ABSTRACT
The levels of PCDDs, PCDFs and DL-PCBs were analyzed both in milk and adipose tissues sampled “in vivo” from lactating, drying off and heifer buffaloes from a Campania farm which had been impounded by the competent authority owing to the high dioxin levels found in bulk milk. The chemical determination was carried out by HRGC-HRMS using US EPA Method 1613b. The range of WHO-TEQ values for the PCDDs/PCDFs in adipose tissues was 1.79 to 68.64 pg g−1 fat and in milk was 8.33 to 13.95 pg g−1 fat. The contamination profile for dioxins and furans was given by 1,2,3,7,8-PeCDD; 2,3,4,7,8-PeCDF; 1,2,3,6,7,8-HxCDD and 2,3,7,8-TCDD. The levels of DL-PCBs in adipose tissue varied from 1.38 to 20.13 pg g−1 fat while ranged from 8.33 to 13.95 pg g−1 fat in milk. The pattern of DL-PCBs in both matrices was dominated by congeners PCB 126 and PCB 169.
Graphical Abstract
RESUMEN
En este estudio se analizaron los niveles de PCDD, PCDF y DL-PCB en leche y en tejido adiposo de búfalos lactantes muestreado in vivo y en periodo de secado, así como de vaquillas de una granja de Campania, confiscada por la autoridad competente debido a los altos niveles de dioxina encontrados en la leche a granel. A través de HRGC-HRMS, utilizando el método 1613b de la epa (Agencia para la Protección del Medioambiente) de Estados Unidos, se realizó la determinación química. El rango de valores establecido por el WHO-TEQ (equivalente tóxico de la Organización Mundial de la Salud) para PCDD y PCDF en tejidos adiposos se sitúa entre 1.79 y 68.64 pg g−1 grasa, ubicándose entre 8.33 y 13.95 pg g−1 grasa en la leche. El perfil de contaminación para dioxinas y furanos se estableció en 1,2,3,7,8-PeCDD; 2,3,4,7,8-PeCDF; 1,2,3,6,7,8-HxCDD y 2,3,7,8-TCDD. Los niveles de DL-PCB en los tejidos adiposos oscilaron entre 1.38 y 20.13 pg g−1 grasa, mientras que en la leche variaron entre 8.33 y 13.95 pg g−1 grasa. Los congéneres PCB 126 y PCB 169 dominaron el patrón de DL-PCB en las dos matrices.
Introduction
Dioxins belong to a group of 75 polychlorinated dibenzo-p-dioxin congeners (PCDDs) and 135 polychlorinated dibenzofuran congeners (PCDFs), among which 17 are of toxicological concern. Polychlorinated biphenyls (PCBs) are a group of 209 different congeners which can be divided into two groups according to their toxicological properties (non-ortho and mono-ortho PCBs). Only 12 congeners of this group exhibit toxicological properties similar to dioxins and are therefore often referred to as “dioxin-like PCBs” (DL-PCBs) (Schecter, Birnbaum, Ryan, & Constable, Citation2006).
PCDDs, PCDFs and DL-PCBs are recognized as Persistent Organic Pollutants (POPs) of mainly anthropogenic origin and are characterized by semi-volatility and resistance to degradation (EPA Citation2004). Because of the large number of compounds and depending on the chlorination level, relevant individual congeners are assigned with a toxic equivalency factor (TEF) that relate their toxicity to 2,3,7,8-tetrachlorodibenzo-p-dioxin, the most toxic compound (TEF 1). Each concentration of an individual congener in a mixture is multiplied with its TEF, and the resulting TCDD equivalents are added up and expressed as toxic equivalents endorsed by WHO (Van Den Berg et al., Citation2006).
