ABSTRACT
Chitosan-microparticles (CM)-coating (CMC) effect on quality and shelf-life of vacuum-packed sea bream (Sparus aurata) fillets was investigated at 0.2% and0.5% (w/w) doses comparatively to untreated control, during refrigerated storage (24 days). Analysis concerned total mesophilic bacteria counts (TMC), total psychrophilic bacteria counts (TPC), proximate composition, pH, total volatile basic nitrogen (TVB-N), trimethylamine (TMA), thiobarbyturic acid (TBA), and fatty acids (FA). Two-way ANOVA and multiple comparison were applied, factors being storage time and treatment. Bacterial counts exceeded the limit in control but remained below in CMC lots. All of TVB-N, TMA and TBA increased faster and reached higher values in control compared to CMC lots, with dose-dependent effect. CMC preserved quality in terms of proximate composition and FA. The 0.5%-CM dose prolonged the shelf-life up to 12 days, corresponding to a two folds extension. CMC showed antimicrobial and antioxidant properties and should be recommended for preserving the quality of fish fillets.
Graphical abstract
RESUMEN
El presente estudio investigó el efecto que provoca el recubrimiento de micropartículas de quitosano (CM) (CMC), a dosis de 0.2% y 0.5% (p/p), en la calidad y la vida útil de filetes de dorada (Sparus aurata) envasados al vacío, en comparación con el control no tratado, durante 24 días de almacenamiento refrigerado. El análisis se centró en el recuento total de bacterias mesófilas (TMC) y psicofílicas (TPC), la composición proximal, el pH, el nitrógeno básico volátil total (TVB-N), la trimetilamina (TMA), el ácido tiobarbitúrico (TBA) y los ácidos grasos (FA). Para efectuarlo se aplicó ANOVA de dos vías y de comparación múltiple, siendo los factores el tiempo de almacenamiento y el tratamiento. Los recuentos bacterianos excedieron el límite permitido en el control, pero se mantuvieron por debajo del límite en los lotes de CMC. El TVB-N, la TMA y el TBA aumentaron más rápido y alcanzaron valores más altos en el control que en los lotes tratados con CMC, mostrando un efecto dependiente de la dosis. El CMC conservó su calidad en términos de composición proximal y FA. La dosis de 0.5% de CM prolongó la vida útil hasta 12 días, lo que significa una extensión del doble. El CMC mostró propiedades antimicrobianas y antioxidantes, por lo que debería recomendarse para preservar la calidad de los filetes de pescado.
1. Introduction
There is worldwide an increasing demand for fish food. This is due to several factors including an awareness of the benefits of consuming seafood, population growth, rising incomes and urbanization (Surathkal, Dey, Engle, Chidmi, & Singh, Citation2017). To meet such growing demand, a large part of aquatic products will be traded and will come from aquaculture (FAO, Citation2018; Natale, Borrello, & Motova, Citation2015). The sea bream (Sparus aurata) is one of the main finfish species farmed in the Mediterranean countries, including Tunisia where aquaculture is well established (Abdou, Aubin, Romdhane, Loc’h, & Lasram, Citation2017). Within these regions, fishes are usually marketed in a whole fresh form as there is still a tradition of fresh seafood consumption (FAO, Citation2018). However, with consumers changing lifestyle; convenient foods such as ready to cook meals are gaining in popularity (Ximena, Rivera, & Azapagic, Citation2019). Accordingly, fish farmers are now opting to offer fresh fish fillets (FAO, Citation2018).
However, fresh-processed fish are particularly perishable and the loss of quality is inevitable because of fish flesh biochemical attributes and the microbial activities that are impacted mostly by the temperature and the manipulation (Smichi et al., Citation2017). Particular interest has been focused on the potential application of natural anti-microbial and anti-oxidant additives, such as essential oils, limonene, allyl isothiocyanate and light salting on the shelf-life of gilthead sea bream (Attouchi & Sadok, Citation2012; Giarratana et al., Citation2016; Goulas & Kontominas, Citation2007; Muscolino et al., Citation2016). Among natural additives, chitosan is being widely used because of its efficiency in improving the product shelf-life (Izci, Ekici, & Günlü, Citation2017; Küçükgülmez, Kadak, & Gӧkçin, Citation2013a; Li, Jiang, Li, & Hu, Citation2016). It is also appreciated for its safety and easy use (Qiu, Chen, Liu, & Yang, Citation2014; Yu et al., Citation2017). It is considered non-toxic, biocompatible, biodegradable and low-cost (Ma, Garrido-Maestu, & Jeong, Citation2017). In most cases, chitosan is used as films (Alves et al., Citation2018; Günlü & Koyun, Citation2013). Few studies are also reported concerning the use of micro/nano-particles forms (Ramezani, Zarei, & Raminnejad, Citation2015; Tapilatu, Nugraheni, Latumahina, Limmond, & Budhijanto, Citation2016; Wu et al., Citation2017). Chitosan microparticles are usually obtained using poly-anionic crosslinking agent by ionic crosslinking polyelectrolyte, coacervation or complexation methods (Yan, Song, Wang, Zhao, & Chen, Citation2018). More recently, the micro and nano-particles forms (micro and nano-chitosan), obtained by spray-drying methods, have been widely applied in industrial processes. Such an application was shown to increase the effectiveness of chitosan antibacterial activity and is particularly appreciated in the food, pharmaceutical and cosmetic industries (Ma et al., Citation2017).
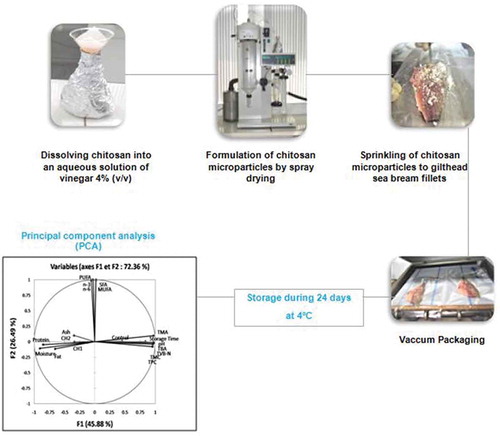
In this study, we opted to apply chitosan-microparticles-coating (CMC) using spray drying technique for fish fillets preservation. To our knowledge, there are no related published studies. The objective of this study was to evaluate the effect of CMC at two different percentages (0.2% and 0.5% w/w) on the quality of vacuum-packed fillets of sea bream from offshore aquaculture during 24 days of refrigerated storage.
2. Materials and methods
2.1. Collection of sea bream (Sparus aurata)
Fresh farmed gilthead sea bream (Sparus aurata; 30 fishes with average weight and length of 300.2 ± 9.5 g and 25.9 ± 0.2 cm, respectively) were obtained from an offshore cages farm located in Monastir (East of Tunisia). They were delivered to the laboratory in insulated boxes containing enough flake ice (ratio of 1:1 (w/w)) within 3 h post mortem. Upon arrival, fishes were immediately measured, gutted, washed and filleted keeping the skin.
