ABSTRACT
Phytochemical profile and activity of anti-oxidative, anti-glycative, anti-α-amylase, anti-α-glucosidase, anti-lipase and anti-acetylcholinesterase (AchE) of aqueous extracts prepared from leaf and stem parts of two edible Amaranthus species, white and red amaranths, were investigated. Phytochemical analyses showed that white amaranth had more total phenolic acids than red one. The content of total flavonoids in four examined aqueous extracts was similar. Red amaranth has greater anthocyanins and carotenoids than white one. All aqueous extracts at three concentrations were used for in vitro bio-activities experiments. Results showed that four aqueous extracts displayed radical scavenging effect, Fe2+ chelating effect, xanthine oxidase inhibition, and reducing power. Aqueous extracts prepared from red amaranth exhibited marked anti-glycative effects, anti-α-amylase, anti-α-glucosidase, anti-lipase, and anti-AchE activities. These findings suggested that these two vegetables could be developed as functional foods for diabetic prevention and/or attenuation.
RESUMEN
En el presente estudio se investigaron el perfil fitoquímico y las actividades antioxidante, anti-glicativa, anti-α-amilasa, anti-α-glucosidasa, anti-lipasa y anti-acetilcolinesterasa (AchE) de extractos acuosos preparados a partir de hojas y tallos de dos especies de Amaranthus comestibles, el amaranto blanco y el rojo. Los análisis fitoquímicos dan cuenta de que el amaranto blanco contiene más ácidos fenólicos totales que el rojo, mientras que el amaranto rojo posee mayor cantidad de antocianinas y carotenoides que el blanco. En cuatro extractos acuosos examinados se constató que el contenido de flavonoides totales era similar. Se utilizaron todos los extractos acuosos a tres concentraciones para realizar experimentos de bioactividad in vitro. Los resultados indican que cuatro extractos acuosos presentaron efecto eliminador de radicales, efecto quelante de Fe2+, efecto inhibidor de la xantina oxidasa, así como poder reductor. Los extractos acuosos preparados a partir de amaranto rojo exhibieron marcados efectos anti-glicativos, anti-α-amilasa, anti-α-glucosidasa, anti-lipasa y anti-AchE. Estos hallazgos sugieren que ambos vegetales podrían desarrollarse como alimentos funcionales para la prevención y/o atenuación de la diabetes.
1. Introduction
Amaranth species are often used as vegetable crops in Asian and African countries including China, Bangladesh, India, Zimbabwe, and Taiwan. Maroyi (Citation2013) reported that many Amaranth species are crop plants with economic importance because of their nutritional and horticultural significance. In addition, some Amaranth species are valuable based on their ornamental or medicinal properties (Rastogi & Shukla, Citation2013). There are two edible Amaranthus species commonly consumed in Taiwan, one is locally called white amaranth (Amaranthus inamoenus Willd), and the other is called red amaranth (Amaranthus gangeticus L.) (). Both leaf and stem parts of these two vegetables are edible. The marked purple-red color appears in leaf and stem parts of red amaranth strongly imply that this plant food contains special phytochemicals like anthocyanins and carotenoids. In fact, less attention is paid to the difference in the phytochemical profile between these two amaranths. Thus, our present study aimed to analyze the content of total phenolic acids, flavonoids, anthocyanins, and carotenoids in aqueous extracts prepared from leaf and stem parts of these two amaranths.
Figure 1. Picture of whole part (upper pictures) and leaf part (lower picture) of white amaranths (Amaranthus inamoenus Willd.) and red amaranths (Amaranthus gangeticus.Linn).
Figura 1. Imagen de la parte completa (imágenes superiores) y la parte de la hoja (imagen inferior) de amarantos blancos (Amaranthus inamoenus Willd) y amarantos rojos (Amaranthus gangeticus.Linn)

Verma, Sisodia, and Bhatia (Citation2002) reported that alcoholic extract of red amaranth leaves protected mice brain against radiation. The study of Anilakumar, Khanum, Sudarshanakrishna, and Santhanam (Citation2004) revealed that the intake of dry red amaranth leaves improved dimethylhydrazine-induced hepatic oxidative injury in rats. Sani, Rahmat, Ismail, Rosli, and Endrini (Citation2004) indicated that the intake of red amaranth aqueous extract decreased the activity of tumor marker enzymes such as gamma-glutamyl transpeptidase, and alkaline phosphatase in rats. These previous studies suggested the medicinal values of red amaranth leaves. However, it is worthy to explore the nutritional benefits of red amaranth stem, white amaranth leaf, and white amaranth stem because these parts are also edible, at least in Taiwan. Therefore, several bio-functions such as anti-oxidative and anti-glycative activities, inhibitory effects against α-amylase, α-glucosidase, lipase, and acetylcholinesterase (AchE) of leaf and stem parts of white and red amaranths were investigated in our present study.
