ABSTRACT
In this study, the effect of carrot puree with enhanced levels of chlorogenic acid, obtained from carrots treated with wounding stress, on Lactobacillus concentration in microbiota, cognitive and brain development in two generations of rats was evaluated. Wistar rats were randomly assigned three different diets: control, 90% (w/w) control + 10% carrot puree (CP), and 90% control + 10% wounding stress carrot puree (WSCP). Wounding stress enhanced chlorogenic acid concentration ~4 times when compared to untreated CP (522 mg/kg). Rats treated with WSCP and CP diets increased the counts of Lactobacillus in the gut microbiota as compared with the control group (p ˂ 0.019) of the second generation. Myelin content of WSPC was significantly higher than control and CP groups in the first generation. In the second generation, myelin raised to 204 mg/g in WSCP group and this group had the highest RNA content. Overall, results indicate that WSCP improves brain development by increasing myelin concentration and RNA.
RESUMEN
En este estudio, se evaluó el efecto del puré de zanahoria con niveles elevados de ácido clorogénico, obtenido de zanahorias tratadas con estrés de corte, sobre la concentración de Lactobacillus en la microbiota y el desarrollo cognitivo y cerebral de dos generaciones de ratas. Las ratas Wistar se dividieron de forma aleatoria en tres dietas diferente: control, 90%(w/w) control + 10% puré de zanahoria(CP), y 90% control + 10% puré de zanahoria obtenida de zanahorias tratadas con estrés de corte(WSCP). El estrés de corte incrementó ~4 veces el contenido de ácido clorogénico comparado con CP(522 mg/kg). Las ratas tratadas con las dietas WSCP y CP incrementaron el conteo de Lactobacillus en la microbiota intestinal de la segundas generación comparado con el grupo control (p ˂ 0.019). El contenido de mielina del grupo WSPC fue significativamente mayor que en los grupos control y CP en la primera generación. En la segunda generación, el contenido de mielina se incrementó a 204 mg/g en el grupo WSCP, el cual mostró mayor contenido de ARN. En general, los resultados indican que la dieta adicionada con WSCP mejora el desarrollo cerebral mediante el incremento de mielita y ARN.
1. Introduction
Carrot is one of the main vegetable crops cultivated worldwide, where China is the largest producer with an estimated 20,000,000 annual ton. Carrots are good source of carbohydrates, minerals (Ca, P, Fe, Mg), dietary fiber, and phytochemicals like carotenes and phenolic compounds (Datt, Swati, Singh, & Surekha, Citation2012; Esmail, Citation2017; Santana-Gálvez, Pérez-Carrillo, Velázquez-Reyes, Cisneros-Zevallos, & Jacobo-Velázquez, Citation2016).
Ingestion of phenolic compounds is associated with numerous health benefits (Chávez Muñoz, Citation2010; Gul, Demircan, Bagdas, & Buyukuysal, Citation2016; Ma, Gao, & Liu, Citation2015; Mubarak et al., Citation2012; Wan et al., Citation2012). Phenolic compounds are synthesized in plants for diverse physiological functions, serving as cell wall components or generated during stress response to protect the cell from external biotic and abiotic agents (Jacobo-Velázquez & Cisneros-Zevallos, Citation2012).
Postharvest abiotic stress has been reported to improve the nutritional profile of vegetables by increasing the concentration of phenolics and other chemical compounds synthesized during stress response (Jacobo-Velázquez & Cisneros-Zevallos, Citation2012). Carrots have served as model system to investigate the effects of post-harvest abiotic stresses such as wounding, UV light, modified atmosphere and phytohormones. Among these techniques, wounding stress is the most studied as it is one of the most effective, fast, and economic approaches (Becerra-Moreno et al., Citation2015; Jacobo-Velázquez & Cisneros-Zevallos, Citation2012; Surjadinata, Jacobo-Velázquez, & Cisneros-Zevallos, Citation2017).
Wounding stress activates the phenylpropanoid metabolic route that generates hydroxycinnamic acid derivatives, mainly chlorogenic acid (Becerra-Moreno et al., Citation2015; Jacobo-Velázquez & Cisneros-Zevallos, Citation2012). In carrots treated with postharvest abiotic stresses, chlorogenic acid is the main phenolic accumulated (~440–920 mg/kg) (Jacobo-Velázquez & Cisneros-Zevallos, Citation2012).
Chlorogenic acid (CA) is absorbed and metabolized by the human body, where in vitro and clinical studies indicate that it has a positive impact against chronic degenerative diseases like diabetes (Ma et al., Citation2015), hypertension (Mubarak et al., Citation2012), obesity (Huang, Liang, Zhong, He, & Wang, Citation2014), dyslipidemia (Wan et al., Citation2012), and metabolic syndrome (Santana-Gálvez, Cisneros-Zevallos, & Jacobo-Velázquez, Citation2017).