2.2. Preparation characterisation of chitosan
Chitin from shrimp shells was purchased from Sigma-Aldrich (Steinheim, Germany) and submitted to deacetylation, according to the method of Galed, Miralles, Paños, Santiago, and Heras (Citation2005) procedure with slight modifications, consisting in an alkali treatment of the chitin using sodium hydroxide (NaOH) (SRL, Maharashtra, India) 1.25M (1:20 w/v) at 100°C for 4 h. The reactants were filtered only once using an organza, washed with distilled water to neutral pH and dried for 3 days at ambient temperature.
Deacetylation degree of the chitosan was determined as 84.66% using a Cary 630 FTIR spectrophotometer (Agilent Technologies, Santa Clara, CA, USA), while the molecular weight was calculated as 2.17 kDa from the intrinsic viscosity values using the Mark-Houwink equation with the constants α = 0.93 and k = 1.81 × 10−3 cm3/g (Roberts, Citation1992). The chitosan solution (0.5% w/v) was prepared using 0.1 g of chitosan dissolved in 20 mL of commercial white vinegar (DesseauxPh., Bardo, Tunis) previously diluted at a ratio of 4% v/v (equivalent of 1M acetic acid solution). Dissolution was obtained by continuous stirring using a magnetic stirrer during 24 h at ambient temperature to achieve complete dispersion. Data of intrinsic viscosity 31.67 cp were measured in an Ubbelohde viscometer Viscosystem® AVS 470 (SI Analytics, Weilheim, Germany) at 25°C.
2.3. Formulation and size of chitosan microparticles
The chitosan microparticles were prepared from the chitosan solution using a Mini Spray Dryer B-290 equipped with a peristaltic pump (BüchiLabortechnik AG, Flawil, Switzerland). Compressed air (4 bar) was used to disperse the chitosan solution into fine droplets through a 0.7 mm nozzle. The droplets were dried and the solid particles were collected in the cyclone at aspiration rate of 100%. The rate of the peristaltic pump was at the levels of 25%. The inlet temperature was kept at 120°C in order to preserve the polymer stability and to limit the excessive material loss during drying. The outlet temperature was set at 23°C. Chitosan microparticles were placed in glass tubes and stored at 4°C.
2.4. Optical microscopy measurement
An optical microscope (Euromex, Arnhem, Holland) equipped with software for processing images and measuring size (ImageFocus 4.0, Arnhem, Holland) was used for chitosan particles measurement. The samples were observed with immersion oil at 100x magnification.
2.5. Treatment of fish fillets and storage conditions
The 60 skinned fillets were maintained over an ice flakes bed until treatment and packing. Chitosan microparticles were spread uniformly using a fine strainer directly onto the muscle side of gilthead sea bream fillets at two different percentage doses (0.2% and 0.5% w/w) corresponding to the experimental treatments CH1 and CH2, respectively. These treatments were studied comparatively to a control (C) in which fish fillets did not receive any chitosan addition. For each sampling date and each treatment or control group, a lot of 4 fillets were prepared. All fillets of all the lots were individually vacuum packed using a Multivac C200 packaging machine (Multivac, Wolfertschwenden, Germany) and then stored in flake ice (ratio of 1:1 (w/w)) into polystyrene boxes provided with holes for drainage and stored in a refrigerator (2–4°C) for up to 24 days. Sampling for both microbiological and biochemical analyses occurred on days 0, 6, 12, 18 and 24 during the storage period, and the analysis was performed in triplicates for each fillet.
2.6. Microbiological analyses
At sampling, a white muscle portion (10 g) from each fillet was transferred aseptically to a sterile blender (Fasyline, Rimini, Italy) containing 90 mL of sterile water with 0.1% peptone (Biokar, Zac de Ther, France) and blended for 2 min at high speed. Volumes of 0.1 mL of decimal dilutions of these homogenates were inoculated on a culture of Plate Count Agar (Biokar Diagnostics, Beauvais, France). The plates were incubated at 30°C for 48 h for total mesophilic bacteria counts (TMC) or incubated at 4°C for 10 days for total psychrophilic bacteria counts (TPC) (Harrigan & McCance, Citation1976).
2.7. Proximate and biochemical analyses
Sampling for proximate and biochemical analysis was performed immediately after sampling for microbiological analysis. Each fillet was individually chopped without the skin in a blender (Russell Hobbs, Mainland, UK) and divided into 5 g aliquots preserved in sealed bags and immediately frozen at −80°C for subsequent analysis.
2.7.1. Moisture and crude ash
Moisture was determined by drying 1 g fish flesh in an oven (Memmert, Schwabach, Germany) at 105°C for 24 h according to the AOAC method (Citation1995). Crude ash content was determined by incineration for 6 h in a muffle furnace oven (Protherm, Ankara, Turkey) at 550°C according to the AOAC method (Citation1995).
2.7.2. Crude protein
Crude protein was determined on homogenized fish flesh according to the method of Lowry modified by Hartree (Citation1972) in which water-insoluble fractions obtained during cell fractionation dissolve readily in reagents at 50°C and no special procedure for insoluble material is necessary. This method was adapted to microtitration in our accredited laboratory. Bovine serum albumin (Sigma-Aldrich, Steinheim, Germany) was used for standard solutions. For instance, a portion of (0.45 g) of flesh was thoroughly homogenized in 9 mL distilled water. Two dilutions were made with a final dilution factor of 200. Small fractions (250 μL) of the standard or diluted sample solutions were taken for subsequent protein analysis in microtubes by adding successively the corresponding stoichiometric reactive solutions and measuring absorbencies at 650 nm using 96 wells microplates containing 500 µL of final solutions.
2.7.3. Crude fat
Crude fat extraction from 1 g chopped fillet was performed according to the method described by Folch, Lees, and Stanley (Citation1957) using a chloroform: methanol (2:1 v/v) (Carlo Erba, Val-de-Reuil, France) extraction solution containing 0.01% butylatedhydroxytoluene (BHT) (Sigma-Aldrich, Barcelona, Spain) as antioxidant. After centrifugation (4000 g, 10 min, 4°C) the lower phase was removed with a Pasteur pipette and the solvent was evaporated to dryness. Fats were established gravimetrically.
2.7.4. pH measurement
Measurement of pH was performed using a digital pH meter (Eutech pH-2700, AyerRajah Crescent, Singapore) which was calibrated using buffer solutions pH (4.01 ± 0.01; 7.00 ± 0.01; 10.01 ± 0.01/25°C) (XS Green Line, Pisa, Italy). This measurement has been done on fillet samples homogenized in distilled water (1:2 w/v) according to AOAC method (Citation1995).