The in vitro anti-oxidative activities including free radical scavenging effect, ferrous ions (Fe2+) chelating effect and reducing power were examined. Glycative stress due to the overproduction of glycative products is an important pathological contributor for the progression of diabetes or Alzheimer’s disease (AD) because glycative products activate many signaling pathways and exacerbate oxidative and inflammatory injury (Rowan, Bejarano, & Taylor, Citation2018). α-Amylase and α-glucosidase are involved in glucose metabolism. Increased activity of these enzymes impairs glycemic control and promotes the development of diabetes (Tundis, Loizzo, & Menichini, Citation2010). The inhibition upon pancreatic lipase is a target for developing agent(s) to prevent or ameliorate obesity (Guo, Liu, Cai, Wang, & Ji, Citation2016) because this enzyme analyzes triglycerides into fatty acids and glycerol in the gastrointestinal tract. When the activity of this lipase is limited, triacylglycerol fails to pass cross the intestinal brush border membrane, and subsequently, the uptake of triacylglycerol into the human body is lowered. AchE is a major neurotransmitter responsible for synaptic transmission, and AchE is the key enzyme in charge of acetylcholine hydrolysis (McHardy, Wang, McCowen, & Valdez, Citation2017). The raised AchE activity in the brain decreases acetylcholine level, which is highly related to the pathogenesis of AD (Kračmarová, Drtinová, & Pohanka, Citation2015). Thus, any agent(s) with the abilities to attenuate oxidative and glycative stresses as well as decrease the activity of α-amylase, α-glucosidase, lipase, and AchE might be able to ameliorate the development of diabetes, obesity, and AD.
A. gangeticus L. is considered as a synonym of Amaranthus tricolor L. (Sarkar et al., Citation2009). However, A. tricolor was usually treated as a leafy vegetable (Kushwaha, Chawla, & Kochhar, Citation2014), and those authors used only the leaf part of A. tricolor for their studies. Thus, it is doubtful that A. gangeticus is identical as A. tricolor because the stem part of the former is edible. In our present study, the pictures of white and red amaranths are shown in order to clearly indicate which plant foods we used. Aqueous extract was used for all measurements because it is easily prepared, without chemical residue and high safety for future applications.
2. Materials and methods
2.1. Materials
Fresh white amaranth and red amaranth, harvested in summer, 2016, were purchased from farms in Nanton County, Taiwan. Leaf part and stem part were separately collected. There were four groups, leaf part of white amaranth (WL), stem part of white amaranth (WS), leaf part of red amaranth (RL) and stem part of red amaranth (RS), for experiments. Each part at 50 g was chopped, and mixed with 200 mL sterile distilled water. After homogenizing in a Waring blender, the homogenate was maintained at 25°C for 12 hr. After filtrating through a filter paper, the aqueous extract was collected and processed to powder by freeze-drying.
2.2. Analyses of phenolic acids, flavonoids, anthocyanins, and carotenoids
The levels of total phenolic acids and total flavonoids were analyzed by the methods of Sarker and Oba (Citation2018). The content of total phenolic acids was expressed as mg gallic acid equivalents/100 g dry weight, and the content of total flavonoids was expressed as mg quercetin equivalents/100 g dry weight. Total anthocyanins content was determined by a pH differential method, and expressed as mg cyanidin-3-glucoside equivalent/100 g dry weight (Huang & Lai, Citation2016). The content of total carotenoids was detected according to the method of Craft (Citation1992) via a UV-Vis spectrophotometer and expressed as mg lutein equivalents/100 g dry weight. The content of total phenolic acids and total anthocyanins was analyzed to standardize the used aqueous extracts for all measurements of nutritional benefits.
2.3. Assays of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and ferrous ions chelating effect
Aqueous extract at 0.25, 0.5 or 1 mg was mixed with 500 μL of 60 μM DPPH ethanolic solution and 500 μL distilled water. After keeping at room temperature for 30 min, the absorbance was measured via a microplate reader at 540 nm (Kitts, Wijewickreme, & Hu, Citation2000). Ascorbic acid was used as a positive control for DPPH scavenging activity. Data are shown as ascorbic acid equivalent (AAE)/g dry weight. The iron-chelating effect of aqueous extract was determined according to the method of Le, Chiu, and Ng (Citation2007). Aqueous extract at 0.25, 0.5 or 1 mg was mixed with a 2 mL solution containing 30 mM hexamine, 9 mM ferrous sulfate and 30 mM potassium chloride. After keeping at room temperature for 10 min, the sample was mixed with 200 μL of 1 mM tetramethyl murexide. Control groups had no test extract. After further keeping for 3 min at 25°C, the absorbance at 485 nm was recorded. EDTA at 1 mg was used to compare the iron-chelating capability of test aqueous extract. Results were shown as % of EDTA.