The International Agency for Research on Cancer (IARC) has classified the DL-PCBs in Group 2A (probably carcinogenic to humans), based on evidence in humans and in animals (Lundqvist et al., Citation2006; Murray, Patterson, & Perdew, Citation2014; Puga, Citation2011; Yamada, Mishima, Fujiwara, Imura, & Sugahara, Citation2006). The 2,3,7,8-TCDD, 2,3,4,7,8-PeCDF and PCB 126 congeners have been classified as a Group 1 carcinogen, indicating that they are carcinogenic to humans (IARC Citation2012). Other PCDD/Fs are classified in Group 3 (not classifiable as to their carcinogenicity in humans) because of the absence of convincing data from experimental animals (IARC Citation1997).
The recognized main exposure to dioxins, furans and dioxin-like PCBs for the general population in Europe occurs via food, especially fish, meat and dairy products (EFSA Citation2012).
To minimize human exposure to dioxins and DL-PCBs, the European Commission established maximum levels for the sum of dioxins, furans, DL-PCBs and non-dioxin-like PCBs in food items, mainly of animal origin (Regulation EU No Citation1259/2011).
Adipose tissues are the pool of POPs in animals. In the context of food safety, many authors carried out studies on the exposure and elimination of these contaminants. Hoogenboom et al. (Citation2004) reported accumulation of residues of dioxins and polychlorinated biphenyls in the fat of the growing pigs and broilers fed with contaminated feed. Several other studies (Covaci et al. Citation2002; Guruge, Seike, Yamanaka, & Miyazaki, Citation2005; Simm et al., Citation2006; Spitaler, Iben, & Tausch, Citation2005; Thorpe, Kelly, Sartin, Harrison, & Rose, Citation2001) reported the occurrence of POPs in animal fat-based foods.
The presence of occasional and diffuse sources of emission, generally represented by accidental fires, burnings and open air disposal of ashes from combustion processes has been reported in some rural areas of Italy, as in the case of the buffalo milk dioxin crises in Campania Region (Borrello et al., Citation2008). The concern about dioxins contamination in Campania Region began in 2001, during the implementation of the National Residue Plan (PNR), when two sheep milk samples resulted non-compliant with contamination levels above the limits set by Commission Regulation 466/Citation2001. The well-known “buffalo milk crisis” re-emerged in 2008 and extensive monitoring plans were set up in order to keep the situation under control. Milk from several buffalo farms of a restricted area of the Caserta province continued to result contaminated for long times, notwithstanding the application of suitable restrictive measures, change of pasture and/or forage in particular, since PCDDs/PCDFs and DL-PCBs, owing to their resistance to degradation and high lipophilicity, may be accumulated into fat tissues and then transferred to milk during lactation period, as reported also by Fürst, Krause, Hein, Delschen, and Wilmers (Citation1993).
In literature there are no in vivo studies on adipose tissues contamination levels in buffaloes from a dairy farm naturally exposed to these chemicals. Marchand et al. (Citation2010) and Shen et al. (Citation2012) reported the possibility of predicting levels of PCDDs/PCDFs and DL-PCBs by an “in vivo” sampling of bovine edible tissues and pig fat tissues, respectively. Aim of the present study was to investigate the levels of the PCCDs, PCDFs and DL-PCBs and evaluate the contamination profile in subcutaneous adipose tissues sampled in vivo from heifers, drying off and lactating buffaloes of an impounded herd of the Campania region during the “dioxin crisis” and to research correlations, if any, with the concentrations in the milk of the lactating animals.
Materials and methods
Animal study
Nine dairy buffaloes (Bubalus bubalis), from a farm (Caserta province, Campania region, southern Italy) which had been impounded for a long time since the bulk milk was non-compliant to the PCDDs, PCDFs and DL-PCBs levels set by Regulation EU No Citation1259/2011, were selected for the study and divided into three groups. The first group included No 3 lactating buffaloes (L1, L2, L3), the second group No 3 drying off buffaloes (D1, D2, D3) and the last group No 3 heifers (H1, H2, H3). Animals of each group were selected for similar age and weight. The lactating buffaloes were randomly chosen among animals of the most abundant class (parity 4–5) and the same lactating period, specifically not beyond the six month of lactation. Moreover they were not pregnant and had been fed in the same way.