In addition to the well-documented benefits against the metabolic syndrome, research studies suggest phenolic compounds may improve neuron performance and help against aging neuron degeneration (Gul et al., Citation2016; Prediger et al., Citation2008; Shukitt-Hale et al., Citation2005). Scientific reports evaluating CA effects on neural health are scarce, although Gul et al. (Citation2016) reported that this compound protects rat brain cortical cells against oxidative stress. Similarly, CA may have anti-amnesic activity in the hippocampus and frontal cortex (Kwon et al., Citation2010). CA is mostly metabolized by the gut microbiota, exerting a prebiotic effect (Cowan et al., Citation2014; Gonthier, Verny, Besson, Rémésy, & Scalbert, Citation2003). On the other hand, recent studies suggest that microbiota may be involved in neuronal development and function (Borre et al., Citation2014; Bravo et al., Citation2011). Phenolic compounds, like CA, may modulate the microbiota in vivo, producing changes in beneficial bacteria such as Lactobacillus spp. (Molan, Liu, & Kruger, Citation2010).
The objective of the present study was to evaluate the effect of carrot puree with enhanced levels of chlorogenic acid obtained from carrots treated with wounding stress on the microbiota, cognitive and brain development of rats.
2. Materials and methods
2.1. Wounding stress application and production of carrot puree
Carrots (Daucus carota L.) were purchased in a local supermarket and visually inspected to discard samples with physical damage. Carrots were washed and disinfected with a chlorine solution (200 mg/L, pH 6.5–7.0) for 5 min. Both carrot ends were manually cut-off with a steel knife and the remaining carrot bodies were sliced with a commercial food processor (Waring Commercial, WFP11, Torrington, CT, USA). Samples intended to serve as non-stressed carrot puree (CP) were blanched (82°C, 6 min), grinded and pasteurized (85°C, 10 min). In the case of samples intended for the production of puree obtained from carrots treated with wounding stress (wounding stress carrot puree, WSCP), carrot slices were placed in plastic containers and stored for 48 h at 15°C before blanching, grinding and pasteurizing. Wounding Stress conditions were established to maximize the phenolic content of carrot based on studies previously performed by our group (Kovacs et al., Citation2011; Santana-Gálvez et al., Citation2016). Puree was cooled down to 20°C, divided into 30-g portions, and stored in 50-mL plastic cryovials at −80°C until used.
2.2. Proximal composition
CP and WSPC were analyzed to determine protein (NMX-F608-Normex-2011), fat (NOM-086-SSA-1-1994), non-soluble dietary fiber (AOAC 991.42), soluble dietary fiber (AOAC 993.19), total dietary fiber (AOAC 985.29), ash (NMX-F-607-Normex-2013), and moisture (NOM-116-SSA-1-1994) contents. All results were expressed as percentage in weight basis.
2.3. Phenolic compounds
Carrot puree samples (5 g) were homogenized with 20-mL of methanol and centrifuged for 15 min (12,000 g, 4°C). The supernatant was collected and filtered with a 0.45-µm syringe, and a 10-µL aliquot was injected to a HPLC-DAD (Agilent Technologies, 1260 Infinity Santa Clara, CA, USA) with a reverse C18 column (250 x 4.6 mm, 15 µm, Luna, Phenomenex, Torrance, CA, USA). Mobile phases consisted of water (A) and 60% methanol (B) solutions adjusted to pH 2.5 with orthophosphoric acid. Phenolics were detected at 320-nm wavelength. Chromatogram information was processed with OpenLAB CDS ChemStation software (Agilent Technologies), and results were expressed as mg/kg on dry weight base (Santana-Gálvez et al., Citation2016).
2.4. Rat individuals and diets
Post-weaning Wistar rats (30 day) were randomly selected and fed according to diets shown in . Each treatment group contained four females and four males, and rodents were individually kept in stainless steel cages with controlled room conditions (21°C, 12 h of alternate light/dark cycles).
Table 1. Treatments and diets.
Tabla 1. Tratamientos y dietas
The control group daily received 30 g of a casein balanced diet (AIN-76). The diet AIN-76 semi-purified casein control diet was formulated with 20% casein, 50% sucrose, 15% corn starch, 5% cellulose, 5% vegetable oil, 3.5% mineral mix and 1% vitamin mix (Bieri, Citation1980). The remaining treatments group were fed with 90% (w/w) of the AIN-76 diet, and the rest 10% (w/w) intake was supplied through carrot puree (CP; Group 2) or wounding-stress carrot puree (WSCP; Group 1) for 30 days (). Ad libitum water intake was set for all treatment groups. Food intake per day and weight gain per week were recorded until sacrifice day.
Table 2. Composition of the experimental diets per day (30 g).
Tabla 2. Composición de las dietas experimentales por día (30 g)
After 30 days, the rats were subjected to cognitive tests described below (60 d of age). Once rats finished cognitive tests two females were randomly paired with one male of the same treatment group up to 10 days for reproduction, and male individuals were rotated after 5 days. Pregnant females were placed in maternity cages and males were placed back in their individual cage. Rats gave birth after 27 days allowing offspring weaning for 20–25 days. Feeding of the first rat generation continued as established in during the reproduction and lactation periods. At 179 d of age, individuals were anesthetized through intraperitoneal injection of sodium pentobarbital (40 mg/kg) and sacrificed with intracardiac puncture.
The small intestine and brain were surgically removed and stored in hermetic, wide opening 60-mL containers. All samples were stored at −20°C until further analysis. Post-weaning survivors of the second rat generation were selected to continue diets for 1 month (). As in the case of the first generation, each treatment group was balanced with four-female and 4-male individuals. Following 30 days, second-generation rats were subjected to cognitive tests and sacrificed for collecting small intestine and brain samples as described above. This experimental protocol was reviewed and approved by the Research Ethics Committee of the Faculty of Biological Sciences (CEIBA-2018-031).