2.7.5. Determination of total volatile basic nitrogen (TVB-N) and trimethylamine (TMA)
A portion (1 g) of chopped fillet was homogenized (DI-25; IKA, Staufen, Germany) on ice in 2 mL ultrapure water for 1 min. Perchloric acid (0.250 mL – 6% solution) (Sigma-Aldrich, Steinheim, Germany) was added and the solution was homogenized for further 2 min. Homogenates were centrifuged at 12 000 g for 15 min, and the supernatants were used for the determination of total volatile basic nitrogen (TVB-N) and trimethylamine (TMA) by flow injection analysis according to the methods of Ruiz-Capillas and Horner (Citation1999) and Sadok, Uglow, and Haswell (Citation1996), respectively.
2.7.6. Determination of thiobarbituric acid value
The thiobarbyturic acid (TBA) values were determined spectrophotometrically according to the procedure described by Hamre, Næss, Espe, Holm, and Lie (Citation2001). A portion of chopped fillet (0.5 g) was homogenized in 4 ml of chloroform:methanol 2:1 (v/v) (Carlo Erba, Val-de-Reuil, France) containing 0.005% butylated hydroxytoluene (BHT) (Sigma-Aldrich, Barcelona, Spain). Thereafter, 2 ml of a saturated EDTA (Suvchem, Mumbai, India) solution was added and the tubes were centrifuged for 20 min at 1500 g. A 2 ml aliquot of the methanol:water layer was transferred to clean screw-capped glass tubes, mixed with 2 ml TBA-reagent (1% thiobarbituric acid in 5% trichloroacetic acid) (Suvchem, Mumbai, India) and heated for 30 min at 100°C. After cooling, the absorption was measured at 532 nm with a Smart Spec-plus spectrophotometer (Bio-Rad, Hercules, CA). The results were expressed as mg of malondialdehyde (MDA)/kg of fresh weight quantified in reference to standards solutions of 1,1,3,3-tetramethoxypropan (TMP) (Sigma, St. Louis, Mo., USA).
2.7.7. Determination of fatty acids
Fatty acid methyl esters (FAMEs) were determined only for the sampling days 0, 12 and 24. They were obtained using the method described by Christie (Citation1993). For instance, the crude fat extract was esterified in 2% sulfuric acid in absolute methanol (Carlo Erba, Val-de-Reuil, France) and incubated overnight at 50°C. Then, water (1 mL) was added and the esters were extracted with hexane (2 mL) (Sigma-Aldrich, Steinheim, Germany) to separate the layers. The resulting methyl esters were analysed using an Agilent Gas chromatograph system 6890N (Agilent Technologies, USA), equipped with a flame ionization detector (FID), a splitless injector and a polar INNOWAX fused silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness). The temperature of the injector and the detector were 220°C and 275°C, respectively. Helium was used as a carrier gas with a flow rate of 1.5 ml.min−1. Peaks were identified by comparison of their retention times with those of known mixture of standard fatty acids (PUFA N°3, Menhaden Oil, SUPELCO Sigma-Aldrich, Laramie, Wyoming USA). The results are expressed as percentages of the total fatty acids' methyl esters.
2.8. Statistical analysis
The results are presented as mean ± standard deviation (SD). STATISTICA software was employed for performing the statistical analysis. To get homogeneity of the variances, the data were log-transformed for microbiological and ABVT data while the percentage values of proximal composition were arcsin(x1/2) transformed. Before performing statistics, the homogeneity of the transformed data was systematically verified using Levene’s test. The data were then submitted to two-way analysis of variance (ANOVA II), the analyzed factors being storage time and experimental treatment along with their interaction effect. The LSD multiple comparison procedure was adopted for post-hoc comparisons when differences were found. For all the tests, significant level was set at P < 0.05. For crude ash and TMA, the nonparametric Kruskal-Wallis test was applied as homogeneity of variances could not be obtained. The compiled data were finally submitted to a principal component analysis (PCA) after scaling variables. Because units differed between study variables, scaling was performed by dividing with means instead of standard deviations as this method makes results independent of the choice of units (Forkman, Josse, & Piepho, Citation2019). The software XL-STAT was used for the PCA.
3. Results and discussion
3.1. Size of chitosan microparticles
In this study, the sizes of chitosan microparticles ranged from 2.350 µm to 3.798 µm, similar to chitosan size microparticles found in other study using the same diameter of nozzle (0.7 mm). Microparticle sizes ranging from 2.585 μm to 3.646 μm are reported in the study of Katsarov, Pilicheva, Manev, Lukova, and Kassarova (Citation2017). The size of chitosan microparticles generated by spray drying is affected by several factors such as the concentration and the molecular weight of chitosan, the nozzle size, the feeding pump rate, inlet temperature, and compressed airflow rate (He, Davis, & Illum, Citation1999).
3.2. Microbial analysis
Changes in TMC and TPC during storage period are shown in . The initial TMC and TPC values in the fish fillets were 3.31 ± 0.06 and 2.88 ± 0.04 log CFU/g, respectively; indicating the good quality of the fresh fish. For fresh-farmed sea bream, values between 2 and 4 log CFU/g are reported (Attouchi & Sadok, Citation2012; Garrido, Hernández, Espinosa, & López, Citation2014).
Figure 1. Bacterial growth evolution in total mesophilic TMC (a) and psychrotrophic TPC (b) counts of control (C) and chitosan-microparticles-coating (CMC) treated fillets (CH1 and CH2) during refrigerated storage. Values with different superscript letters (a-j) are significantly different (P < 0.05) according to ANOVA II and LSD post-hoc analysis. The dotted lines indicated the limit of acceptability.
Figura 1. Evolución del crecimiento bacteriano en recuentos de TMC mesofílica total (a) y TPC psicrotrófica (b) del control (C) y los filetes tratados con el revestimiento de micropartículas de quitosano (CMC) (CH1 y CH2) durante el almacenamiento refrigerado. Los valores con diferentes letras superíndice (a-j) son significativamente diferentes (P < 0.05) según ANOVA II y el análisis LSD post hoc. Las líneas punteadas indican el límite de aceptabilidad
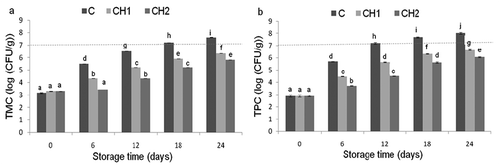
Values of TMC and TPC increased gradually within each lot during the storage period. For both TMC and TPC, the increase was significantly higher in control fillets compared to CMC treated fillets. The value of 7 log CFU/g, which is considered as the maximum permissible limit for marine fish (ICMSF, Citation1986), was reached on the 12th and 18th day for TPC and TMC, respectively, in the control group. These results are in agreement with the reported shelf-life for sea bream fillets, which commonly ranges between 10 and 15 days (Attouchi & Sadok, Citation2012; Chouliara, Savvaidis, Riganakos, & Kontominas, Citation2005; Garrido et al., Citation2014). In CMC treated fillets, the TMC and TPC remained below this limit during the entire storage period. Both CMC doses were able to inhibit bacterial growth and the highest dose (0.5%) was more effective as the bacterial growth was less important in that experimental group. It appears clear that CMC of fish fillets is effective for delaying bacterial growth and thus could be recommended for extending the shelf-life of sea bream fillets.