Subcutaneous fat biopsies were performed in the milking parlor by a veterinarian of the National Sanitary Service after local anesthesia, following the animal welfare rules. After the sanitization procedures, a 3 cm incision was realized near the ischial tuberosity of each animal and a subcutaneous fat sample (about 1 g) was taken. The incision was sutured and each buffalo remained under observation for a couple of hours. The whole sampling procedure was realized within not more than 15 minutes.
The total morning milking was collected from the three lactating buffaloes prior to the in vivo fat sampling. Adipose tissues and milk samples were immediately stored at −25°C and sent under the same temperature conditions to Eurofins GfA GmbH laboratory Hamburg (Germany) for the analysis.
GC–HRMS analyses
Analysis of the 17 PCDDs/PCDFs congeners and 12 DL-PCBs congeners were carried out by high-resolution gas chromatography coupled to high resolution mass spectrometry (HRGC-HRMS) in agreement with EPA method 1613-b (Citation1997). Samples were analyzed at Eurofins GfA GmbH laboratory Hamburg (Germany) according to accepted European directives and certified standard operating procedures (Decision Citation2002/657/EC and subsequent amendments and additions). The 6 non-dioxin-like PCBs (NDL-PCBs) set by Regulation EU No Citation1259/2011 were not considered to be consistent with previous survey programs performed in Campania region.
The fat percentage and the cumulative PCDDs, PCDFs and DL-PCBs in milk and adipose tissues from individual buffaloes are expressed on WHO2005 TEQ scale on a fat basis (pg g−1) () following the upper bound (ub) approach. Data are reported for the current World Health Organization Toxicity Equivalent factors (TEF-WHO2005) as required by the CitationRegulation (EU) No. 1259/2011. The total PCDDs/PCDFs concentration is expressed as the sum of TEQs of ten furans and seven dioxins congeners having chlorine atoms in 2,3,7,8-positions, the total DL-PCBs concentration is expressed as the sum of TEQs of twelve non-ortho and mono-ortho PCB congeners (PCB 77, 81, 105, 114, 118, 123, 126, 156, 157, 167, 169 and 189).
Table 1. PCDDs, PCDFs and DL-PCBs congeners distribution expressed in WHO-TEQ 2005 in milk and adipose tissue of lactating buffaloes and in adipose tissues of drying off animals and heifers. Values expressed on lipid base in UB mode.
Tabla 1. Distribución de congéneres PCDD, PCDF y DL-PCBs expresada en WHO-TEQ 2005 en la leche y los tejidos adiposos de búfalos lactantes, así como en los tejidos adiposos de animales en el periodo de secado de vaquillas. Los valores se presentan en la base lípida de modo UB.
Results and discussion
Levels of PCDDs/PCDFs and DL-PCBs
In adipose tissues the total levels of PCDDs and PCDFs varied from 1.79 to 68.64 pg TEQ g−1 fat in lactating animals, from 18.25 to 43.2 pg TEQ g−1 fat in drying off buffaloes and from 6.88 to 18.52 pg TEQ g−1 fat in heifers. DL-PCBs ranged from 1.38 to 20.13 pg TEQ g−1 fat, 9.63 to 17.59 pg TEQ g−1 fat and 5.11 to 11.6 pg TEQ g−1 fat in lactating animals, drying off and heifers, respectively.
In milk the sum of PCDDs and PCDFs congeners ranged from 8.33 to 13.95 pg TEQ g−1 fat and those of DL-PCBs from 3.89 to 5.95 pg TEQ g−1 fat. Therefore, on the whole, only in adipose tissue of the buffalo L2 the sums of PCDDs/PCDFs and that of PCDDs/PCDFs and DL-PCBs resulted lower than the limits of 2.5 and 4.0 pg/g fat set by EU Regulation Citation1259/2011 ().