2.5. Cognitive development
Each rat generation was subjected to the Morris maze test (Morris, Citation1984). A circular water tank was subdivided into four sections (northwest, northeast, southwest, southeast). A blue-coloured squared platform was placed in the water tank. Blue-tainted water filled the tank to hide the platform. The submerged platform was moved 180° from its original position (southeast). Rats started on the northern position (platform on the south). The platform location was subsequently rotated to the south, east and west of the water tank, and the rat drop-off point was moved along accordingly. Each trial was repeated four times during three consecutive days. Latency and error were recorded for each individual (Amaya-Guerra, Serna Saldívar, & Alanis-Guzman, Citation2006).
2.6. Gut microbiota DNA
Surgically removed small intestines were homogenized with 1:10 (w/v) of peptone water in a sterile laminar flow hood (Santacruz et al., Citation2012). DNA extraction was done with DNeasy Clean Microbial kit (QIAGEN, 6MBH, QIAGEN STRASSEL, Hilden, Germany).
2.7. RT-PCR microbial quantification
Lactobacillus spp. and total count were determined by real-time PCR using specific primers for each microbial group. Amplification and detection were determined with a Rotor-Gen R6-3000, QIAGEN using the SyBR Green PCR kit consisting of 12.5 µL forward primer, 12.5-µL reverse primer, 12.5 µL of SyBR Green PCR Master Mix, and 3 µL of DNA. The sequence of the forward and reverse primers were 5ʹAGCAGTAGGGAATCTTCCA and 3ʹATTYCACCGCTACACATG for Lactobacillus spp.; 5ʹTGGCTCAGGACGAACGCTGGCGGC and 3ʹCCTACTGCTGCCTCCCGTAGGAGT for total bacteria (Liu et al., Citation2014).
2.8. Myelin determination
Brain and cerebellum samples were weighed with an analytical balance prior to extraction. Half of the surgically removed brains and cerebellums were mixed with a chloroform/methanol/water 8:4:3 (v/v/v) solution. Butylated hydroxytoluene (BHT) was added (0.02g/L) to prevent lipid oxidation. Samples were diluted (1:10) and homogenized in sucrose solution (0.8 mol/L). The mixture was centrifuged at 12,000 rpm for 70 min. Supernatants were collected and resuspended in 30 mL of distilled water. Samples were placed in ice and manually agitated for 20 min. Centrifuging followed by washing was performed two more times, and the final extract was dried in a vacuum incubator (Model 1400 E; VWR, West Chester, PA, USA) at 60°C. Dried samples were weighed in an analytical balance (VE204-B) (Folch, Lees, & Sloane Stanley, Citation1957).
2.9. Brain’s protein determination
Protein content of brain was determined with 2-mL aliquot taken in duplicate and digested with 3 mL of H2SO4 and 1 g of K2SO4: CuSO4 (25:1) in a micro-Kjeldahl. The nitrogen content was determined after the sample was adjusted to 25 mL with distilled water using an Orion 901 ion analyzing microprocessor (Orion Research, Inc. Cambridge, MA). Bovine serum albumin and ammonium sulfate were used to standardize the procedure. Protein content was estimated by multiplying nitrogen content by 6.25 (Bernocchi & Scherini, Citation1980).
2.10. Cerebral DNA and RNA determination
Brain tissue (0.5 g) was suspended in a trichloroacetic acid solution (TCA; 50% v/v) and centrifuged at 2,500 rpm for 20 min. The pellet was collected, resuspended in 2.0 mL of 5% (v/v) TCA and immersed for 30 min in a boiling water bath. Tubes were centrifuged at 2,500 rpm for 15 min (Morse & Carter, Citation1949). Supernatant was collected for colorimetric DNA and RNA determination (Dische, Citation1983). An aliquot (1.2 mL) was mixed with 2.4 mL of orcinol and placed for 15 min in a boiling water bath. Samples were cooled down and absorbance was measured at 665 nm to determine RNA content. For DNA quantification, 320 µL of diphenylamine were added to each sample. The mixture was incubated at 37°C during 4 h, and absorbance was measured at 600-nm wavelength in a Spekol-spectrocolorimeter (VEB-Carl Seizz, Jena, Germany).
2.11. Statistical analysis
Average and standard deviation values of phytochemical concentration for each diet treatment group were analyzed with a one-factor ANOVA using SPSS Statistics software (v22). Lactobacillus spp., total bacteria count, and Morris cognitive tests results were analyzed using the Tukey mean comparison tests. Statistical analysis was done with 0.05 significance.