2.4. Assays of xanthine oxidase (XO) inhibition and reducing power
Aqueous extract at 0.25, 0.5 or 1 mg was mixed with 1 mL distilled water. Control groups were distilled water only. One-milliliter sample was further mixed with 1 mL solution containing 100 μM xanthine and 0.4 U/mL XO. After keeping at room temperature for 5 min, the generation of uric acid was assayed by measuring the variation of absorbance at 295 nm. The XO activity inhibition (%) was calculated by the formula (Acontrol – Asample)/Acontrol x 100. Reducing power was assayed via the method of Hue, Boyce, and Somasundram (Citation2012). Aqueous extract at 0.25, 0.5 or 1 mg was mixed with a solution consisting of 1 mL of 1% potassium ferricyanide and 1 mL of 200 mM PBS (pH 6.6). After incubating for 20 min at 50°C, the sample was further mixed with 1 mL of 10% TCA and followed by centrifugation for 10 min at 650x g. The supernatant was collected and mixed with 1 mL of 0.1% ferric chloride and 1 mL of deionized water. The absorbance (Abs) at 700 nm was recorded and reported.
2.5. Evaluation of anti-glycative effect
The anti-glycative effect of aqueous extract was evaluated by using the BSA-fructose assay according to the method reported in Ma et al. (Citation2015). Aqueous extract at 0.25, 0.5, or 1 mg was added into 2 mL sterilized distilled water, and followed by mixing with glycation reaction mixture consisted of 100 mM D-fructose and 10 mg/mL BSA. After incubated for 21 days at 37°C, intrinsic fluorescence was measured at 360 nm and 435 nm for excitation and emission, respectively. Aminoguanidine (AG), an anti-glycative agent, at 50 μg/mL, was used as the positive control.
2.6. Measurement of α-amylase and α-glucosidase inhibition
The inhibitory effects of aqueous extract upon α-amylase and α-glucosidase activities were measured according to the method of Kazeem, Adamson, and Ogunwande (Citation2013). For α-amylase inhibition, aqueous extract at 0.25, 0.5, or 1 mg was added into 2 mL PBS (20 mM, pH 6.9). Subsequently, the sample was further mixed with 2 mL α-amylase (0.5 mg/mL) and followed by incubating for 30 min at room temperature. Then, 2 mL of 1% soluble potato starch was added as substrate and followed by incubating for another 10 min. The reaction was terminated by adding 2 mL of 1% dinitrosalicylic acid. After heating for 5 min at a boiling water bath, an orange red color was developed. For α-glucosidase inhibition, all steps were similar, but α-amylase was replaced by α-glucosidase, and 2 mL of 3 mM p-nitrophenyl glucopyranoside was used as substrate. After incubating for 20 min at 37°C, the reaction was terminated by adding 2 mL of 100 M Na2CO3. The absorbance was read at 550 and 405 nm, respectively, for measuring α-amylase and α-glucosidase activities. PBS buffer at 2 mL was used as controls, and a sample without adding enzyme was used to determine the basal level of reducing sugar presented in the sample. The % inhibition of α-amylase or α-glucosidase was calculated as follows (Acontrol – Asample)/Acontrol x 100.
2.7. Assay of lipase inhibition
The inhibitory effect of aqueous extract against lipase was assayed according to a method described in de Camargo, Regitano-d’Arce, Biasoto, and Shahidi (Citation2016). Lipase could convert p-nitrophenyl butyrate (p-NPB) to p-nitro-phenol and produce a yellow color, which could be monitored in order to quantify the extent of lipase activity. p-NPB at 5 mM was prepared in DMSO. A solution of type 2 porcine pancreatic lipase was prepared at 5 mg/mL in distilled water, and followed by centrifugation at 10,000x g for 5 min. The supernatant was used as an enzyme solution. Each extract at 0.25, 0.5 or 1 mg was dissolved in 1 mL 10% DMSO and further mixed with 50 μL enzyme solution and 500 μL Tris-HCl buffer (pH 7.4). After incubation for 15 min at 37°C, 30 μL p-NPB was added and followed by further incubation for 25 min at 37°C. After the sample was cooled for 2 min, the absorbance was read by using a microplate reader. Orlistat at 1 mg was used as the positive control. Inhibition % was presented as a percentage of Orlistat.
2.8. Determination of ache inhibition
The inhibitory effect of aqueous extract upon AchE activity was determined by an acetylcholinesterase assay kit (BioVision, Milpitas, CA, USA). Aqueous extract at 0.25, 0.5, or 1 mg was added into 2 mL PBS (20 mM, pH 6.9). Then, 100 µL sample and 100 μL assay buffer were mixed, followed by adding 100 µL of the reaction mixture. Donepezil hydrochloride (DH) at 1 mg was used as a positive control for AchE inhibitory activity. After incubation for 20 min at 37°C, the absorbance was measured by using a microplate reader at 570 nm (PowerWave XS; Bio-Tek Instruments, Winooski, VT, USA). Inhibition % of AchE activity was calculated as follows (Acontrol – Asample)/Acontrol × 100.