Congener patterns of PCDDs/PCDFs and DL-PCBs
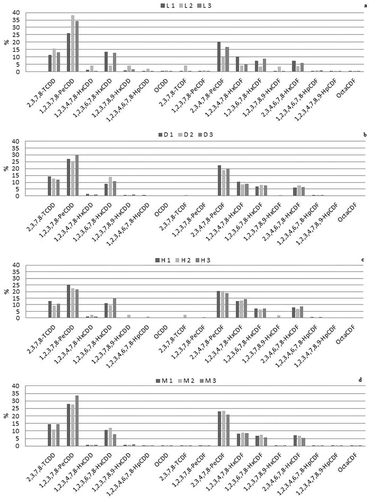
Among the PCDD and PCDF congeners, the highest contributions to total TEQs in all samples were given, in the order, by 1,2,3,7,8-PeCDD (TEF 1); 2,3,4,7,8-PeCDF (TEF 0.3); 2,3,7,8-TCDD (TEF 1) and 1,2,3,6,7,8-HxCDD (TEF 0.1) congeners ().
Figure 1. Relative contribution of PCDDs and PCDFs congeners in adipose tissues of lactating buffaloes L1 – L2 – L3 (a), drying off buffaloes D1, D2, D3 (b), heifers H1, H2, H3 (c) and milk (d). The values are expressed in percentage to total WHO2005-TEQ (PCDDs + PCDFs + DL-PCBs).
Figura 1. Aportación relativa de los congéneres PCDD y PCDF en los tejidos adiposo de búfalos lactantes L1 – L2 – L3 (a), en el periodo de secado D1, D2, D3 (b), en vaquillas H1, H2, H3 (c) y en la leche (d). Los valores se presentan como porcentajes de los totales de WHO2005-TEQ (PCDDs + PCDFs + DL-PCBs).
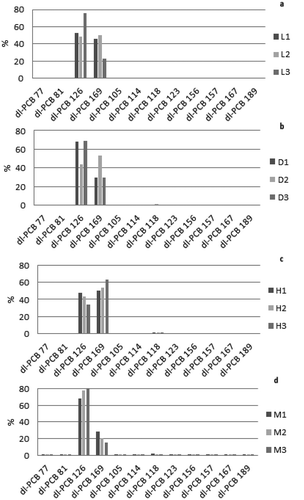
As far as the DL-PCBs are concerned, the pattern in adipose tissues, referred to total DL PCB-s (WHO2005TEQ) was dominated by PCB 126 and PCB 169 (49–76% and 23–50% in lactating animals; 44–69% and 30–53% in drying off subjects; 34–47% and 50–63% in heifers). Also in the milk samples the major contributing congener was PCB 126 (68–83%) followed by PCB 169 (15–29%) (). Cumulated contributions of the remaining DL-PCBs congeners were no higher than 3% in all examined samples and the occurrence of DL-PCBs to total PCDDs/PCDFs and DL-PCBs (WHO2005TEQ) ranged from 18 to 30% in lactating animals, 22 to 26% in drying off subjects and from 24 to 30% in heifers. In the three milk samples DL-PBCs contributed to the total TEQ for 21, 24 and 26%, respectively.
Figure 2. Non-ortho and mono-ortho-PCB congeners profile in adipose tissues of lactating buffaloes L1 – L2 – L3 (a), drying off buffaloes D1, D2, D3 (b), heifers H1, H2, H3 (c) and milk (d). The values are expressed in percentage to total WHO2005-TEQ (DL-PCBs).
Figura 2. Perfiles de los congéneres de PCB no ortos y mono ortos en los tejidos adiposos de búfalos lactantes L1 – L2 – L3 (a), en el periodo de secado D1, D2, D3 (b), en vaquillas H1, H2, H3 (c) y en la leche (d). Los valores se presentan como porcentajes de los totales de WHO2005-TEQ (DL-PCBs).