The chitosan microparticles antimicrobial activity is also reported in other studies. Ramezani et al. (Citation2015) and Tapilatu et al. (Citation2016) showed the effectiveness of nano-chitosan in extending the shelf-life of fresh silver carp up to 12 days and yellowfin tuna up to 24 days, respectively. Such an effect is related to the destructing action of nano-chitosan on the bacterial cell walls making them prone to lysis with lethal consequences (Tapilatu et al., Citation2016). The antimicrobial action of chitosan can be executed by the interactions between the positively charged chitosan and negatively charged microbial cell membranes and thus cause the leakage of intracellular electrolytes and proteinaceous constituents (Ramezani et al., Citation2015). Other mechanisms mentioned in the literature are the interaction of diffused hydrolysis products with microbial DNA which leads to the inhibition of the mRNA and the protein synthesis (Devlieghere, Vermeulen, & Debevere, Citation2004).
3.3. Proximate composition
The proximate composition of gilthead sea bream fillets for the different experimental groups and sampling dates during the 24 days of refrigerated storage are given in .
Table 1. Changes in proximate composition of control (C) and coated fillets (CH1 and CH2) during the storage*.
Tabla 1. Cambios en la composición proximal del control (C) y los filetes recubiertos (CH1 y CH2) durante el almacenamiento*
The ash content did not show any significant difference between control and treated fillets throughout storage. For the other proximal indicators, significant differences are detected between control and treated fillets but not between the fillets of the two assayed doses. According to the ANOVA II, these variations appeared to be related to the treatments, the storage time along with their interaction factors for moisture and crude protein contents. The variations appeared exclusively related to the storage time factor.
The initial mean moisture content measured of fresh fillets was 70.0 ± 0.1% which is in agreement with the value (71.2 ± 1.5%) reported for farmed S. aurata (Marco et al., Citation2017). The control fillets showed a significant decrease after 12 days (69.1 ± 0.1%) and reached (67.2 ± 0.1%) at the end of the storage. For treated fillets the decrease was observed later, from 18 days; with values of 69.02 ± 0.3% and 69.2 ± 0.2% for CH1 and CH2 groups, respectively.
Concerning crude fat changes, the level found on the first day in fresh fillets (9.27 ± 0.12%) was within the values reported for farmed sea bream (Grigorakis, Citation2007; Marco et al., Citation2017). Throughout storage, crude fat content decreased only for the control group, reaching 7.68 ± 0.24% at the end of the storage period while no significant change was detected throughout the storage for coated fillets CH1 and CH2.
The crude protein content in fresh fillets (19.60 ± 0.13%) was also in the range of values (18.0% to 22.4%) reported for the species (Attouchi & Sadok, Citation2012; Marco et al., Citation2017). Up to the 12th day of storage, crude protein content remained unchanged in all treatments. Afterwards, from days 18, a significant decrease was observed in all groups with a more pronounced variation in the control comparatively to CMC treated fillets.
Such a decrease in proximate composition is commonly observed during the storage of seafood products and it is due to the bacterial and enzymatic degradation (Chaijan, Benjakul, Visessanguan, & Faustman, Citation2005). The different changes observed between the two doses of CMC treated fillets and the control fillets clearly indicate that chitosan microparticles treatment showed a preserving effect on proximate composition during storage. Such an effect could be due to the inhibition of enzymatic and bacterial alteration of gilthead sea bream by micro-chitosan. Sathivel (Citation2005) found that chitosan coating was effective in reducing moisture loss and in prolonging the storage life of pink salmon fillets. In the same way, the immersion in nano-chitosan solution of yellowfin tuna seemed to inhibit bacterial activity, to the point that protein degradation was also delayed (Tapilatu et al., Citation2016). It appears clear that CMC of fish fillets is effective for protecting quality characteristics in terms of proximate composition.
3.4. Changes in pH
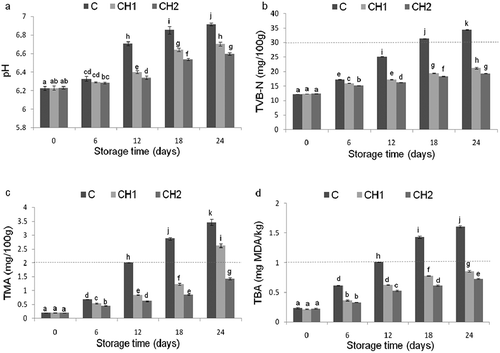
Changes in pH values of sea bream fillets during refrigerated storage are illustrated in ). At the first day the pH value was 6.22 ± 0.01, which is in agreement with the value of 6.12 mentioned by Goulas and Kontominas (Citation2007) for fresh-farmed sea bream. A slow increase was recorded up to 6th day in all groups. Subsequently, the increased became more pronounced in the control group reaching the maximal value of 6.91 ± 0.01 at the end of storage. These results confirmed that the control fillets were degrading at a faster rate during the storage period. The higher pH values may be related to the formation of alkaline autolysis compounds, such as ammonia and trimethylamine mainly derived from microbial action in the muscle during the post-mortem period (Qiu et al., Citation2014). In CMC treated fillets, pH values increased more slowly and the value reached in CH2 at the end of storage was the lowest among the experimental groups (6.59 ± 0.02). Thus, the CMC maintained the pH lower for a longer time compared to the control fillets with the higher ratio of CMC being more effective. Similar results were established for other fish species coated with chitosan such as salmon, sea bream and silver carp fillets as reported by Alves et al. (Citation2018), Küçükgülmez et al. (Citation2013a) and Ramezani et al. (Citation2015). The lower pH values in coated fillets were related to the antimicrobial action of chitosan that causes protein degradation and the production of basic compounds responsible for the pH increase. Therefore, CMC was effective in inhibiting microbial and endogenous proteases' activities during refrigerated storage contributing to the extension of the shelf-life in treated fillets.
Figure 2. Changes in quality indicators: pH (a), TVB-N (b), TMA (c) and TBA (d) of control (C) and coated fillets (CH1 and CH2) with chitosan microparticles during refrigerated storage. Values with different superscript letters (a-k) are significantly different (P < 0.05) according to ANOVA II and LSD post-hoc analysis. For TMA was analyzed by Kruskal-Wallis test. The dotted lines indicated the limit of acceptability.