2.9. Statistical analyses
Each value was obtained from eight different preparations (n = 8). Data were reported as means ± standard deviation (SD). Statistical analyses were handled by one-way analysis of variance, and processed by using SAS (SAS Institute, Cary, NC, USA). Least Significance Difference Test was performed to determine the differences among means. It was considered as significant when P < 0.05.
3. Results
3.1. Phytochemical profiles
As shown in , white amaranth had more total phenolic acids than red one (P < 0.05). The content of total flavonoids in four examined aqueous extracts was similar (P > 0.05). The content of total anthocyanins followed the order, RL >RS >WL = WS (P < 0.05). The content of total carotenoids followed this order: RL>RS>WS = WL (P < 0.05).
Table 1. Content (mg/100 g dry weight) of total phenolic acids, total flavonoids, total anthocyanins, total carotenoids in aqueous extracts prepared from leaf part of white amaranth (WL), stem part of white amaranth (WS), leaf part of red amaranth (RL) and stem part of red amaranth (RS). Data were expressed as mean ± SD (n = 8).
Tabla 1. Contenido (mg/100 g de peso seco) de ácidos fenólicos totales, flavonoides totales, antocianinas totales, carotenoides totales en extractos acuosos preparados a partir de la hoja de amaranto blanco (WL), el tallo de amaranto blanco (WS), la hoja de amaranto rojo (RL) y el tallo de amaranto rojo (RS). Los datos se expresan como media ± DE (n = 8)
3.2. Anti-oxidative and anti-glycative effects
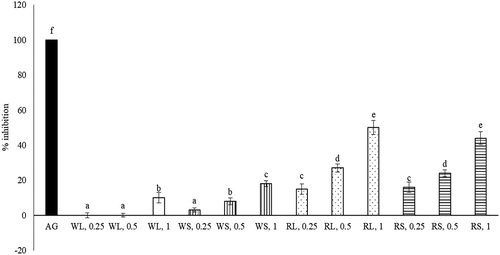
As shown in , each aqueous extract at test doses displayed DPPH scavenging effect, Fe2+ chelating effect, XO inhibition, and reducing power (P < 0.05), in which dose-dependent manner was presented in scavenging effect, Fe2+ chelating effect and reducing power (P < 0.05). When each aqueous extract at 1 mg was used, RL exhibited greater activities in scavenging DPPH, chelating Fe2+, inhibiting XO and reducing power than other aqueous extracts (P < 0.05). As shown in , the anti-glycative effects of aqueous extracts prepared from RL and RS dose-dependently increased (P < 0.05), in which aqueous extracts at 0.5 mg were greater than 20%, and at 1 mg were greater than 40% when compared with AG (P < 0.05).
Table 2. Anti-oxidative activities of aqueous extracts prepared from leaf part of white amaranth (WL), stem part of white amaranth (WS), leaf part of red amaranth (RL) and stem part of red amaranth (RS). Data were expressed as mean ± SD (n = 8).
Tabla 2. Actividades antioxidantes de extractos acuosos preparados a partir de la hoja de amaranto blanco (WL), el tallo de amaranto blanco (WS), la hoja de amaranto rojo (RL) y del tallo de amaranto rojo (RS). Los datos se expresan como media ± DE (n = 8)
Figure 2. Anti-glycative effects of aqueous extracts prepared from leaf part of white amaranth (WL), stem part of white amaranth (WS), leaf part of red amaranth (RL) and stem part of red amaranth (RS). Aminoguanidine (AG) at 50 μg/ml was used for comparison. Data were expressed as mean ± SD (n = 8). a–fValues among bars without a common letter differ, P < 0.05.
Figura 2. Efectos anti-glicativos de los extractos acuosos preparados a partir de la hoja de amaranto blanco (WL), el tallo de amaranto blanco (WS), la hoja de amaranto rojo (RL) y el tallo de amaranto rojo (RS). Para fines de comparación, se usó aminoguanidina (AG) a 50 μg/ml. Los datos se expresaron como media ± DE (n = 8). a–fLos valores entre las barras sin una letra en común difieren, P < 0,05
3.3. Inhibitory effects upon α-amylase, α-glucosidase, lipase, and ache
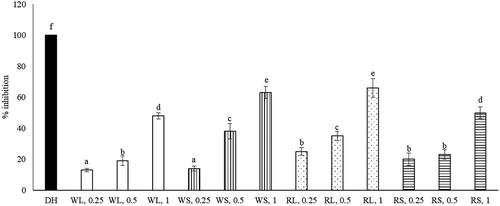
The inhibitory effects of WL, WS, RL, and RS upon α-amylase and α-glucosidase activities dose-dependently increased from 0.25 to 1 mg (, P < 0.05). When 1 mg was tested and compared, the anti-α-amylase activity followed the order: RL >RS = WS >WL (P < 0.05); and the anti-α-glucosidase activity followed the order: RL >RS = WL >WS (P < 0.05). The inhibitory effects of each extract upon pancreatic lipase activity dose-dependently raised from 0.25 to 1 mg (, P < 0.05). When 1 mg was tested and compared, the anti-lipase activity was 20-68% of ORL and followed the order: RL = RS >WS > WL (P < 0.05). As shown in , the anti-AchE effects of four test aqueous extracts at 3 doses were 18-66% of DH. Each test aqueous extract increased its anti-AchE activity from 0.25 to 1 mg (P < 0.05). When 1 mg was tested, the anti-AchE activity followed the order: RL = WS > RS = WL (P < 0.05).