The results concerning the congener contribution are in agreement only for 2,3,4,7,8-PeCDF with those found by Esposito et al. (Citation2010) during an extraordinary plan of official controls carried out in 2008 in Campania region (Italy) with the aim to assess PCDDs, PCDFs and DL-PCBs levels in buffalo milk and to detect the contaminated farms, mainly located in Caserta province. The outcome of this plan showed that the main contribution to the WHO-TEQ was due to PCDFs congeners; in particular 2,3,4,7,8 PeCDF and 1,2,3,4,7,8 HxCDF were dominant. It is however to underline that the different data obtained during the present research are limited to one farm and therefore are indicative only of a restricted situation of the “dioxin buffaloes crisis” in the Campania region. During a more recent study regarding buffaloes tissues, milk and dairy products from three different herds located in Caserta province (De Filippis et al., Citation2013), the most represented congeners in the perirenal and retrobulbar fat of the three animals were 1,2,3,6,7,8-HxCDD for dioxins and 1,2,3,4,6,7,8-HpCDF; 1,2,3,4,7,8-HxCDF; 2,3,4,7,8-PeCDF for furans. In our study the highest contributions to total TEQs were given by 1,2,3,7,8-PeCDD (TEF1); 2,3,4,7,8-PeCDF (TEF 0.3); 2,3,7,8-TCDD (TEF 1) and 1,2,3,6,7,8-HxCDD (TEF 0.1).
Among the DL-PCBs, the toxicological contribution of PCB 126 is particularly relevant in relation to both the high toxicity value (TEF = 0.1) and concentration. This result might be due to PCB 126 high resistance to metabolic degradation. Corsolini, Focardi, Kannan, Tanabe, and Tatsukawa (Citation1995) relate this resistance to the enzymes involved in the detoxification pathways and report that in their studies DL-PCB 118, 156 and 126 were the main contributors to toxicity in humans and foxes, suggesting that the significant contribution of mono-ortho congeners in humans and non-ortho congeners in foxes might be due to differences in metabolic potential affecting the PCB toxicity pattern. Storelli et al. (Citation2012) found only PCB 118 in 51% of 80 sheep milk samples from an industrialized area of Sardinia (Italy). PCB 118, PCB 126 and PCB 169 were the most represented DL-PCB in buffaloes milk samples from Campania herds exposed to these chemicals (De Filippis et al., Citation2013).
The fat content in milk of the lactating buffaloes L1, L2 and L3 were in the order 6.2, 6.0 and 8.1% (). According to Zicarelli (Citation2004), only the animal L3 showed fat percentage close to the physiologic buffalo milk composition. This buffalo also showed the highest contaminants concentration in adipose tissue (88 pg/g fat sum of PCDDs/PCDFs/DL-PCBs TEQ), at a level more than seven times higher than the corresponding in milk (12.22 pg/g fat sum of PCDDs/PCDFs/DL-PCBs TEQ). Contamination levels in milk and adipose tissues of L1 resulted rather close, whereas PCDD/F and DL-PCB concentrations in milk resulted higher than those found in fat only for L2, that resulted lower than the limits of 2.5 (WHO-PCDD/F TEQ) and 4.0 pg/g fat (WHO-PCDDs/PCDFs/DL-PCBs TEQ) set by EU Regulation Citation1259/2011.
This result might be related to the unsatisfactory state of nutrition of the subject, also demonstrated by a degenerated fat, characterized by a macroscopic gelatinous consistence, possibly caused by trials of starvation carried out in some instance by the farmers in an effort to decontaminate the animals since milk represents the main route of excretion of the lipophilic contaminants in dairy animals (Schulz et al., Citation2005).
Analyses of edible matrices for the determination of PCDDs, PCDFs and DL-PCBs levels is not always realizable on living animals. For milk and dairy products sampling at the farm or in the dairy plants is practicable, but sampling in the live animal appears inappropriate because of reasons of animal welfare. However, the assessment of the contamination profile in fat, using a weakly invasive biopsy of subcutaneous adipose tissue in living buffaloes from impounded farms (fast recovery, no major side effects), could be used to evaluate dioxins, furans and DL-PCBs congeners pattern transferred in milk and mozzarella cheese intended to human consumption. This approach could be considered a useful tool after acute or chronic exposure events.
