in combination resulted in a 2.5 to 4.7 log cycle reduction while yielded 3.2 to 5.3 log cycle reduction at 60 min exposure time.
Although nisin underwhelms inactive against Gram-negative bacteria such as E. coli and Salmonella spp. due to their outer membranes, the antimicrobial activity of nisin has to be promoted with a chelating agent like EDTA, which increases the susceptibility to nisin by destabilizing the LPS layer (Boziaris & Adams, Citation1999; Khan et al., Citation2015; Martin-Visscher, Yoganathan, Sit, Lohans, & Vederas, Citation2011). As EDTA derivate reduces the interaction between LPS molecules, the alterations in the structure of LPS layer may lead to the appearance of phospholipids in the outer membranes and form channels through the pores into which hydrophobic residues (N-terminal position) of nisin could diffuse (Gill & Holley, Citation2003; Stevens et al., Citation1991) and disrupt the cytoplasmic membrane of susceptible bacteria (Shai, Citation1999). In the current study, nisin could then improve the antimicrobial activity against Salmonella Enteritidis and TVBC incorporation of EDTA. Both nisin and EDTA have been found to have a positive influence on the bactericidal activity against argued bacteria.
The results of the current study demonstrate a change in the behaviour of the bacterial population previously exposed to the combination of nisin with EDTA under specific packaging conditions, especially in reduced O2 concentrations. Days of storage at refrigerator temperature and packaging conditions were the factors (P < 0.05) affecting each treatment group. Significant differences were determined by the interaction of packaging systems (P < 0.05) with a magnitude of lower microbial loads in MAP and CO compared to in air packaging. Microbial growth was more inhibitory in CO packaged meatballs, whereas bacterial inhibition was enhanced with increasing concentrations of EDTA ().
In the light of these data, air packaged samples showed higher Salmonella Enteritidis and total viable bacteria counts compared to MAP and CO packages during whole storage time (P < 0.05), with approximately 1 log CFU/g higher than modified atmosphere. Meatballs which were treated with 1000 IU/g nisin and 50 mM EDTA and packaged with CO differed to 2.1–2.5 log CFU/g in every examined day of the storage in terms of Salmonella Enteritidis (). These findings are conspicuous because this is the first study to observe the effect of nisin combined with EDTA in low level of CO atmosphere on Salmonella Enteritidis, which is a considerable application to protect the consumer’s health.
Previous researches demonstrated the beneficial effect of modified gas composition in retail packages by proving that fresh meats in high-oxygen MAP (70–80% O2) or CO-MAP both have longer shelf-lives compared to meats in PVC overwrap due to the inhibitory effects of CO2 on bacterial growth (AMSA, Citation2008). Jayasingh, Cornforth, Carpenter, and Whittier (Citation2001) emphasized that aerobic and anaerobic bacteria count of minced beef in aerobic PVC packages reached spoilage levels (< 106 CFU/g) by 2 weeks of storage at 2°C, compared to 3 weeks for aerobic and 5 weeks for anaerobic bacteria counts in 0.5% CO-MAP. Additionally, Hunt et al. (Citation2004), Brooks et al. (Citation2008) and Rogers et al. (Citation2014) observed a considerable reduction in the number of spoilage bacteria in CO-MAP packages compared to air and high-O2 MAP ones. These researchers concluded that CO-MAP was a viable solution for the extension of shelf-life in minced beef packages. Similarly, minced beef stored in low CO-MAP (<0.5% CO) had also less growth of pathogenic bacteria compared to similar foods packaged in aerobic conditions (AMSA, Citation2008). Nissen, Alvseike, Bredholt, Holck, and Nesbakken (Citation2000) stated that the growth of E. coli O157:H7, Y. enterocolitica and L. mononcytogenes in ground beef was inhibited by low CO-MAP. Brashears, Brooks, and Miller. (Citation2006) also reported that a modified atmosphere environment consisting of 0.4:30:69.60/CO:CO2:N2 reduced E. coli O157:H7 and Salmonella spp. in minced beef patties by 1 × 102 log CFU/g compared to traditional packages. These results are in agreement with the present study where a considerable inhibition could be observed in the growth of bacteria.