Figura 2. Cambios en los indicadores de calidad: pH (a), TVB-N (b), TMA (c) y TBA (d) del control (C) y los filetes recubiertos (CH1 y CH2) con micropartículas de quitosano durante el almacenamiento refrigerado. Los valores con diferentes letras superíndice (a-k) son significativamente diferentes (P < 0.05) según ANOVA II y el análisis LSD post hoc. Para El TMA se analizó mediante la prueba de Kruskal–Wallis. Las líneas punteadas indican el límite de aceptabilidad
3.5. Changes in total volatile basic nitrogen
The changes in TVB-N contents in all fish fillet-lots during storage are presented in ). Initially, the TVB-N contents of all fillets were 12.20 ± 0.03 mg/100g, indicating their freshness. Küçükgülmez et al. (Citation2013a) reported similar results (12.66 ± 1.16 mg/100g) for fresh gilthead sea bream. The TVB-N values increased gradually during the storage period. For control, the increase was faster and reached higher values compared to CMC groups. The limit of acceptability (30–35 mg TVB-N/100g) established for most fresh marine fish species (CEC, Citation1995) was exceeded on 18th day of storage for control group while in CMC groups the values remained far below in both CH1 and CH2 coated fillets with at last day of storage values of 21.15 ± 0.27 and 19.27 ± 0.07 mg/100g, respectively. These differences may be attributed to the antimicrobial activity of chitosan microparticles, contributing to the reduction of bacterial population and decreasing the capacity of microorganisms for oxidative deamination of non-protein nitrogen compounds. Similar results were reported for chitosan treated fillets of salmon, sea bream and sea bass (Alves et al., Citation2018; Izci et al., Citation2017; Li et al., Citation2016). In a study carried out on silver carp, it is reported that nanochitosan (2%) had a stronger ability to inhibit the TVB-N content than chitosan. In this study, the TVB-N value in nanochitosan group was 30.8 ± 1.5 mg/100g while in chitosan group it reached 44.4 ± 3.3 mg/100g after 12 days of cold storage (Ramezani et al., Citation2015).
3.6. Changes in trimethylamine
As shown in ), a low initial TMA value (0.19 ± 0.01mg/100g) was detected, indicating a good freshness. This type of level is commonly observed in fresh-farmed sea breams as reported by Attouchi and Sadok (Citation2012) for example. During the storage period, the TMA contents increased significantly in all groups and similarly to TVB-N, the increase was faster and reached higher values in the control group compared to CMC groups. In control the values reached the acceptable limit of 2 mg/100g on the 12th days of storage, reflecting the rapid deterioration of quality in uncoated fillets. A wide range of TMA values have been recommended as acceptability limits for fish species but no official limit has been fixed. However, for sea bream, a limit of 2–3 mg/100 g for sea bream is considered a threshold (Attouchi & Sadok, Citation2012). On the basis of this proposed limit value, on the last day of storage, the fillets of CH1 treatment should be rejected while those of treatment CH2 remained acceptable as the last value was 1.43 ± 0.04 mg/100g. This result was consistent with the other quality indicators (TMC and TPC, pH and TVB-N) and apparently the CMC’s inhibitory effect on bacterial development limited the accumulation of TMA. Indeed, it is clearly demonstrated that chitosan can reduce the TMA levels, maintaining quality for longer by inhibiting bacterial growth fish fillets of different species such as sea bass and grass carp (Günlü & Koyun, Citation2013; Yu et al., Citation2017).
3.7. Changes in thiobarbituric acid reactive substances
The variations of TBA values of all groups during refrigerated storage are compiled in ). Low initial TBA content was observed in fresh fillet sea bream (0.22 ± 0.01 mg MDA/kg) emphasizing the good quality and freshness of the starting product. Almost similar value (0.18 ± 0.04 mg MDA/kg) was mentioned by Goulas and Kontominas (Citation2007) for fresh-farmed sea bream. Similarly to both profiles of TVB-N and TMA, contents of TBA increased significantly in all groups and the increase was faster and reached higher values in the control group compared to CMC groups. According to Connell (Citation1995), TBA levels of 1–2 mg MDA/kg of fish flesh are generally regarded as the limit beyond which fish will normally develop off-flavours and off-odours. The limit TBA content was exceeded on the 12th day in the control group, while it was never reached in coated fillets' groups throughout the whole storage period with values of 0.85 ± 0.01 and 0.72 ± 0.01 mg MDA/kg for CH1 and CH2, respectively. These results clearly indicate that CMC has antioxidant properties. Similar observation is reported for chitosan coating in sea bream fillets (Küçükgülmez et al., Citation2013a) or nano-chitosan coating in silver carp fillets (Ramezani et al., Citation2015) during refrigerated storage. According to Qiu et al. (Citation2014), the chitosan may reduce oxidation by chelating ferrous ions present in fish proteins, thus eliminating their pro-oxidant activity.
3.8. Changes in fatty acids
Changes in fatty acid composition of all groups during cold storage of sea bream fillets are given in . As expected, fatty acids' profiles of fresh fillets did not differ between the three experimental treatments; thus the data are presented as pooled means. The polyunsaturated fatty acids (PUFA; 52.74 ± 0.04%) represent the bulk of the total fatty acids, followed by the monounsaturated fatty acids (MUFA; 27.78 ± 0.07%) and the saturated fatty acids (SFA; 16.56 ± 0.02%). This distribution of fatty acids is in agreement with several studies concerning farmed gilthead sea bream (Sparus aurata) (Grigorakis, Citation2007; Senso, Suarez, Ruiz-Cara, & Garcia-Gallego, Citation2007).
Table 2. Changes in fatty acid composition of control (C) and coated fillets (CH1 and CH2) during the storage (%)*.
Tabla 2. Cambios en la composición de ácidos grasos del control (C) y los filetes recubiertos (CH1 y CH2) durante el almacenamiento (%)*
In the present study, within SFA, palmitic acid (C16:0; 11.65 ± 0.07%) was found as the predominant saturated fatty acid, while in MUFA the major FA was oleic acid (C18:1n-9; 25.07 ± 0.07%). Regarding PUFA, it was found that linoleic acid – LA (C18:2n-6; 43.13 ± 0.26%) was the most important among all the n-6 fatty acids, while the linolenic acid (C18:3n-3; 4.12 ± 0.05%), the docosahexaenoic acid – DHA (C22:6n-3; 2.64 ± 0.17%) and the eicosapentaenoic acid – EPA (C20:5n-3; 1.28 ± 0.03%) were the substantial fatty acids of total n-3 fatty acids. The particularly high level of LA is explained by the use plant oils in the feed of cultured fish and the fact that it accumulates mostly unchanged in the fat of marine fish due to their reduced ability for chain elongation and desaturation (Alasalvar, Taylor, Zubvoc, Shahidi, & Alexis, Citation2002).