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials
Acknowledgments
Authors wish to thank Prof. Maria Luisa Cortesi who provided insight and expertise that greatly assisted this research.
Disclosure statement
No author had a financial or personal relationship with people or organizations that could inappropriately influence or bias the content of this paper.
References
- Borrello, S., Brambilla, G., Candela, L., Diletti, G., Gallo, P., Iacovella, N., Iovane, G., Limone, A., Migliorati, G., Pinto, O., Sarnelli, P., Serpe, L., Scortichini, G., di Domenico, A. (2008). Management of the 2008 “buffalo milk crisis” in the Campania Region under the perspective of consumer protection. Organohalogen Compound, 70, 891–893.
- Commission Regulation (EC) 466/2001 setting maximum levels for certain contaminants in foodstuffs. (2001). Official Journal of the European Communities, L 77, 1–13.
- Corsolini, S., Focardi, S., Kannan, K., Tanabe, S., & Tatsukawa, R. (1995). Isomer-specific analysis of polychlorinated biphenyls and 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin equivalents (TEQs) in red fox and human adipose tissue from central Italy. Archives of Environmental Contamination and Toxicology, 29, 61–68.
- Covaci, A., Ryan, J. J., & Schepens, P. (2002). Patterns of PCBs and PCDD/PCDFs in chicken and pork fat following a Belgian food contamination incident. Chemosphere, 47, 207–217.
- De Filippis, S. P., Chirollo, C., Brambilla, G., Anastasio, A., Sarnelli, P., De Felip, E., … Cortesi, M. L. (2013). Polychlorodibenzodioxin and −furan and dioxin-like polychlorobiphenyl distribution in tissues and dairy products of dairy Buffaloes. Journal of Agricultural and Food Chemistry, 61, 6552–6561.
- Environmental Protection Agency (EPA). 2004. Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. National Academy of Sciences (NAS) review draft. Retrieved February 11, 2005, from http://www.epa.gov/ncea/pdfs/dioxin/nas-review/
- Esposito, M., Serpe, F. P., Neugebauer, F., Cavallo, S., Gallo, P., Colarusso, G., … Serpe, L. (2010). Contamination levels and congener distribution of PCDDs, PCDFs and dioxin-like PCBs in buffalo’s milk from Caserta province (Italy). Chemosphere, 79, 341–348.
- European Commission. (2002). 2002/657/EC. Commission Decision implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Official Journal of the European Communities, L221, 8–36.
- European Food Safety Authority (EFSA). (2012). Update of the monitoring of levels of dioxins and PCBs in food and feed. EFSA Journal, 10(7), 2832–2914.
- Fürst, P., Krause, G. H. M., Hein, D., Delschen, T., & Wilmers, K. (1993). PCDD/PCDF in cow’s milk in relation to their levels in grass and soil. Chemosphere, 27, 1349–1357.
- Guruge, K. S., Seike, N., Yamanaka, N., & Miyazaki, S. (2005). Polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in domestic animal food stuff and their fat. Chemosphere, 58(7), 883–889.
- Hoogenboom, L. A. P., Kan, C. A., Bovee, T. F. H., van der We, G., Onstenk, C., & Traag, W. A. (2004). Residues of dioxins and PCBs in fat of growing pigs and broilers fed with contaminated feed. Chemosphere, 57, 35–42.
- International Agency for Research on Cancer (IARC). (1997). Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. In IARC monogr eval carcinog risks hum (Vol. 69). Geneva: IARC Press. Retrieved from http://monographs.iarc.fr/ENG/Monographs/vol69/volume69.pdf
- International Agency for Research on Cancer (IARC). (2012). A review of human carcinogens-Part F: Chemical agents and related occupations. In IARC monogr eval carcinog risks hum (Vol. 100F). Geneva: IARC Press. Retrieved from http://monographs.iarc.fr/ENG/Monographs/vol100F/index.php
- Lundqvist, C., Zuurbier, M., Leijs, M., Johansson, C., Ceccatelli, S., Saunders, M., … Koppe, J. G. (2006). The effects of PCBs and dioxins on child health. Acta Paediatrica, 95, 55–64.