3. Results and discussion
3.1. Carrot puree phytochemical and physicochemical characterization
CP and WSCP had similar carbohydrate, fiber, fat and protein content (). On the other hand, wounding stress enhanced chlorogenic acid concentration ~4 times (2296 ± 217 mg/kg) when compared to control carrot puree (522 ± 23 mg/kg). The results for CA content reported herein are in agreement with a previous report, where carrots treated with wounding stress, were further processed into juice by applying additional treatments such as blanching and pasteurization (Santana-Gálvez, Santacruz, Cisneros-Zevallos, & Jacobo-Velázquez, Citation2019). Likewise, WSCP showed 2–5-fold higher concentrations of coumaric acid (p-CA) and p-CA derivative p-CADA when compared to control samples. Santana-Gálvez et al. (Citation2016) observed a similar increase of these phenolic compounds after wounding stress. Results showed 522% CA and 69% p-CADA increments in wounding stress shredded carrots when compared to the control. Hydroxycinnamic acid derivatives like chlorogenic acid and coumaric acid are synthesized via the phenylpropanoid metabolic route which is triggered as part of the plant cell mechanisms against cellular damage induced by wounding (Jacobo-Velázquez & Cisneros-Zevallos, Citation2012).
Table 3. Carrot puree proximal and phytochemical composition.
Tabla 3. Composición proximal y fitoquímica de la papilla de zanahoria
3.2. Learning development
Food intake and weight gain were not statistically different between treatments. There were no significant differences between the three groups in the first two days of the learning development test in the first rat generation (). Nevertheless, WSCP improved the number of errors from 2 to 1 when compared with the CP group (p ˂ 0.019) in day 3. On the second generation, subjects under CP and WSPC diets significantly improved, averaging 1 error and <10 s to find the hidden platform. Both parameters were significantly lower (p < .020) when compared to the control diet group. Although there are no previous reports on the application of CA in cognitive development, a previous study of Jang et al. (Citation2013) investigated the consumption of instant decaffeinated coffee (120 mg/g per day) in scopolamine rats for 2 weeks. Coffee contains high amounts of CA. In agreement with the results obtained herein, scopolamine rats treated with instant decaffeinated coffee showed improved performance in Morris water maze as compared with the untreated rats.
Figure 1. Learning Performance errors and latency in two generations of rats. Figures: (a) Learning performance errors in first generation; (b) Learning performance latency in first generation; (c) Learning performance errors in second generation; (d) Learning performance latency in second generation. Values are means and bars indicate standard error of the mean. * indicates statistically significant difference by the Tukey’s Test (p ˂ 0.005). WSCP: wound stress carrot puree; CP: control carrot puree; AIN-76: base diet complemented with casein.
Figura 1. Errores y latencia en el desempeño de aprendizaje de dos generaciones de ratas. Figuras: (a) Errores en el desempeño de aprendizaje en la primera generación; (b) latencia en el desempeño de aprendizaje en la primera generación; (c) errores en el desempeño de aprendizaje en la segunda generación; (d) Latencia en el desempeño de aprendizaje en la segunda generación. Los valores están expresados como medias y las barras indican el error estándar de la media. * indica una diferencia estadísticamente significativa mediante la prueba de Tukey (p ˂ 0.005). WSCP: puré de zanahoria obtenido de zanahorias tratadas con estrés de corte; CP: puré de zanahoria control. AIN-76: Dieta base complementada con caseína
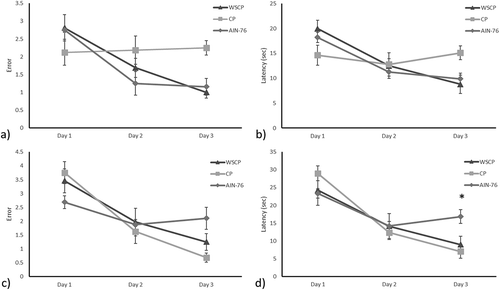
Research studies suggest that phenolic compounds could act in different ways on learning development. For example, ellagitannins act through the hippocampus, proanthocyanidins in the brain sulcus and flavonols are efficient to regulated the neurotrophic factor derived from the brain metabolism (Kwon et al., Citation2010; Rendeiro et al., Citation2013). Chlorogenic acid acts on Central Nervous System through the blood-brain barrier in its original chemical composition or as a secondary metabolite. Once inside, it triggers serotonin in the brain hippocampus which in turn lowers the anxiety of the individual (Bouayed, Rammal, Dicko, Younos, & Soulimani, Citation2007). It should be noted that on day 3, group WSCP of both generations made fewer errors/latencies than the control group in the first generation or with the group CP in the second generation. Therefore, chlorogenic acid present in WSCP could have helped rats to experience less stress during trials and perform better on cognitive test (Prediger et al., Citation2008).
3.3. Lactobacillus spp. concentration in gut microbiota
The beneficial effects of gut microbiota are well documented and recent research studies suggest that it may also influence the Central Nervous System, particularly the brain (Borre et al., Citation2014; Bravo et al., Citation2011). Moreover, it has been demonstrated that phenolic compounds can regulate the composition of microbiota (Filosa, Meo, & Crispi, Citation2018). Lactobacillus spp. are Gram-positive bacteria present in the microbiota and they are usually present as probiotics. This genus of bacteria is capable to produce gamma-aminobutyric acid (GABA), which is an activity biogenic substance present in CNS and implicated in neurotransmission and brain metabolism (Yunes et al., Citation2016).