Changes in pH and water activity are also shown in . There were no significant interactions for pH and aw (P > 0.05) in terms of treatment and packaging conditions; whereas, significant differences were generally found among treatment and packaging groups during storage period (P < 0.05). The pH value of meatballs increased with storage time, while a decrease was monitored in aw value until the end of storage time for all samples.
Figure 1. pH and aw values of experimental meatball samples during storage at refrigerator temperature.(a) pH values of experimental meatball samples under air packaging during storage at 4°C, (b) pH values of experimental meatball samples under modified atmosphere packaging during storage at 4°C, (c) pH values of experimental meatball samples under CO packaging during storage at 4°C, (d) aw values of experimental meatball samples under air packaging during storage at 4°C, (e) aw values of experimental meatball samples under modified atmosphere packaging during storage at 4°C, (f) aw values of experimental meatball samples under CO packaging during storage at 4°C, (C) Control (without Nisin or EDTA), (NIS) 1000 µg/g nisin; no EDTA, (E10) 10 mM EDTA; no nisin, (E50) 50 mM EDTA; no nisin, (NE10) 1000 µg/g nisin and 10 mM EDTA, (NE50) 1000 µg/g nisin and 50 mM EDTA.
Figura 1. Valores del pH y aw de la muestras experimentales de albóndigas durante su almacenamiento a temperaturas de refrigerador.(a) Valores de pH de muestras experimentales de albóndigas envasadas con aire durante su almacenamiento a 4°C, (b) valores de pH de muestras experimentales de albóndigas envasadas en atmósfera modificada durante su almacenamiento a 4°C, (c) valores de pH de muestras experimentales de albóndigas envasadas con CO durante su almacenamiento a 4°C, (d) valores de aw de muestras experimentales de albóndigas envasadas con aire durante su almacenamiento a 4°C, (e) valores de aw de muestras experimentales de albóndigas envasadas en atmósfera modificada durante su almacenamiento a 4°C, (f) valores de aw de muestras experimentales de albóndigas envasadas con CO durante su almacenamiento a 4°C, (C) Control (sin nisina ni EDTA), (NIS) 1000 µg/g nisina; sin EDTA, (E10) 10 mM EDTA; sin nisina, (E50) 50 mM EDTA; sin nisina, (NE10) 1000 µg/g nisina y 10 mM EDTA, (NE50) 1000 µg/g nisina y 50 mM EDTA.
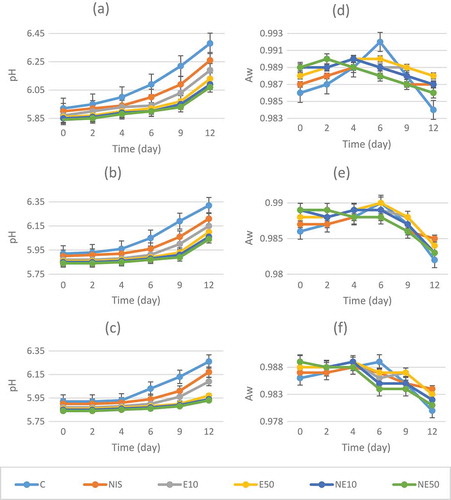
The initial pH value of control (C-Air) meatball sample was 5.92 and reached to 6.38 and varied from 6.07 to 6.26 for treated meatballs at the end of the storage under air packaging condition. Identically, samples packaged with MAP and CO reached to 6.32 for C-MAP and 6.26 for C-CO and varied from 6.04 to 6.21 and 5.93 to 6.17, respectively, for treated meatballs at the end of the storage. For water activity, the initial aw value was 0.986 and decreased to 0.984, 0.982 and 0.980 for control samples of each packaging condition, while the counts for treated samples varied from 0.986 to 0.988 for air packaging, 0.983 to 0.985 for MAP and 0.981 to 0.984 for CO packaging on day12th.