Figure 3. Inhibitory effects of aqueous extracts prepared from leaf part of white amaranth (WL), stem part of white amaranth (WS), leaf part of red amaranth (RL) and stem part of red amaranth (RS) on α-amylase and α-glucosidase. Data were expressed as mean ± SD (n = 8). a–fValues among bars without a common letter differ, P < 0.05.
Figura 3. Efectos inhibidores de extractos acuosos preparados a partir de la hoja de amaranto blanco (WL), el tallo de amaranto blanco (WS), la hoja de amaranto rojo (RL) y el tallo de amaranto rojo (RS) en α-amilasa y α-glucosidasa. Los datos se expresan como media ± DE (n = 8). a–fLos valores entre las barras sin una letra en común difieren, P < 0,05
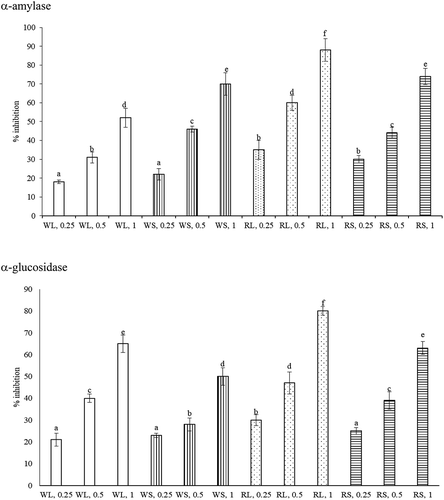
Figure 4. Anti-lipase effects of aqueous extracts prepared from leaf part of white amaranth (WL), stem part of white amaranth (WS), leaf part of red amaranth (RL) and stem part of red amaranth (RS). Orlistat (ORL) at 1 mg was used for comparison. Data were expressed as mean ± SD (n = 8). a–gValues among bars without a common letter differ, P < 0.05.
Figura 4. Efectos anti-lipasa de extractos acuosos preparados a partir de la hoja de amaranto blanco (WL), el tallo de amaranto blanco (WS), la hoja de amaranto rojo (RL) y el tallo de amaranto rojo (RS). Para efectos de comparación, se usó orlistat (ORL) a 1 mg. Los datos se expresaron como media ± DE (n = 8). a–gLos valores entre las barras sin una letra en común difieren, P < 0,05
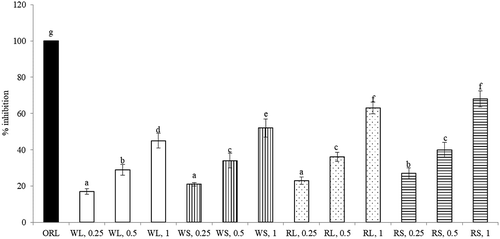
Figure 5. Anti-AchE effects of aqueous extracts prepared from leaf part of white amaranth (WL), stem part of white amaranth (WS), leaf part of red amaranth (RL) and stem part of red amaranth (RS). Donepezil hydrochloride (DH) at 1 mg was used for comparison. Data were expressed as mean ± SD (n = 8). a–fValues among bars without a common letter differ, P < 0.05.
Figura 5. Efectos anti-AchE de extractos acuosos preparados a partir de la hoja de amaranto blanco (WL), el tallo de amaranto blanco (WS), la hoja de amaranto rojo (RL) y el tallo de amaranto rojo (RS). Para fines de comparación, se usó hidrocloruro de donepezilo (DH) a 1 mg. Los datos se expresan como media ± DE (n = 8). a–fLos valores entre las barras sin una letra en común difieren, P < 0,05
4. Discussion
Both white and red amaranthus are popular vegetables consumed in Asian countries. The results of our present study revealed that stem and leaf parts of these two plant foods were rich in phytochemicals including phenolic acids, flavonoids, anthocyanins, and carotenoids. Furthermore, these aqueous extracts displayed anti-oxidative, anti-diabetic, anti-AchE and anti-glycative activities. These observed bio-activities might be due to the presence of certain component compounds or the interactions between multiple component compounds. These novel findings suggest that these two vegetables through their phytochemical ingredients could be applied to prevent or attenuate diseases like diabetes and AD. Further in vivo studies are necessary to verify these anti-diabetic and anti-AD effects of these two vegetables.