Diet did not influence gut microbiota in the first generation on the three groups (). Nonetheless, WSCP and CP had higher concentration of Lactobacillus spp. than the control group (p ˂ 0.019) of the second generation. Lactobacillus spp. prevents memory dysfunction by normalizing the expression of neurotrophic factor CA1 on rat hippocampus (Bouayed et al., Citation2007; Bravo et al., Citation2011). Phenolic compounds affect microbiota composition by exerting as probiotics for beneficial microorganisms while sensitizing pathogens. For example, flavonols favor Lactobacillus spp. and Bifidobacteria spp. growth (Cardona, Andrés-Lacueva, Tulipani, Tinahones, & Queipo-Ortuño, Citation2013; Cueva et al., Citation2013), although the mechanisms that enhance beneficial bacteria growth remain unknown (Cardona et al., Citation2013). Higher Lactobacillus spp. contents in WSCP and CP coincide with shorter latencies in learning performance of the second generation at day 3 ()). On the other hand, the total bacteria count in the gut microbiota of the first generation is similar among the three treatments. However, groups of the second generation that consumed the carrot purees (WSCP and CP) did not increase the number of bacteria as carbon sources, in comparison with the control (p ˂ 0.045). These results indicate that CA helps to maintain gut health by modulating the gut microbial balance through stimulating the growth of beneficial bacteria such as Lactobacillus spp.
Table 4. Bacterial counts in gut microbiota.
Tabla 4. Recuento de bacterias en la microbiota del intestino
3.4. Brain development
Diet did not influence brain (1183–1296 mg) or cerebellum (280–312 mg) weight of the first generation (). Over the next generation, CP and WSCP averaged more brain and cerebellum weight, although significant differences were only found while comparing the control and CP treatments. Myelin content of WSPC was significantly higher (183 ± 45 mg/g) than CP (166 ± 14 mg/g) group. In the second generation, myelin raised to 204 ± 6 mg/g, although significant differences were only observed with the CP group.
Table 5. Effect of diet on brain and cerebellar weight and myelin.
Tabla 5. Efecto de la dieta sobre el peso del cerebro y cerebelo y mielina
Chlorogenic acid could be modulating nucleotides by activating the ecto-nucleoside triphosphatase and ecto-50-nucleotidase to increase myelin content (Leal et al., Citation2016). Myelin is directly involved with signals of the central neuro system, thus rats consuming WSCP can process neuro signals faster and may be reflected on cognitive results (Amaya-Guerra et al., Citation2006; Elaine & Katja, Citation2012). The CP group registered the lowest brain and cerebellum weights, which are directly related to motor development and long-term memory in animals (Warren & Bedi, Citation1984). No significant differences between treatments in protein and RNA concentrations were observed in the first generation (). However, the control group brains could have a greater number of brain neurons than the CP group (p ˂ 0.011), because neurons may be related to brain DNA content (Amaya-Guerra et al., Citation2006). In generation two, the WSCP group had the highest RNA content, followed by the control group and finally the CP group (p ˂ 0.000). This parameter is used to determine RNA/DNA, which is an indicator of metabolic activity of brain cells, so WSCP group showed greater metabolic activity than the control and CP groups (p ˂ 0.000).
Table 6. Effect of diet on brain protein, RNA and DNA.
Tabla 6. Efecto de la dieta sobre el contenido de proteína, ARN y ADN en el cerebro
Some researchers have related a low DNA and RNA brain content with memory loss and chronic diseases like Alzheimer. An increase of free radicals may induce lipid peroxidation, which in turn affects polyunsaturated fatty acids present in the brain (Chu et al., Citation2009; Gul et al., Citation2016; Markesbery & Lovell, Citation2007). Chlorogenic acid is a potent antioxidant that may inhibit lipid peroxidation as it sequesters free radicals, providing a protective effect against oxidative stress in the brain (Elaine & Katja, Citation2012; Gul et al., Citation2016; Kwon et al., Citation2010). Furthermore, chlorogenic acid derivatives like ferulic, coumaric, and caffeic acids may also go through the blood-brain barrier and diffuse to different brain regions. Successful diffusion is also affected by other factors like charge state, lipophile and transport proteins (Amaya-Guerra et al., Citation2006; Ferlemi et al., Citation2015; Shukitt-Hale et al., Citation2005). Likewise, results observed in the second could be attributed to an epigenetic regulation capacity exerted by CA present in WSCP (Lee & Zhu, Citation2006).
Parameters on the brain development results and the Morris water maze do not coincide between treatments. However, Morris water maze is complex, since many times the success or failure of the animals in the search for the platform is influenced by several factors such as attention, motivation and sensorimotor function (Prediger et al., Citation2008). On the other hand, it should be noted that myelin, brain weight, cerebellum weight, DNA and RNA content are strictly related to adequate protein nutrition (Cruz-Rizzolo et al., Citation2017; Griffin, Woodward, & Chanda, Citation2018) and it should be considered that the CP and WSCP groups consumed approximately 90% of their daily proteins as carrot puree contains a small amount of protein than casein. Therefore, if the carrot puree will be used as a supplement to diets covering 100% of protein requirement, the WSCP may result in improved brain development, which may subsequently result in better results on Morris water maze cognitive tests.