- Marchand, P., Cariou, R., Vénisseau, A., Brosseaud, A., Antignac, J. P., & Le Bizec, B. (2010). Predicting PCDD/F and dioxin-like PCB contamination levels in bovine edible tissues from in vivo sampling. Chemosphere, 80, 634–640.
- Murray, I. A., Patterson, A. D., & Perdew, G. H. (2014). AH RECEPTOR LIGANDS IN CANCER: FRIEND AND FOE. Nature Reviews. Cancer, 14, 801–814.
- Puga, A. (2011). Perspectives on the potential involvement of the AH receptor–Dioxin axis in cardiovascular disease. Toxicological Sciences : an Official Journal of the Society of Toxicology, 120, 256–261.
- Regulation (EC) No 1259/2011 of 2 December 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for dioxins, dioxin-like PCBs and non dioxin-like PCBs in foodstuffs. (2011). Official Journal of the European Union, L 320, 18.
- Schecter, A., Birnbaum, L., Ryan, J. J., & Constable, J. D. (2006). Dioxins: An overview. Environmental Research, 101, 419–428.
- Schulz, A. J., Wiesmüller, T., Appuhn, H., Stehr, D., Severin, K., Landmann, D., & Kamphues, J. (2005). Dioxin concentration in milk and tissues of cows and sheep related to feed and soil contamination. Journal of Animal Physiology and Animal Nutrition, 89, 72–78.
- Shen, H., Henkelmann, B., Rambeck, W. A., Mayer, R., Wehr, U., & Schramm, K. W. (2012). The predictive power of the elimination of dioxin-like pollutants from pigs: An in vivo study. Environment International, 38, 73–78.
- Simm, M., Roots, O., Kotta, J., Lankov, A., Henkelmann, B., Shen, H., & Schramm, K. W. (2006). PCDD/Fs in sprat (Sprattus sprattus balticus) from the Gulf of Finland, the Baltic Sea. Chemosphere, 65(9), 1570–1575.
- Spitaler, M., Iben, C., & Tausch, H. (2005). Dioxin residues in the edible tissue of finishing pigs after dioxin feeding. Journal of Animal Physiology and Animal Nutrition, 89, 65–71.
- Storelli, M. M., Scarano, C., Spanu, C., De Santis, E. P., Busco, V. P., Storelli, A., & Marcotrigiano, G. O. (2012). Levels and congener profiles of polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs) in sheep milk from an industrialised area of Sardinia, Italy. Food and Chemical Toxicology : an International Journal Published for the British Industrial Biological Research Association, 50(5), 1413–1417.
- Thorpe, S., Kelly, M., Sartin, J., Harrison, N., & Rose, M. (2001). Concentration changes for 5 PCDD/F congeners after administration in beef cattle. Chemosphere, 43, 869–879.
- US EPA, US EPA method 1613, Revision B, Tetra- through octa-chlorinated dioxins and furans by isotope dilution HRGC/HRMS. (1997, September 15), 40 CFR 136 (FR 48405). Washington, DC.
- Van Den Berg, M., Birnbaum, L., Denison, M., De Vito, M., Farland, W., Feeley, M., … Peterson, R. E. (2006). The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicological Sciences : an Official Journal of the Society of Toxicology, 93, 223–241.
- Yamada, T., Mishima, K., Fujiwara, K., Imura, H., & Sugahara, T. (2006). Cleft lip and palate in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin: A morphological in vivo study. Congenital Anomalies, 46, 21–25.
- Zicarelli, L. (2004). Buffalo milk: Its properties, dairy yield and mozzarella production. Veterinary Research Communications, 28, 127–135.