Economou et al. (Citation2009) stated that the pH values of fresh chicken muscle showed no statistically significant (P > 0.05) changes for all samples containing nisin and/or EDTA during the storage period under a gas mixture of 65:30:5/CO2:N2:O2 modified atmosphere package and remained in the range of approximately 6.2–6.4. Govaris et al. (Citation2010) indicated that the initial pH value of minced sheep meat was 5.27 and increased to 5.63 and 5.85 by the end of storage at 4°C and 10°C, respectively. They also added that the pH values of nisin (500 or 1000 IU/g) treated samples were not significantly different (P > 0.05) than those of control. Similarly, Colak et al. (Citation2008) determined that the initial pH of meatballs was 6.0 and increased during storage time, reaching nearly similar values (6.32–6.39) at the end of the shelf-life and added that the pH of treated meatballs with different nisin concentrations was not significantly affected (P > 0.05). Moreover, they observed that the aw values in the treated meatballs were in accordance with the results obtained in the control sample. Nisin had no effect on aw values of the meatballs during refrigerated storage under aerobic PVC packages. Water activity takes an active part in the exchange with the ambient humidity during storage and the reduction in aw improves the shelf-life and safety of meat products, because they are more stable to microorganisms that can cause spoilage or food poisoning (Troller & Christian, Citation1978).
4. Conclusions
Nisin has been used in combination with EDTA on various foods, such as fresh beef, meat products and chicken meat to control of spoilage and pathogenic bacteria. The present study demonstrates that increasing concentrations of the chelating agent EDTA allows the reduction of Salmonella Enteritidis. Addition of nisin and EDTA together in the product delays the microbial growth significantly compared to their individual usage.
The shelf-life of Turkish type meatballs packaged by various modified atmosphere conditions were improved compared to aerobic packaging. MAP with a low level of CO applications limited the growth of microorganisms and appeared as a substantial choice for the refrigerated storage of meatballs. The combined effect of nisin and EDTA incorporation with a low level of CO (<0.5% CO) atmosphere appears to be an important strategy to increase food security and to minimize the incidence of foodborne diseases.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AMSA. (2008). Low-oxygen packaging of fresh meat with carbon monoxide. American meat science association. White Paper Series Number, 2(10), 1–12.
- Anar, S. (2010). Et ve Et Ürünleri Teknolojisi. Pastırma, Kavurma, Konserve, Hazırlanmış Et Karışımları, Jöle Işkembe ve Füme Dil Üretim Teknolojileri (Chapter 6, pp. 400–405). Bursa, Istanbul: DORA Basım.
- AOAC. (1990). Official methods of analysis of AOAC international (15th ed.). Washington DC: Association of Official Analytical Chemists.
- Arqués, J. L., Rodríguez, E., Nuñez, M., & Medina, M. (2011). Combined effect of reuterin and lactic acid bacteria bacteriocins on the inactivation of food-borne pathogens in milk. Food Control, 22, 457–461.
- Baskaya, R., Karaca, T., Sevinc, I., Cakmak, O., Yildiz, A., & Yoruk, M. (2004). The histological, microbiological and serological quality of ground beef marketed in İstanbul. Yuzuncu Yıl Universitesi Veteriner Fakültesi Dergisi, 15, 41–46.
- Bingol, E. B., Colak, H., Cetin, O., & Hampikyan, H. (2012). Effects of sodium lactate on the shelf life and sensory characteristics of cig kofte – A turkish traditional raw meatball. Journal of Food Processing and Preservation, 38, 1024–1036.
- Boziaris, I. S., & Adams, M. R. (1999). Effect of chelators and nisin produced in situ on inhibition and inactivation of Gram negatives. International Journal of Food Microbiology, 53, 105–113.
- Brashears, M. M., Brooks, J. C., & Miller., M. F. (2006). Effect of meat packaging technologies on the safety and spoilage-indicating characteristics of ground beef – phase 1: Safety characteristics. Final Report submitted to the National Cattlemen’s Beef Association.
- Brooks, J. C., Alvarado, M., Stephens, T. P., Kellermeier, J. D., Tittor, A. W., & Miller, M. F. (2008). Spoilage and safety characteristics of ground beef packaged in traditional and modified atmosphere packages. Journal of Food Protection, 71, 293–301.
- Bruno, M. E., Kaiser, A., & Montville, T. J. (1992). Depletion of the proton motive force by nisin in Listeria monocytogenes cells. Applied Environmental Microbiology, 58, 2255–2259.
- Cetin, O., Bingol, E. B., Colak, H., Ergun, O., & Demir, C. (2010). The microbiological, serological and chemical qualities of mincemeat marketed in Istanbul. Turkish Journal of Veterinary & Animal Sciences, 34, 407–412.