4. Conclusions
Results indicated that WSCP have a positive effect on improving brain development, myelin concentration and brain of rats. Rats treated with WSCP showed a higher concentration of Lactobacillus spp and lower total bacterial content compared to CP group, indicating that CA exerts a prebiotic effect. Now that the potential positive effects of CA on Lactobacillus spp have been identified, future research should consider the evaluation of a dose response. Furthermore, to better understand these results more studies evaluating a larger rodent population considering longer study duration, more generations and the effect of gender should be performed. Indeed, the latter (effect of gender) should be specially considered since although reports indicate that gender does not affect the gut microbiota (Lay et al., Citation2005), other authors have reported that gender affect gut microbiota, metabolism and neural system (Bridgewater et al., Citation2017; Bullock, Gemzik, Johnson, Thomast, & Parkinson, Citation1991; Czerniak, Citation2001; Fukuno, Nagai, Horii, Yamamoto, & Konishi, Citation2018; Fukushima et al., Citation2015; Jasarevic´, Morrison, & Bale, Citation2016; Org et al., Citation2016), suggesting that compounds may undergo gender-dependent metabolism.
Acknowledgments
This study is based upon research supported by Universidad Autónoma de Nuevo León and Tecnológico de Monterrey Bioprocess and NutriOmics Research Groups. Author J.M.L.-M acknowledges the scholarship [#373289] from CONACYT.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Amaya-Guerra, C., Serna Saldívar, S., & Alanis-Guzman, M. G. (2006). Soyabean fortification and enrichment of regular and quality protein maize tortillas affects brain development and maze performance of rats. British Journal of Nutrition, 96, 161. doi:10.1079/BJN20061804
- Becerra-Moreno, A., Redondo-Gil, M., Benavides, J., Nair, V., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2015). Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Frontiers in Plant Science, 6, 837. doi:10.3389/fpls.2015.00837
- Bernocchi, G., & Scherini, E. (1980). Cytochemical data on DNA and protein nuclear content during the prenatal cerebellar histogenesis in the rat. Effects of maternal protein malnutrition. Cellular and Molecular Biology, 26, 405–413.
- Bieri, J. G. (1980). Second report of the ad hoc committee on standards for nutritional studies. Journal of Nutrition, 110, 1726. doi:10.1093/jn/110.8.1726
- Borre, Y. E., O’Keeffe, G. W., Clarke, G., Stanton, C., Dinan, T. G., & Cryan, J. F. (2014). Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends in Molecular Medicine, 20, 509–518. doi:10.1016/j.molmed.2014.05.002
- Bouayed, J., Rammal, H., Dicko, A., Younos, C., & Soulimani, R. (2007). Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. Journal of the Neurological Sciences, 262, 77–84. doi:10.1016/j.jns.2007.06.028
- Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., & Cryan, J. F. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences, 108, 16050–16055. doi:10.1073/pnas.1102999108
- Bridgewater, L. C., Zhang, C., Wu, Y., Hu, W., Zhang, Q., Wang, J., … Zhao, L. (2017). Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Scientific Reports, 7, 10776. doi:10.1038/s41598-017-11069-4
- Bullock, P., Gemzik, B., Johnson, D., Thomast, P., & Parkinson, A. (1991). Evidence from dwarf rats that growth hormone may not regulate the sexual differentiation of liver cytochrome P450 enzymes and steroid 5a-reductase. Proceedings of the National Academy of Sciences of the United States of America, 88, 5227–5231. doi:10.1073/pnas.88.12.5227
- Cardona, F., Andrés-Lacueva, C., Tulipani, S., Tinahones, F. J., & Queipo-Ortuño, M. I. (2013). Benefits of polyphenols on gut microbiota and implications in human health. The Journal of Nutritional Biochemistry, 24, 1415–1422. doi:10.1016/j.jnutbio.2013.05.001
- Chávez Muñoz, M. (2010). Composición de Alimentos. Segunda (Ed.). México, DF: Mc Graw Hill.
- Chu, Y.-F., Brown, P. H., Lyle, B. J., Chen, Y., Black, R. M., Williams, C. E., … Cheng, I. H. (2009). Roasted coffees high in lipophilic antioxidants and chlorogenic acid lactones are more neuroprotective than green coffees. Journal of Agricultural and Food Chemistry, 57, 9801–9808. doi:10.1021/jf902095z
- Cowan, T. E., Palmnäs, M. S., Yang, J., Bomhof, M. R., Ardell, K. L., Reimer, R. A., … Shearer, J. (2014). Chronic coffee consumption in the diet-induced obese rat: Impact on gut microbiota and serum metabolomics. Journal of Nutritional Biochemistry, 25, 489–495. doi:10.1016/j.jnutbio.2013.12.009
- Cruz-Rizzolo, R. J., Limieri, L. L., de Paiva, I. R., Ribeiro, J. O. B., Pimenta, T. F., Pinato, L., & Liberti, E. A. (2017). Protein malnutrition during gestation and early life decreases neuronal size in the medial prefrontal cortex of post-pubertal rats. IBRO Reports, 3, 65–71. doi:10.1016/j.ibror.2017.08.002
- Cueva, C., Sánchez-Patán, F., Monagas, M., Walton, G. E., Gibson, G. R., Martín-Álvarez, P. J., & Moreno-Arribas, M. V. (2013). In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: Changes in microbial groups and phenolic metabolites. FEMS Microbiology Ecology, 83, 792–805. doi:10.1111/1574-6941.12037
- Czerniak, R. (2001). Gender-based differences in pharmacokinetics in laboratory animal models. International Journal of Toxicology, 20, 161–163. doi:10.1080/109158101317097746
- Datt, S. K., Swati, K., Singh, T. N., & Surekha, A. (2012). Chemical composition, functional properties and processing of carrot-a review. Journal of Food Science and Technology, 49, 22–32. doi:10.1007/s13197-011-0310-7
- Dische, E. (1983). Physiological studies on the herbicide “Cotorane” S.S. Roushdy. Cairo, Egypt: University of Cairo.