Our results concerning a particularly high level of LA (>40%) with conversely very low levels of highly unsaturated fatty acids (HUFA) with DHA + EPA (<4%) may be questioning for marine fish. However, similar findings are reported by few studies. Indeed, Wassef, Saleh, and El-Abd El-Hady (Citation2009) report LA contents of 39.91% and 41.12% in sea bream fillets of fish fed diets containing blends of sunflower, cottonseed and linseed oils or sunflower, cottonseed and soybean oils, respectively. In the same way, Benedito-Palos et al. (Citation2008) demonstrated that replacement of fish oil (FO) by vegetable oils (VO) in fish diets induces and to particularly low contents of EPA (1.34 ± 0.05%) and DHA (3.15 ± 1.15%) in fillets of sea bream fed with 100% vegetable oils for 8 months. Also, Izquierdo et al. (Citation2005) reported that the partial dietary replacement of FO by VO causes low levels of EPA (2.31%) and DHA (3.88%) in fillets of sea bream fed with 80% linseed or soybean oils in diets for 204 days, respectively. They concluded that the main constraint for the use of vegetable oils in marine fish feeds is the lack of n-3 long-chain PUFA. In contrast and recently, Castro et al. (Citation2015) mentioned a considerable content of EPA (6.67%) and DHA (10.52%) in sea bream fillets of fish fed diets containing 70% of linseed oil for 330 days. Thus, the use of well-balanced plant protein diets with a suitable inclusion of marine raw materials and with an adequate substitution of FO by VO, in a dietary of sea bream, covers essential fatty acid needs and particularly omega-3 highly unsaturated fatty acids.
During refrigerated storage, a significant increase in the SFA was detected in all groups on the 12th day of storage but no difference appeared between control and treated lots. At the end of the experiment, a difference was detected between the control and treatment CH2, while the CH1 showed an intermediary value. The MUFA content of the control and CH1 treated fillets increased significantly to reach at the end of storage 29.68 ± 0.22% and 29.09 ± 0.13%, respectively. However, no significant increase was detected in CH2-treated fillets (28.50 ± 0.11%). A concomitant PUFA decreases down to 49.85 ± 0.22% was observed in control lot and to lesser extent in CH1 treated fillets (50.63 ± 0.36%) and CH2 treated fillets (51.74 ± 0.16%). The change in PUFA value observed during the storage period is generally caused by the oxidation of fatty acids and enzymatic hydrolysis of lipids (Attouchi & Sadok, Citation2012; Grigorakis, Citation2007; Turhan, Sagir, & Temiz, Citation2009). It appears that such degradation was inhibited by CMC treatment which thus has preservative effects on fatty acids. Similar findings were reported in a study examining the effects of natural antioxidant extract isolated from shrimp shells on the changes in fatty acid profile of anchovy during refrigerated storage (Küçükgülmez & Celik, Citation2013b).
4. Conclusions
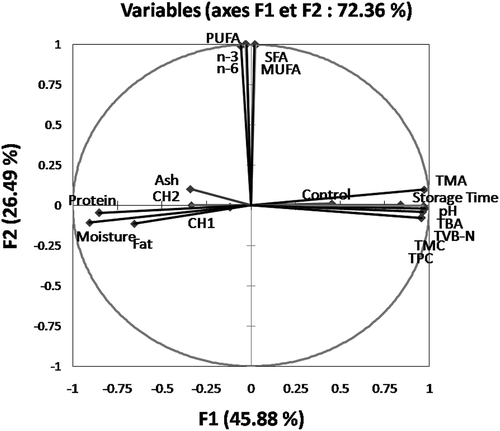
Results of the present study showed clearly that CMC is effective for preserving the quality of vacuum-packed fish fillets during refrigerated storage. It inhibits bacterial growth which limits the increase of pH as well as the accumulation of TMA, TVB-N and TBA. The CMC treatment also shows antioxidant properties and inhibits the degradation of unsaturated fatty acids. All these beneficial effects are dose dependent, with the highest dose being more effective. Moreover, the treatment has protective effects on quality characteristics in terms of proximate composition. These observations could be corroborated by the PCA performed to assess the correlations between CMC treatment and doses with the various studied parameters (storage time, the proximate composition, biochemical, microbial and fatty acids' attributes). Indeed, the first principal component explained 72.36% of the total variations in the dataset with the axis F1 and F2 accounting for 45.88% and 26.49%, respectively (). Along the F1 axis, all indicators evaluating quality (pH, TVB-N, TMA and TBA), storage time, TMC and TPC were positively correlated with the control group. Inversely, these mentioned parameters were negatively correlated with both CMC treated groups, which were positively correlated with proximate composition. The F2 axis was strongly related to fatty acids. Thus, CMC should be recommended for extending the shelf-life and preserving the quality of fish fillets during refrigerated storage. The highest tested dose (0.5%) allows prolonging the shelf-life for 12 days longer, which correspond to a two folds extension of potential storage conservation.
Figure 3. The Principal Component Analysis (PCA) carried out on all variables (Storage time, pH, TMA, TVB-N, TBA), Bacterial counts (TMC and TPC), fatty acids and batches: Control, CH1 and CH2.
Figura 3. El análisis de componentes principales (PCA) se realizó en todas las variables (tiempo de almacenamiento, pH, TMA, TVB-N, TBA, recuentos bacterianos (TMC y TPC), ácidos grasos y lotes: control, CH1 y CH2
Acknowledgments
This work was supported by the project (PromAqua) within the action “Promotion and Innovations of Tunisian Aquaculture Products-PromAqua.Tn” (2016-2018) funded by a grant from the “Institution de la Recherche et de l’Enseignement Scientifique Agricoles” within the Tunisian Ministry of Agriculture and Hydraulic Resources (IRESA).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdou, K., Aubin, J., Romdhane, M. S., Loc’h, F. L., & Lasram, F. B. R. (2017). Environmental assessment of seabass (Dicentrarchus labrax) and seabream (Sparus aurata) farming from a life cycle perspective: A case study of a Tunisian aquaculture farm. Aquaculture, 471, 204–212. doi:10.1016/j.aquaculture.2017.01.019
- Alasalvar, C., Taylor, K. D. A., Zubvoc, E., Shahidi, F., & Alexis, M. (2002). Differentiation of cultured and wild sea bass (Dicentrarchus labrax): Total lipid content, fatty acid and trace mineral composition. Food Chemistry, 79, 145–150. doi:10.1016/S0308-8146(02)00122-X
- Alves, V. L. C. D., Rico, B. P. M., Cruz, R. M. S., Vicente, A. A., Khmelinskii, I., & Vieira, M. C. (2018). Preparation and characterization of a chitosan film with grape seed extract carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT – Food Science and Technology, 89, 525–534. doi:10.1016/j.lwt.2017.11.013
- AOAC. (1995). Official methods of analysis (16th ed.). Washington, DC: Association of Official Chemists.