It has been documented that dietary phenolic acids and flavonoids including gallic acid, ellagic acid, quercetin, or rutin could provide many health benefits (Adefegha, Citation2018). The prevention of these two groups of phytochemicals upon hypertension, diabetes, and cancers has attracted a lot of attention (Huang, Davidge, & Wu, Citation2013; Kalita, Holm, LaBarbera, Petrash, & Jayanty, Citation2018). Our data indicated that both white and red amaranthus contained phenolic acids and flavonoids. These results suggest that these two vegetables are good food sources of these two groups of phytochemicals. The detected anthocyanins in stem and leaf parts of red amaranthus explained its purple-red color. It is indicated that anthocyanins such as malvidin and petunidin offered cardioprotective effects by promoting the defensive activities of endogenous anti-oxidative elements (Wei et al., Citation2017), and ameliorate photooxidation of autofluorescent pigments in retinal pigment epithelial cells by quenching singlet oxygen (Jang, Zhou, Nakanishi, & Sparrow, Citation2005). Since red amaranthus was rich in anthocyanins, the intake of this vegetable might increase the bio-available anthocyanins, and further protect heart and eyes. On the other hand, a clinical study revealed that supplements of carotenoids such as astaxanthin or lutein improved glycemic control and blood pressure management in diabetic patients (Mashhadi et al., Citation2018), and protected the brain against ischemia/reperfusion injury (Li et al., Citation2012). Because red amaranthus contained substantial level of carotenoids, the consumption of this vegetable might increase the available carotenoids in circulation and organs, which in turn benefits diabetic management.
We found that four aqueous extracts exhibited non-enzymatic anti-oxidative activities including free radical scavenging effects and reducing power. Based on the detectable phenolic acids, flavonoids, anthocyanins, and carotenoids in leaf and stem parts of both vegetables, it seems reasonable to observe the anti-oxidative effects of these aqueous extracts because the anti-oxidative activities of some phytochemicals are already well known. Glycation favors the development of diabetes and AD because the glycative products promote oxidative and inflammatory reactions in circulation and organs, which in turn facilitate the deterioration of diabetes and AD (Rowan et al., Citation2018). We found that leaf and stem parts of red amaranthus had more anthocyanins and carotenoids than white amaranthus, and displayed marked anti-glycative activity. In fact, the anti-glycative effects of pelargonidin, an anthocyanin, and astaxanthin, a carotenoid, have been reported (Roy, Sen, & Chakraborti, Citation2008; Sun et al., Citation2011). Thus, it is possible that the observed anti-glycative effects of red amaranthus were due to the presence of anthocyanins and carotenoids. These results suggest that these aqueous extracts via suppressing oxidative and glycative response could prevent or ameliorate oxidative and glycative stress-associated diseases such as diabetes and AD.
α-Amylase catalyzes long-chain carbohydrates to small carbohydrates. α-Glucosidase presented in small intestine catalyzes carbohydrate to glucose. The inhibition upon these two enzymes may decelerate the production of postprandial blood glucose, which in turn ameliorates hyperglycemia. We found that four test aqueous extracts could limit the activity of both α-amylase and α-glucosidase, in which leaf part of red amaranthus displayed the greatest inhibitory effects upon these two enzymes. These findings implied that these two vegetables might benefit glycemic control by suppressing the activity of α-amylase and α-glucosidase. It has been reported that polyphenolic compounds and flavonoids could inhibit the activity of these two enzymes (Kalita et al., Citation2018; Li et al., Citation2018). In addition, Różańska and Regulska-Ilow (Citation2018) reported that anthocyanins could improve insulin secretion and decrease insulin resistance. Since four aqueous extracts tested in our present study also contained these phytochemicals, the observed anti-diabetic potent of these aqueous extracts could be partially explained. Pancreatic lipase is responsible for catabolizing exogenous and endogenous lipids to fatty acids and glycerol, which subsequently pass through intestinal brush border and increase the available lipids for the human body. The limitation upon the activity of this enzyme may slow down intestinal lipid catabolism and decrease lipid availability, and contribute to alleviate obesity and diabetes (Buchholz & Melzig, Citation2016). Our data indicated that the aqueous extracts from both white and red amaranthus had substantially inhibitory effects upon lipase activity when compared with orlistat. These data indicated that both white and red amaranthus could attenuate hyperlipidemia or steatosis via restricting lipase activity. AchE hydrolyzes acetylcholine, a neurotransmitter, to choline and acetic acid. The increased AchE activity in the brain leads to decrease available acetylcholine for brain functions, and promotes AD pathogenesis. The inhibitory effects of phenolic acids and flavonoids upon AchE activity have been reported (Choi et al., Citation2016; Szwajgier, Baranowska-Wojcik, & Borowiec, Citation2018). Since the aqueous extracts from both white and red amaranthus had substantial levels of phenolic acids and flavonoids, it seems reasonable to observe their suppression on AchE activity.
