 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The crude polysaccharides obtained by different extraction methods have different uses in food and cosmetics, mainly due to the significant differences in physicochemical and rheological properties. Crude Tremella fuciformis polysaccharides (TPs) were extracted by five different methods: hot-water extraction (TP1), high-pressure water extraction (TP2), alkali extraction (TP3), acid extraction (TP4) and enzyme-assisted extraction (TP5). Their extraction yield, chemical composition, molecular weight, micromorphological structure and rheological properties were investigated and compared. They all were acid heteropolysaccharides with mannose as the main chain. Five TPs had similar dynamic viscoelasticity, exhibited viscous characteristics at low vibration frequencies and elastic characteristics at higher vibration frequencies. TP3, TP4 and TP5 solutions are not easily deformed and are suitable for applying in the products that require shape retention. This paper provides some theoretical data support for the scientific research of crude Tremella fuciformis polysaccharide and its application in food, medicine and cosmetics.
RESUMEN
Los polisacáridos crudos obtenidos utilizando diferentes métodos de extracción tienen diferentes usos en la alimentación y la cosmética, lo cual responde, principalmente, a las importantes diferencias en sus propiedades fisicoquímicas y reológicas. Se extrajeron polisacáridos crudos de Tremella fuciformis (TPs) empleando cinco métodos diferentes: extracción con agua caliente (TP1), extracción con agua a alta presión (TP2), extracción con alcalinos (TP3), extracción con ácidos (TP4) y extracción asistida por enzimas (TP5). Se investigó y se comparó rendimiento de extracción, composición química, peso molecular, estructura micromorfológica y propiedades reológicas. Todos los polisacáridos extraídos eran ácidos heteropolisacáridos con manosa como cadena principal. Se constató que cinco TP poseen una viscoelasticidad dinámica similar, presentan características viscosas a bajas frecuencias de vibración y características elásticas a frecuencias de vibración más altas. Se observó que las soluciones TP3, TP4 y TP5 no se deforman fácilmente y son adecuadas para ser aplicadas a productos que requieren retener su forma. El presente estudio proporciona algunos datos teóricos que apoyan la investigación científica sobre el polisacárido crudo de Tremella fuciformis y su aplicación en la alimentación, la medicina y la cosmética.
1. Introduction
Tremella fuciformis, belonging to the order of the Tremellales and Tremellaceae family (Zhang & Wang, Citation2016), has been regarded as a precious tonic in China. T. fuciformis is a nutritional food low in energy and fats but rich in various nutrients including polysaccharides, proteins, dietary fiber (Wu et al., Citation2019), potassium and some other trace minerals. Tremella fuciformis contains acidic, neutral and basic heteropolysaccharides being acidic heteropolysaccharide as the main one. So far, many studies have shown that Tremella fuciformis polysaccharides have various biological activities such as lowering blood glucose, improving immunity (Wang et al., Citation2015), promoting wound healing (Zhang et al., Citation2015), anti-aging and anti-tumor (Chen, Citation2010).
The molecular weight, physicochemical and rheological properties of polysaccharides are the main factors affecting their processing application in food. Molecular weight is an important factor influencing the physicochemical and processing properties of polymers. The rheological properties of polysaccharides can reflect their structure, physical properties, and directly affect the stirring, mixing, molding and other processes and the quality of the final products (Fischer et al., Citation2009). The dynamic rheological properties of Tremella fuciformis polysaccharide solution are still very limited, and the gel properties are rarely reported. Few studies have systematically studied the physicochemical and rheological properties of Tremella fuciformis polysaccharide obtained by different extraction methods. Based on this, five crude Tremella fuciformis polysaccharides (TPs) were obtained using different extraction methods, and their related properties were systemically investigated. This paper provide a theoretical basis for their application in food, medicine and cosmetics industries.
2. Materials and methods
2.1. Materials
Tremella fuciformis powder was obtained by grinding commercially dry Tremella fuciformis which was further dehydrated at 70°C for 2 h and then passed through a 60 mesh sieve.
10,000 U/g and papain 80,000 U/g were purchased from Shandong Longcotase Preparation Co. LTD. Sodium hydroxide, citric acid, anhydrous ethanol, potassium bromide, iodine, potassium iodide and sucrose are all pure analytical reagents made in China.