- Colak, H., Hampikyan, H., Bingol, E. B., & Aksu, H. (2008). The effect of nisin and bovine lactoferrin on the microbiological quality of Turkish style meatball (Tekirdag kofte). Journal of Food Safety, 28, 355–375.
- Delves-Broughton, J. (1993). The use of EDTA to enhance the efficacy of nisin towards Gram-negative bacteria. International Biodeterioration & Biodegradation, 32, 87–97.
- Dischinger, J., Chipalu, S. B., & Bierbaum, G. (2014). Lantibiotics: Promising candidates for future applications in health care. International Journal of Medical Microbiology, 304, 51–62.
- Economou, T., Pournis, N., Ntzimani, A., & Savvaidis, I. N. (2009). Nisin–EDTA treatments and modified atmosphere packaging to increase fresh chicken meat shelf-life. Food Chemistry, 114, 1470–1476.
- Fang, T. J., & Tsai, H.-C. (2003). Growth patterns of Escherichia coli O157:H7 in ground beef treated with nisin, chelators, organic acids and their combinations immobilized in calcium alginate gels. Food Microbiology, 20, 243–253.
- Farber, J. M. (1991). Microbiological aspects of modified-atmospheres packaging technology: A review. Journal of Food Protection, 54, 58–70.
- Galvao, M. F., Prudencio, C. V., & Vanetti, M. C. D. (2015). Stress enhances the sensitivity of Salmonella enterica serovar Typhimurium to bacteriocins. Journal of Applied Microbiology, 118, 1137–1143.
- Gänzle, M. G., Hertel, C., & Hammes, W. P. (1999). Resistance of Escherichia coli and Salmonella against nisin and curvacin A. International Journal of Food Microbiology, 48, 37–50.
- Gill, A. O., & Holley, R. A. (2003). Interactive inhibition of meat spoilage and pathogenic bacteria by lysozyme, nisin and EDTA in the presence of nitrite and sodium chloride at 24°C. International Journal of Food Microbiology, 80, 251–259.
- Gokmen, M., & Alisarli, M. (2003). Investigation of some pathogenic microorganisms in minced meat consumed in Van. Yuzuncu Yıl Universitesi Veteriner Fakültesi Dergisi, 14, 27–34.
- Gould, G. W. (1995). New methods of food preservation (1st ed). US: Springer Science & Business Media.
- Govaris, A., Solomakos, N., Pexara, A., & Chatzopoulou, P. S. (2010). The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. International Journal of Food Microbiology, 137, 175–180.
- Hotchkiss, J. H. (1988). Experimental approaches to determining the safety of food packaged in modified atmospheres. Food Technology, 42, 55–64.
- Hunt, M. C., Mancini, R. A., Hachmesiter, K. A., Kropf, D. H., Merriman, M., de Lduca, G., & Milliken, G. (2004). Carbon monoxide in modified atmosphere packaging affects color, shelf life, and microorganisms of beef steaks and ground. Journal of Food Science, 69, 45–52.
- ISO 4833 (05/2003). General guidance for the enumeration of micro-organisms. Colony-count technique at 30°C.. Geneva, Switzerland: International Organization for Standardization.
- ISO 6579/A1 (02/2006). Microbiology of food and animal feeding stuffs Horizontal method for the detection of Salmonella spp. Geneva, Switzerland: International Organization for Standardization.
- Jayasingh, P., Cornforth, D. P., Carpenter, C. E., & Whittier, D. (2001). Evaluation of carbon monoxide treatment in modified atmosphere packaging or vacuum packaging to increase color stability of fresh beef. Meat Science, 59, 317–324.
- Jeremiah, L. E. (2001). Packaging alternatives to deliver fresh meats using short-or long-term distribution. Food Research International, 34, 749–772.
- Khan, A., Vu, K. D., Riedl, B., & Lacroix, M. (2015). Optimization of the antimicrobial activity of nisin, Na-EDTA and pH against gram-negative and gram-positive bacteria. Journal of Food Science and Technology, 61, 124–129.
- Koutsoumanis, K. P., Stamatiou, A. P., Drosinos, E. H., & Nychas, G. J. E. (2008). Control of spoilage microorganisms in minced pork by a self-developed modified atmosphere induced by the respiratory activity of meat microflora. Food Microbiology, 25, 915–921.