- Elaine, M., & Katja, H. (2012). Human anatomy & physiology. (8th ed.). Pearson (Ed.). San Francisco, California: Benjamin Cummings.
- Esmail, A.-S. A. (2017). Nutritional and therapeutic importance of daucus carota-A review. Journal of Pharmacy, 7, 72–82.
- Ferlemi, A.-V., Mermigki, P. G., Makri, O. E., Anagnostopoulos, D., Koulakiotis, N. S., Margarity, M., & Lamari, F. N. (2015). Cerebral area differential redox response of neonatal rats to selenite-induced oxidative stress and to concurrent administration of highbush blueberry leaf polyphenols. Neurochemical Research, 40, 2280–2292. doi:10.1007/s11064-015-1718-7
- Filosa, S., Meo, F. S., & Crispi, S. (2018). Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regeneration Research, 13, 2055–2059. doi:10.4103/1673-5374.241429
- Folch, J., Lees, M., & Sloane Stanley, G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. The Journal of Biological Chemistry, 226, 497–509.
- Fukuno, S., Nagai, K., Horii, A., Yamamoto, K., & Konishi, H. (2018). Pharmacokinetics and metabolic elimination of tolbutamide in female rats: Comparison with male rats. Biopharmaceutics & Drug Disposition, 39, 321–327. doi:10.1002/bdd.2148
- Fukushima, A., Hagiwara, H., Fujioka, H., Kimura, F., Akema, T., & Funabashi, T. (2015). Sex differences in feeding behavior in rats: The relationship with neuronal activation in the hypothalamus. Frontiers in Neuroscience, 9, 88. doi:10.3389/fnins.2015.00088
- Gonthier, M. P., Verny, M. A., Besson, C., Rémésy, C., & Scalbert, A. (2003). Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. Journal of Nutrition, 133, 1853–1859. doi:10.1093/jn/133.6.1853
- Griffin, W. S. T., Woodward, D. J., & Chanda, R. (2018). Malnutrition and brain development: Cerebellar weight, DNA, RNA, protein and histological correlations. Journal of Neurochemistry, 28, 1269–1279. doi:10.1111/j.1471-4159.1977.tb12320.x
- Gul, Z., Demircan, C., Bagdas, D., & Buyukuysal, R. L. (2016). Protective effects of chlorogenic acid and its metabolites on hydrogen peroxide-induced alterations in rat brain slices: A comparative study with resveratrol. Neurochemical Research, 41, 2075–2085. doi:10.1007/s11064-016-1919-8
- Huang, K., Liang, X., Zhong, Y., He, W., & Wang, Z. (2014). 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. Journal of the Science of Food and Agriculture, 95, 1903–1910. doi:10.1002/jsfa.6896
- Jacobo-Velázquez, D. A., & Cisneros-Zevallos, L. (2012). An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture, 2, 259–271. doi:10.3390/agriculture2030259
- Jang, Y. J., Kim, J., Shim, J., Kim, C. Y., Jang, J. H., Lee, K. W., & Lee, H. J. (2013). Decaffeinated coffee prevents scopolamine-induced memory impairment in rats. Behavioural Brain Research, 15, 113–119. doi:10.1016/j.bbr.2013.02.003
- Jasarevic´, E., Morrison, K. E., & Bale, T. L. (2016). Sex differences in the gut microbiome– Brain axis across the lifespan. Philosophical Transactions of the Royal Society B, 371, 1–11. doi:10.1098/rstb.2015.0122
- Kovacs, A., Ben-Jacob, N., Tayem, H., Halperin, E., Iraqi, F. A., & Gophna, U. (2011). Genotype is a stronger determinant than sex of the mouse gut microbiota. Environmental Microbiology, 61, 423–428. doi:10.1007/s00248-010-9787-2
- Kwon, S.-H., Lee, H.-K., Kim, J.-A., Hong, S.-I., Kim, H.-C., Jo, T.-H., … Jang, C.-G. (2010). Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. European Journal of Pharmacology, 649, 210–217. doi:10.1016/j.ejphar.2010.09.001
- Lay, C., Rigottier-Gois, L., Holmstrøm, K., Rajilic, M., Vaughan, E. E., de Vos, W. M., … Doré, J. (2005). Colonic microbiota signatures across five Northern European countries. Applied and Environmental Microbiology, 71, 4153–4155. doi:10.1128/AEM.71.7.4153–4155.2005
- Leal, C. A. M., Leal, D. B. R., Adefegha, S. A., Morsch, V. M., Beckmann, D. V., Castilhos, L. G., … Schetinger, M. R. C. (2016). Effects of chlorogenic acid on adenine nucleotides hydrolyzing enzyme activities and expression in platelets of rats experimentally demyelinated with ethidium bromide. Biomedicine & Pharmacotherapy, 81, 363–370. doi:10.1016/j.biopha.2016.04.003
- Lee, W. J., & Zhu, B. T. (2006). Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis, 27, 269–277. doi:10.1093/carcin/bgi206
- Liu, X., Blouin, J., Santacruz, A., Lan, A., Andriaamihaja, M., Wilkanowicz, S., … Davila, A. (2014). High-protein diet modifies colonic microbiota and luminal environment but not colonocyte metabolism in the rat model: The increased luminal bulk connection. The American Journal of Physiology-Gastrointestinal and Liver Physiology, 307, 459–470. doi:10.1152/ajpgi.00400.2013