2.2. Preparation of crude TPs
Five crude TPs were extracted from Tremella fuciformis by hot water extraction (30 g tremella powder was mixed with 2400 g deionized water and kept in a constant temperature water bath at 95°C for 4 h; Duan & Wang, Citation2016), high-pressure water extraction (30 g tremella powder was mixed with 2400 g deionized water and kept in a pressure cooker at 121°C for 1 h; Tao et al., Citation2015), alkali extraction (30 g tremella powder was mixed with 2400 g NaOH solution with pH 9.0 and kept in a water bath at 95°C for 4 h; Lai et al., Citation2010), acid extraction (30 g tremella powder was mixed with 2400 g citric acid solution with pH 2.0 and kept in a water bath at 95°C for 100 min.) and enzyme-assisted extraction (30 g tremella powder was mixed with 2400 g deionized water, 0.3 g cellulase and 0.3 g papain, adjusting the pH to 6, then kept in a constant temperature culture oscillator (100 r/min) at 50°C for 1 h, after enzyme inactivation, it was kept in a water bath at 95°C for 1 h; Bao, Citation2015), respectively, followed by a centrifugation at 2900 g for 15 min, thus resulting in the extracts of TP1, TP2, TP3, TP4 and TP5.
Each extract was concentrated to 1/3 of its original volume in a vacuum at 60°C (Chen et al., Citation2018). The concentrate obtained was mixed with 95% (v/v) ethanol solution of 4 times its volume and stirred continuously until flocculent precipitation formed. The resulting mixture was kept at 4°C for 24 h, and it was then centrifuged at 2900 g for 15 min. The precipitate obtained was rinsed with an appropriate amount of anhydrous ethanol 3–4 times and then freeze-dried to a constant weight, thus five crude TPs samples were obtained. Each sample was weighed, and its yield was calculated according to the following formula:
Where, m is the mass of dried crude polysaccharide (g), and M is the mass of Tremella fuciformis powder (g).
2.3. Physicochemical properties of crude TPs
2.3.1. Determination of total sugar, protein, moisture and ash contents
The total sugar content of TPs was determined using phenol-sulfuric acid method (W. H. Zhang et al., Citation2020). The Bradford assay was adopted to calculate the amount of protein where bovine serum albumin was a standard reagent (Bradford, Citation1976). Ash and moisture determinations were done based on the method reported by the Association of Official Analytical Chemists in 2000.
2.3.2. Determination of molecular weights of TPs
The molecular weights(MP) of TPs were determined using a Gel permeation chromatography (GPC) instrument (Waters Breeze GPC, Waters, USA) according to the previous method (Geng et al., Citation2009). The chromatographic conditions were set as follows, the chromatographic column of Ultrahyrogel Linear (7.8 mm×300 mm), 2400 differential refractive index detector, a mobile phase of 0.2 M NaNO3 solution, a flow rate of 0.6 mL/min, column temperature of 40°C. GPC software was used to fit and calculate the weight average molecular weight, number average molecular weight and polydispersion coefficient of each crude TPs sample.
2.3.3. UV and FT-IR analyses of crude TPs
The Ultraviolet spectra of each crude TPs solutions at 1.0 mg/mL was scanned on a UV-vis spectrophotometer (UV-1600, Meipuda, Shanghai) in the range of 200–400 nm. The proteins and nucleic acids were analyzed by examining whether absorption peaks appeared at 260 nm and 280 nm.
The mixture of 4 mL of each crude TPs solution (1 mg/mL) and 2.4 mL of iodine reagent (0.2% KI solution containing 0.02% I2) was scanned on a UV-vis spectrophotometer in the range of 300–700 nm to analyze the branches and side chains of crude TPs.
The FT-IR spectra of TPs were recorded by the Nicolet 6700 FT-IR spectrometer (Thermo Electron, Madison, WI, US; Jiang et al., Citation2013). Briefly, 2 mg of TPs samples were mixed with 200 mg KBr powder separately, and then compressed into tablets for FT-IR measurement in the frequency range of 4000–400 cm−1.
2.3.4. Micromorphology analysis of crude TPs
TPs solution of 50 μg/mL (5 μL) was taken onto a mica sheet until it was naturally dried. Then, it was scanned by an atomic force microscope (AFM, SPM-9600, Shimadzu, Japan) at room temperature. Tapping mode was used and the scanning frequency was 1 Hz per line.