- Attouchi, M., & Sadok, S. (2012). The effects of essential oils addition on the quality of wild and farmed Sea Bream (Sparus aurata) stored in ice. Food and Bioprocess Technology, 5, 1803–1816.
- Benedito-Palos, L., Navarro, J. C., Sitjà-Bobadilla, A., Bell, J. G., Kaushik, S., & Pérez-Sànchez, J. (2008). High levels of vegetable oils in plant protein-rich diets fed to gilthead sea bream (Sparus aurata L.): Growth performance, muscle fatty acid profiles and histological alterations of target tissues. British Journal of Nutrition, 100, 992–1003. doi:10.1017/S0007114508966071
- Castro, P. L., Caballero, M. J., Ginés, R., Penedo, J. C., Montero, D., Lastilla, M. T., & Izquierdo, M. (2015). Linseed oil inclusion in sea bream diets: Effect on muscle quality and shelf life. Aquaculture Research, 46, 75–85. doi:10.1111/are.12161
- CEC. (1995). Commission of the European community, decision 95/149/EC of 8 March 1995 fixing the total volatile basic nitrogen (TVB-N) limit values for certain categories of fishery products and specifying the analysis methods to be used. Brussels, Belgium: Author.
- Chaijan, M., Benjakul, S., Visessanguan, W., & Faustman, C. (2005). Changes of pigments and color in Sardine (Sardinella gibbosa) and Mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chemistry, 93, 607–617. doi:10.1016/j.foodchem.2004.10.035
- Chouliara, I., Savvaidis, I. N., Riganakos, K., & Kontominas, M. G. (2005). Shelf-life extension of vacuum-packaged sea bream (Sparus aurata) fillets by combined γ-irradiation and refrigeration: Microbiological, chemical and sensory changes. Journal of the Science of Food and Agriculture, 85, 779–784. doi:10.1002/jsfa.2021
- Christie, W. W. (1993). Preparation of ester derivatives of fatty acids for chromatographic analysis. Advances in Lipid Methodology, 2(69), e111.
- Connell, J. J. (Ed.). (1995). Methods of assessing and selecting for quality in “Control of fish quality.” (4th ed., pp. 133–164). Oxford, UK: Fishing News Books.
- Devlieghere, F., Vermeulen, A., & Debevere, J. (2004). Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiology, 21, 703–714. doi:10.1016/j.fm.2004.02.008
- FAO. (2018). Market integration between wild and farmed fish in Mediterranean countries. Retrieved from http://www.fao.org/3/i8220en/I8220EN.pdf
- Folch, J., Lees, M., & Stanley, G. H. S. (1957). A simple method for the isolation and purification of total lipids from animal tissue. Journal of Biological Chemistry, 226, 497–509. doi:10.1007/s10858-011-9570-9
- Forkman, J., Josse, J., & Piepho, H. P. (2019). Hypothesis tests for principal component analysis when variables are standardized. Journal of Agricultural, Biological, and Environmental Statistics, 24(2), 289–308. doi:10.1007/s13253-019-00355-5
- Galed, G., Miralles, B., Paños, I., Santiago, A., & Heras, A. (2005). N-Deacetylation and depolymerization reactions of chitin/chitosan: Influence of the source of chitin. Carbohydrate Polymers, 62, 316–320. doi:10.1016/j.carbpol.2005.03.019
- Garrido, M. D., Hernández, M. D., Espinosa, M. C., & López, M. B. (2014). Enhanced quality characteristics of refrigerated seabream (Sparus Aurata) fillets packed under differents systems (Modified atmosphere vs Vacuum). Journal of Aquatic Food Product Technology, 25(2), 156–168. doi:10.1080/10498850.2013.838814
- Giarratana, F., Muscolino, D., Beninati, C., Ziino, G., Giuffrida, A., & Panebianco, A. (2016). Activity of R(+) limonene on the maximum growth rate of fish spoilage organisms and related effects on shelf-life prolongation of fresh gilthead sea bream fillets. International Journal of Food Microbiology, 237, 109–113. doi:10.1016/j.ijfoodmicro.2016.08.023
- Goulas, A. E., & Kontominas, M. G. (2007). Combined effect of light salting, modified atmosphere packaging and oregano essential oil on the shelf-life of sea bream (Sparus aurata): Biochemical and sensory attributes. Food Chemistry, 100, 287–296. doi:10.1016/j.foodchem.2005.09.045
- Grigorakis, K. (2007). Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: A review. Aquaculture, 272, 55–75. doi:10.1016/j.aquaculture.2007.04.062
- Günlü, A., & Koyun, E. (2013). Effects of vacuum packaging and wrapping with chitosan-based edible film on the extension of the shelf life of sea bass (Dicentrarchus labrax) fillets in cold storage (4°C). Food and Bioprocess Technology, 6, 1713–1719. doi:10.1007/s11947-012-0833-6
- Hamre, K., Næss, T., Espe, M., Holm, J. C., & Lie, O. (2001). A formulated diet for Atlantic halibut (Hippoglossus hippoglossus, L.) larvae. Aquaculture Nutrition, 7, 123–132. doi:10.1046/j.1365-2095.2001.00162.x
- Harrigan, W. F., & McCance, M. E. (1976). Laboratory methods in food and dairy microbiology. London, UK: Academic Press Incorporated.
- Hartree, E. F. (1972). Determination of protein: A modification of lowry method that gives a linear photometric response. Analytical Biochemistry, 48, 422–427. doi:10.1016/0003-2697(72)90094-2
- He, P., Davis, S. S., & Illum, L. (1999). Chitosan microspheres prepared by spray drying. International Journal of Pharmaceutics, 187, 53–65. doi:10.1016/S0378-5173(99)00125-8
- ICMSF. (1986). International commission on microbiological specifications for foods, sampling plans for fish and shellfish. In Microorganisms in foods. Sampling for microbiological analysis: Principles and scientific applications (2nd ed.). (Vol. 2, p. 310). Toronto, Canada: University of Toronto Press.