In conclusion, white and red amaranthus were rich in phytochemicals including phenolic acids, flavonoids, anthocyanins, and carotenoids. The aqueous extracts prepared from leaf and stem parts of these two amaranthus exhibited several anti-diabetic and anti-AchE potentials. These findings suggested that these two vegetables could be developed as functional foods for diabetic prevention and/or attenuation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adefegha, S. A. (2018). Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. Journal of Dietary Supplements, 15, 977–1009. doi:10.1080/19390211.2017.1401573
- Anilakumar, K. R., Khanum, F., Sudarshanakrishna, K. R., & Santhanam, K. (2004). Effect of amaranth leaves on dimethylhydrazine-induced changes in multicomponent antioxidant system of rat liver. Indian Journal of Experimental Biology, 42, 595–600.
- Buchholz, T., & Melzig, M. F. (2016). Medicinal plants traditionally used for treatment of obesity and diabetes mellitus - screening for pancreatic lipase and α-amylase inhibition. Phytotherapy Research, 30, 260–266. doi:10.1002/ptr.v30.2
- Choi, J. S., Bhakta, H. K., Fujii, H., Min, B. S., Park, C. H., Yokozawa, T., & Jung, H. A. (2016). Inhibitory evaluation of oligonol on α-glucosidase, protein tyrosine phosphatase 1B, cholinesterase, and β-secretase 1 related to diabetes and Alzheimer’s disease. Archives of Pharmacal Research, 39, 409–420. doi:10.1007/s12272-015-0682-8
- Craft, N. E. (1992). Relative solubility, stability, and absorptivity of lutein and β-carotene in organic solvents. Journal of Agricultural and Food Chemistry, 40, 431–434. doi:10.1021/jf00015a013
- de Camargo, A. C., Regitano-d’Arce, M. A., Biasoto, A. C., & Shahidi, F. (2016). Enzyme-assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chemistry, 212, 395–402. doi:10.1016/j.foodchem.2016.05.047
- Guo, X., Liu, J., Cai, S., Wang, O., & Ji, B. (2016). Synergistic interactions of apigenin, naringin, quercetin and emodin on inhibition of 3T3-L1 preadipocyte differentiation and pancreas lipase activity. Obesity Research & Clinical Practice, 10, 327–339. doi:10.1016/j.orcp.2015.08.004
- Huang, W. Y., Davidge, S. T., & Wu, J. (2013). Bioactive natural constituents from food sources-potential use in hypertension prevention and treatment. Critical Reviews in Food Science and Nutrition, 53, 615–630. doi:10.1080/10408398.2010.550071
- Huang, Y. P., & Lai, H. M. (2016). Bioactive compounds and antioxidative activity of colored rice bran. Journal of Food and Drug Analysis, 24, 564–574. doi:10.1016/j.jfda.2016.01.004
- Hue, S. M., Boyce, A. N., & Somasundram, C. (2012). Antioxidant activity, phenolic and flavonoid contents in the leaves of different varieties of sweet potato (Ipomoea batatas). Australian Journal of Crop Science, 6, 375–380.
- Jang, Y. P., Zhou, J., Nakanishi, K., & Sparrow, J. R. (2005). Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochemistry and Photobiology, 81, 529–536. doi:10.1562/2004-12-14-RA-402.1
- Kalita, D., Holm, D. G., LaBarbera, D. V., Petrash, J. M., & Jayanty, S. S. (2018). Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PloS One, 13, e0191025. doi:10.1371/journal.pone.0191025
- Kazeem, M. I., Adamson, J. O., & Ogunwande, I. A. (2013). Modes of inhibition of alphaamylase and alpha -glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Research International, 2013, 527570. doi:10.1155/2013/527570
- Kitts, D. D., Wijewickreme, A. N., & Hu, C. (2000). Antioxidant properties of north American ginseng extract. Molecular and Cellular Biology, 203, 1e10.