- Kuplulu, O., Sarimehmetoglu, B., & Oral, N. (2003). The microbiological quality of ciğ kofte sold in Ankara. Turkish Journal of Veterinary & Animal Sciences, 27, 325–329.
- Lappe, R., Motaa, A. S., Sant’anna, V., & Brandelli, A. (2009). Inhibition of Salmonella Enteritidis by cerein 8A, EDTA and sodium lactate. International Journal of Food Microbiology, 135, 312–316.
- Martin-Visscher, L. A., Yoganathan, S., Sit, S. C., Lohans, C. T., & Vederas, J. C. (2011). The activity of bacteriocins from Carnobacterium maltaromaticum UAL307 against gram-negative bacteria in combination with EDTA treatment. FEMS Microbiology Letters, 317, 152–159.
- Nissen, H., Alvseike, O., Bredholt, S., Holck, A., & Nesbakken, T. (2000). Comparison between the growth of Yersinia enterocolitica, Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella spp. in ground beef packed by three commercially used packaging techniques. International Journal of Food Microbiology, 59, 211–220.
- Ozturk, A., Yilmaz, N., & Gunes, G. (2010). Effect of different modified atmosphere packaging on microbial quality, oxidation and colour of a seasoned ground beef product (Meatball). Packaging Technology and Science, 23, 19–25.
- Prudêncio, C. V., Mantovani, H. C., Cecon, P. R., Prieto, M., & Vanetti, M. C. D. (2016). Temperature and pH influence the susceptibility of Salmonella Typhimurium to nisin combined with EDTA. Food Control, 61, 248–253.
- Prudêncio, C. V., Vanetti, M. C. D., & Prieto, M. (2015). Tolerance of Salmonella enterica serovar Typhimurium to nisin combined with EDTA is accompanied by changes in cellular composition. Food Research International, 69, 281–288.
- Rahman, M. S. (2007). Handbook of food preservation (2nd ed.). CRC Press, Taylor & Francis Group, Boca Raton, FL.
- Rogers, H. B., Brooks, J. C., Martin, J. N., Tittor, A., Miller, M. F., & Brashears, M. M. (2014). The impact of packaging system and temperature abuse on the shelf life characteristics of ground beef. Meat Science, 97, 1–10.
- Sancak, Y. C., Boynukara, B., & Agaoglu, S. (1993). The microbiological quality of ground meat marketed in Van. Yuzuncu Yıl Universitesi Veteriner Fakültesi Dergisi, 4, 73–86.
- Serdaroglu, M., & Degirmencioglu, O. (2004). Effects of fat level (5%, 10%, 20%) and corn flour (0%,2%,4%) on some properties of Turkish type meatballs (koefte). Meat Science, 68, 291–296.
- Shai, Y. (1999). Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochimica et Biophysica Acta, 1462, 55–70.
- SPSS. (2001). Statistical package for the social sciences. Chicago, IL: Author.
- Stevens, K. A., Sheldon, B. W., Klapes, N. A., & Klaenhammer, T. R. (1991). Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Applied and Environmental Microbiology, 57, 3613–3615.
- Temelli, S., Sen, M. K. C., & Anar, S. (2011). Assessment of microbiological changes in fresh uncooked Inegol meatballs stored under two different modified atmosphere packaging conditions. Ankara Universitesi Veteriner Fakultesi Dergisi, 58, 273–278.
- Troller, J. A., & Christian, J. H. B. (1978). Water activity and food (1st ed.). New York: Academic Press.
- Ulu, H. (2004). Effect of wheat flour whey protein concentrate and soya protein isolate on oxidative processes and textural properties of cooked meatballs. Food Chemistry, 87, 523–529.
- Wan Norhana, M. N., Poole, S. E., Deeth, H. C., & Dykes, G. A. (2012). Effects of nisin, EDTA and salts of organic acids on Listeria monocytogenes, Salmonella and native microflora on fresh vacuum packaged shrimps stored at 4°C. Food Microbiology, 31, 43–50.
- Yilmaz, I., Yetim, H., & Ockerman, H. W. (2002). The effect of different cooking procedures on microbiological and chemical quality characteristics of Tekirdag meatballs. Nahrung-Food, 46, 276–278.
- Zeuthen, P., & Bøgh-Sørensen, L. (2003). Food preservation techniques (1st ed.). Cambridge, UK: Woodhead Publishing.