- Izci, L., Ekici, F., & Günlü, A. (2017). Coating with chitosan film of sea bream (Sparus aurata) fillets: Determining shelf life in refrigerator conditions. Food Science and Technology, Campinas, 38(1), 54–59. doi:10.1590/1678-457x.38416
- Izquierdo, M. S., Montero, D., Robaina, L., Caballero, M. J., Rosenlund, G., & Ginés, R. (2005). Alterations in fillet fatty acid profile and flesh quality in gilthead seabream (Sparus aurata) fed vegetable oils for a long term period. Recovery of fatty acid profiles by fish oil feeding. Aquaculture, 250, 431–444. doi:10.1016/j.aquaculture.2004.12.001
- Katsarov, P. D., Pilicheva, B. A., Manev, H. M., Lukova, P. K., & Kassarova, M. I. (2017). Optimization of chitosan microspheres spray drying via 32 full factorial design. Folia Medica, 59(3), 310–317. doi:10.1515/folmed-2017-0037
- Küçükgülmez, A., & Celik, M. (2013b). The effects of natural antioxidant extract isolated from Giant Red Shrimp (Aristaeomorpha foliacea) shells on fatty acid profiles of anchovy (Engraulis encrasicolus) during refrigerated storage. Journal of Aquatic Food Product Technology, 22(1), 66–76. doi:10.1080/10498850.2011.625519
- Küçükgülmez, A., Kadak, A. E., & Gӧkçin, M. (2013a). Antioxidative and antimicrobial activities of shrimp chitosan on gilthead sea bream (Sparus aurata) during refrigerated storage. International Journal of Food Science and Technology, 48, 51–57. doi:10.1111/j.1365-2621.2012.03157.x
- Li, T., Jiang, Y., Li, J., & Hu, W. (2016). An investigation on quality of japanese sea bass (Lateolabrax japonicas) using chitosan assisted with Origanum vulgare oil and allicin. Journal of Food Processing and Preservation, 41, e12918. doi:10.1111/jfpp.12918
- Ma, Z., Garrido-Maestu, A., & Jeong, K. C. (2017). Application, mode of action, and in vivo activity of Chitosan and its micro- and nanoparticles as antimicrobial agents: A review. CarbohydratePolymers, 176, 257–265. doi:10.1016/j.carbpol.2017.08.082
- Marco, P. D., Petochi, T., Marino, G., Priori, A., Finoia, M. G., Tomassetti, P., … Poli, B. M. (2017). Insights into organic farming of European sea bass Dicentrarchus labrax and gilthead sea bream Sparus aurata through the assessment of environmental impact, growth performance, fish welfare and product quality. Aquaculture, 471, 92–105. doi:10.1016/j.aquaculture.2017.01.012
- Muscolino, D., Giarratana, F., Beninati, C., Ziino, G., Giuffrida, A., & Panebianco, A. (2016). Effects of allyl isothiocyanate on the shelf-life of gilthead sea bream (Sparus aurata) fillets. Czech Journal of Food Sciences, 34, 160–165. doi:10.17221/374/2015-CJFS
- Natale, F., Borrello, A., & Motova, A. (2015). Analysis of the determinants of international seafood trade using a gravity model. Marine Policy, 60, 98–106. doi:10.1016/j.marpol.2015.05.016
- Qiu, X., Chen, S., Liu, G., & Yang, Q. (2014). Quality enhancement in the Japanese sea bass (Lateolabrax japonicas) fillets stored at 4°C by chitosan coating incorporated with citric acid or licorice extract. Food Chemistry, 162, 156–160. doi:10.1016/j.foodchem.2014.04.037
- Ramezani, Z., Zarei, M., & Raminnejad, N. (2015). Comparing the effectiveness of chitosan and nanochitosan coatings on the quality of refrigerated silver carp fillets. Food Control, 51, 43–48. doi:10.1016/j.foodcont.2014.11.015
- Roberts, G. A. F. (1992). Chitin Chemistry (1st ed.). London, UK: MacMillan.
- Ruiz-Capillas, C., & Horner, W. F. A. (1999). Determination of the trimethylamine and total volatile basic nitrogen in flesh fish by flow injection analysis. Journal of the Science of Food and Agriculture, 79, 1982–1986. doi:10.1002/(SICI)1097-0010(199911)79:14<1982::AID-JSFA459>3.0.CO;2-G
- Sadok, S., Uglow, R. F., & Haswell, S. J. (1996). Determination of trimethylamine in fish by flow injection analysis. Analytica Chimica Acta, 321, 69–74. doi:10.1016/0003-2670(95)00559-5
- Sathivel, S. (2005). Chitosan and protein coatings affect yield, moisture loss, and lipid oxidation of pink salmon (Oncorhynchus gorbuscha) fillets during frozen storage. Journal of Food Science, 70(8), 455–459. doi:10.1111/j.13652621.2005.tb11514.x
- Senso, L., Suarez, M. D., Ruiz-Cara, T., & Garcia-Gallego, M. (2007). On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream. Food Chemistry, 101, 298–307. doi:10.1016/j.foodchem.2006.01.036
- Smichi, N., Abdelmalek, B. E., Kharrat, N., Sila, A., Bougatef, A., Gargouri, Y., & Fendri, A. (2017). The effects of storage on quality and nutritional aspects of farmed and wild sea bass (Dicentrachus labrax) muscle: In vitro oils digestibility evaluation. Fisheries Research, 188, 74–83. doi:10.1016/j.fishres.2016.12.003
- Surathkal, P., Dey, M. M., Engle, C. R., Chidmi, B., & Singh, K. (2017). Consumer demand for frozen seafood product categories in the United States. Aquaculture Economics and Management, 21, 9–24. doi:10.1080/13657305.2017.1265020
- Tapilatu, Y., Nugraheni, P. S., Latumahina, M., Limmond, G. V., & Budhijanto, W. (2016). Nano-chitosan utilization for fresh yellowfin tuna preservation. Aquatic Procedia, 7, 285–295. doi:10.1016/j.aqpro.2016.07.040
- Turhan, S., Sagir, I., & Temiz, H. (2009). Oxidative stability of brined anchovies (Engraulis encrasicholus) with plant extracts. International Journal of Food Science and Technology, 44, 386–393. doi:10.1111/j.1365-2621.2008.01777.x
- Wassef, E. A., Saleh, N. E., & El-Abd El-Hady, H. A. (2009). Vegetable oil blend as alternative lipid resources in diets for gilthead seabream. Sparus Aurata. Aquaculture International, 17, 421–435. doi:10.1007/s10499-008-9213-7
- Wu, T., Wu, C., Fang, Z., Ma, X., Chen, S., & Hu, Y. (2017). Effect of chitosan microcapsules loaded with nisin on the preservation of small yellow croaker. Food Control, 79, 317–324. doi:10.1016/j.foodcont.2017.04.016
- Ximena, C., Rivera, S., & Azapagic, A. (2019). Life cycle environmental impacts of ready-made meals considering different cuisines and recipes. Science of the Total Environment, 660(10), 1168–1118. doi:10.1016/j.scitotenv.2019.01.069
- Yan, X., Song, G., Wang, X., Zhao, Y., & Chen, Y. (2018). The preparation and medical applications of chitosan microspheres. Current Organic Chemistry, 22(7), 720–733. doi:10.2174/1385272821666170830112633
- Yu, D., Xu, Y., Regenstein, J. M., Xia, W., Yang, F., Jiang, Q., & Wang, B. (2017). The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Food Chemistry, 242, 412–420. doi:10.1016/j.foodchem.2017.09.037