- Kračmarová, A., Drtinová, L., & Pohanka, M. (2015). Possibility of acetylcholinesterase overexpression in Alzheimer disease patients after therapy with acetylcholinesterase inhibitors. Acta Medica (Hradec Kralove) / Universitas Carolina, Facultas Medica Hradec Kralove, 58, 37–42. doi:10.14712/18059694.2015.91
- Kushwaha, S., Chawla, P., & Kochhar, A. (2014). Effect of supplementation of drumstick (Moringa oleifera) and amaranth (Amaranthus tricolor) leaves powder on antioxidant profile and oxidative status among postmenopausal women. Journal of Food Science and Technology, 51, 3464–3469. doi:10.1007/s13197-012-0859-9
- Le, K., Chiu, F., & Ng, K. (2007). Identification and quantification of antioxidants in Fructus lycii. Food Chemistry, 105, 353–363. doi:10.1016/j.foodchem.2006.11.063
- Li, K., Yao, F., Xue, Q., Fan, H., Yang, L., Li, X., … Liu, Y. (2018). Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure-activity relationship of its eight flavonoids by a refined assign-score method. Chemistry Central Journal, 12, 82. doi:10.1186/s13065-018-0445-y
- Li, S. Y., Yang, D., Fu, Z. J., Woo, T., Wong, D., & Lo, A. C. (2012). Lutein enhances survival and reduces neuronal damage in a mouse model of ischemic stroke. Neurobiology Disease, 45, 624–632. doi:10.1016/j.nbd.2011.10.008
- Ma, H., Liu, W., Frost, L., Wang, L., Kong, L., Dain, J. A., & Seeram, N. P. (2015). The hydrolysable gallotannin, penta-O-galloyl-b-D-glucopyranoside, inhibits the formation of advanced glycation endproducts by protecting protein structure. Molecular bioSystems, 11, 1338e1347. doi:10.1039/C4MB00722K
- Maroyi, A. (2013). Use of weeds as traditional vegetables in Shurugwi District, Zimbabwe. Journal of Ethnobiology and Ethnomedicine, 9, 60. doi:10.1186/1746-4269-9-60
- Mashhadi, N. S., Zakerkish, M., Mohammadiasl, J., Zarei, M., Mohammadshahi, M., & Haghighizadeh, M. H. (2018). Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pacific Journal of Clinical Nutrition, 27, 341–346. doi:10.6133/apjcn.052017.11
- McHardy, S. F., Wang, H. L., McCowen, S. V., & Valdez, M. C. (2017). Recent advances in acetylcholinesterase inhibitors and reactivators: An update on the patent literature (2012-2015). Expert Opinion on Therapeutic Patents, 27, 455–476. doi:10.1080/13543776.2017.1272571
- Rastogi, A., & Shukla, S. (2013). Amaranth: A new millennium crop of nutraceutical values. Critical Reviews in Food Science and Nutrition, 53, 109–125. doi:10.1080/10408398.2010.517876
- Rowan, S., Bejarano, E., & Taylor, A. (2018). Mechanistic targeting of advanced glycation end-products in age-related diseases. Biochimica Et Biophysica Acta-Molecular Basis of Disease, 1864, 3631–3643. doi:10.1016/j.bbadis.2018.08.036
- Roy, M., Sen, S., & Chakraborti, A. S. (2008). Action of pelargonidin on hyperglycemia and oxidative damage in diabetic rats: Implication for glycation-induced hemoglobin modification. Life Science, 82, 1102–1110. doi:10.1016/j.lfs.2008.03.011
- Różańska, D., & Regulska-Ilow, B. (2018). The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Advances in Clinical and Experimental Medicine, 27, 135–142. doi:10.17219/acem/64983
- Sani, H. A., Rahmat, A., Ismail, M., Rosli, R., & Endrini, S. (2004). Potential anticancer effect of red spinach (Amaranthus gangeticus) extract. Asia Pacific Journal of Clinical Nutrition, 13, 396–400.
- Sarkar, R., Nandan, C. K., Mandal, S., Patra, P., Das, D., & Islam, S. S. (2009). Structural characterization of a heteropolysaccharide isolated from hot water extract of the stems of Amaranthus tricolor Linn. (Amaranthus gangeticus L.). Carbohydrate Research, 344, 2412–2416. doi:10.1016/j.carres.2009.09.014
- Sarker, U., & Oba, S. (2018). Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under salinity stress. Scientific Report, 8, 12349. doi:10.1038/s41598-018-30897-6
- Sun, Z., Liu, J., Zeng, X., Huangfu, J., Jiang, Y., Wang, M., & Chen, F. (2011). Astaxanthin is responsible for antiglycoxidative properties of microalga Chlorella zofingiensis. Food Chemistry, 126, 1629–1635. doi:10.1016/j.foodchem.2010.12.043
- Szwajgier, D., Baranowska-Wojcik, E., & Borowiec, K. (2018). Phenolic acids exert anticholinesterase and cognition-improving effects. Current Alzheimer Research, 15, 531–543. doi:10.2174/1567205014666171128102557
- Tundis, R., Loizzo, M. R., & Menichini, F. (2010). Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini-Reviews in Medicinal Chemistry, 10, 315–331. doi:10.2174/138955710791331007
- Verma, R. K., Sisodia, R., & Bhatia, A. L. (2002). Radioprotective role of Amaranthus gangeticus Linn.: A biochemical study on mouse brain. Journal of Medicinal Food, 5, 189–195. doi:10.1089/109662002763003339
- Wei, H., Li, H., Wan, S. P., Zeng, Q. T., Cheng, L. X., Jiang, L. L., & Peng, Y. D. (2017). Cardioprotective effects of malvidin against isoproterenol-induced myocardial infarction in rats: A mechanistic study. Medical Science Monitor, 23, 2007–2016. doi:10.12659/MSM.902196