- Ma, Y., Gao, M., & Liu, D. (2015). Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in Mice. Pharmaceutical Research, 32, 1200–1209. doi:10.1007/s11095-014-1526-9
- Markesbery, W. R., & Lovell, M. A. (2007). Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Archives of Neurology, 64, 954–956. doi:10.1001/archneur.64.7.954
- Molan, A.-L., Liu, Z., & Kruger, M. (2010). The ability of blackcurrant extracts to positively modulate key markers of gastrointestinal function in rats. World Journal of Microbiology and Biotechnology, 26, 1726–1735. doi:10.1007/s11274-010-0352-4
- Morris, R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods, 11, 47–60. doi:10.1016/0165-0270(84)90007-4
- Morse, M., & Carter, E. (1949). The synthesis of nucleic acid in cultures of Escherichia coli, strain B and B/R. Journal of Bacteriology, 58, 317. doi:10.1128/JB.58.3.317-326.1949
- Mubarak, A., Bondonno, C. P., Liu, A. H., Considine, M. J., Rich, L., Mas, E., & Hodgson, J. M. (2012). Acute effects of chlorogenic acid on nitric oxide status, Endothelial function, and blood pressure in healthy volunteers: A randomized trial. Journal of Agricultural and Food Chemistry, 60, 9130–9136. doi:10.1021/jf303440j
- Org, E., Mehrabian, M., Parks, B., Shipkova, P., Liu, X., Drake, T. A., & Lusis, A. J. (2016). Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes, 7, 313–322. doi:10.1080/19490976.2016.1203502
- Prediger, R. D. S., Fernandes, M. S., Rial, D., Wopereis, S., Pereira, V. S., Bosse, T. S., & Costa-Campos, L. (2008). Effects of acute administration of the hydroalcoholic extract of mate tea leaves (Ilex paraguariensis) in animal models of learning and memory. Journal of Ethnopharmacology, 120, 465–473. doi:10.1016/j.jep.2008.09.018
- Rendeiro, C., Vauzour, D., Rattray, M., Waffo-Téguo, P., Mérillon, J. M., Butler, L. T., & Spencer, J. P. E. (2013). Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. Plos One, 8. doi:10.1371/journal.pone.0063535
- Santacruz, A., Marcos, A., Wärnberg, J., Martí, A., Martin-Matillas, M., Campoy, C., & Sanz, Y. (2012). Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity, 17, 1906–1915. doi:10.1038/oby.2009.112
- Santana-Gálvez, J., Cisneros-Zevallos, L., & Jacobo-Velázquez, A. D. (2017). Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules, 22, 358. doi:10.3390/molecules22030358
- Santana-Gálvez, J., Pérez-Carrillo, E., Velázquez-Reyes, H. H., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2016). Application of wounding stress to produce a nutraceutical-rich carrot powder ingredient and its incorporation to nixtamalized corn flour tortillas. Journal of Functional Foods, 27, 655–666. doi:10.1016/J.JFF.2016.10.020
- Santana-Gálvez, J., Santacruz, A., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. (2019). Postharvest wounding stress in horticultural crops as a tool for designing novel functional foods and beverages with enhanced nutraceutical content: Carrot juice as a case study. Journal of Food Science, 84(5), 1151–1161. doi:10.1111/1750-3841.14588
- Shukitt-Hale, B., Galli, R. L., Meterko, V., Carey, A., Bielinski, D. F., McGhie, T., & Joseph, J. A. (2005). Dietary supplementation with fruit polyphenolics ameliorates age-related deficits in behavior and neuronal markers of inflammation and oxidative stress. Age, 27, 49–57. doi:10.1007/s11357-005-4004-9
- Surjadinata, B. B., Jacobo-Velázquez, A. D., & Cisneros-Zevallos, L. (2017). UVA, UVB and UVC light enhances the biosynthesis of phenolic antioxidants in fresh-cut carrot through a synergistic effect with wounding. Molecules, 22, 668. doi:10.3390/molecules22040668
- Wan, C.-W., Wong, C. N.-Y., Pin, W.-K., Wong, M. H.-Y., Kwok, C.-Y., Chan, R. Y.-K., & Chan, S.-W. (2012). Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytotherapy Research, 27, 545–551. doi:10.1002/ptr.4751
- Warren, M. A., & Bedi, K. S. (1984). A quantitative assessment of the development of synapses and neurons in the visual cortex of control and undernourished rats. Journal of Comparative Neurology, 227, 104–108. doi:10.1002/cne.902270111
- Yunes, R. A., Poluektova, E. U., Dyachkova, M. S., Klimina, K. M., Kovtun, A. S., Averina, O. V., … Danilenko, V. N. (2016). GABA production and structure of gadB/gadC genes in lactobacillus and bifidobacterium strains from human microbiota. Anaerobe, 42, 197–204. doi:10.1016/j.anaerobe.2016.10.011