2.4. Rheological properties of TPs solutions
2.4.1. Detection of linear viscoelastic region
The changes of storage modulus (G’) and loss modulus (G′’) of 0.5% TPs as a function of strain were measured by a rheometer (AR 2000-EX, TA, USA) equipped with a parallel plate geometry of 20 mm diameter (plate space 0.1 mm). The linear viscoelastic region was determined to 2% of strain through an amplitude sweep at 25°C and a constant shear rate (50 s-1). The measurement was performed with the angular frequency that increased from 0.1 rad/s to 100 rad/s at 30°C.
2.4.2. Rheological measurements
The static rheological properties of TPs in distilled water were measured by a rheometer equipped with a 20 mm parallel plate at 30°C. The shear rate range was from 0 to 600 s-1 (7 points for each order of magnitude). The dynamic viscoelastic behaviors of TPs solutions were measured under the angular frequency range from 0.1 to 100 rad/s.
The thixotropy of 1% TPs solution was determined at 30°C. A two-step steady-state flow program was adopted to measure the change of shear stress of TPs solution in the process when the shear rate first increased from 0.1 to 600 s-1, and then returned from 600 to 0.1 s-1at the same rate.
2.5. Statistical analysis
The results are shown as mean ± standard deviation (mean ± SD) based on three separate experiments. SPSS 17.0 was used for data statistics and significance difference analysis, and Origin 8.0 was used for mapping.
3. Results and discussion
3.1. Physicochemical properties of crude TPs
3.1.1. Major chemical compositions of crude TPs
The physicochemical and processing characteristics of a polysaccharide are greatly affected by its chemical composition. For example, the thickening (Cho & Yoo, Citation2015), gelation (Florina et al., Citation2015) and emulsification (García et al., Citation2015) of polysaccharides are closely related to its contents of total sugar and protein.
Main chemicals of crude TPs were shown in . It can be seen that their yields and the contents of total sugar and ash were significantly different. TP2 had the highest yield followed by TP4, TP3, TP1 and TP5. TP4 contained the highest total sugar followed by TP3, TP5, TP2 and TP1. High pressure and high temperature can facilitate the dissolution of polysaccharides and non-polysaccharides from Tremella fuciformis by destroying its cell wall and increasing their solubility, thus resulting in the highest yield and lower total sugar content in TP2. (Y. Xu et al., Citation2016). The hot acid condition can destroy the cell wall of Tremella fuciformis, hydrolyze the proteins combined with polysaccharides and inhibit the dissolution of acid impurities, therefore leading to the second-high yield and the highest content of total sugar in TP4. The main factors influencing the yield of polysaccharides by enzyme-assisted extraction include the concentration of enzyme added, the extraction temperature, the ratio of extraction liquid to material, the extraction time and so on. The lowest yield of TP5 was due to its shortest extraction time (Vierhuis et al., Citation2001).
Table 1. Yields and chemical compositions of crude TPs. (TP1, hot water extraction. TP2, high-pressure water extraction. TP3, alkali extraction. TP4, acid extraction. TP5, enzyme-assisted extraction).
Tabla 1. Rendimientos y composiciones químicas de los TP crudos (TP1, extracción con agua caliente; TP2, extracción con agua a alta presión; TP3, extracción con alcalinos; TP4, extracción con ácido; TP5, extracción asistida por enzimas)
3.1.2. Molecular weights of crude TPs
Molecular weight and its distribution are important factors affecting the physicochemical properties of a polymer. In general, the larger the molecular weight of the polymer, the greater its tensile strength, impact resistance and elasticity. However, too large molecular weight will affect the solubility, solution rheological properties and processing applications of a polymer (Hou et al., Citation2012; Zhang et al., Citation2013).
As shown in , TP5 had the largest molecular weight and the smallest polydispersity coefficient (PC) followed by TP2. TP4 possessed the smallest molecular weight and relatively high PC. TP1 had a molecular weight and PC close to those of TP3. These results demonstrate that TP5 and TP2 contain larger polysaccharides and have a relatively concentrated molecular weight distribution, suggesting that mild enzyme-assisted extraction and short time of high-pressure extraction can better maintain the structure of Tremella fuciformis polysaccharides. TP4 contains more decomposed polysaccharides and its molecular weight distribution is not concentrated, indicating that acid extraction can cause the decomposition of polysaccharides (Yao et al., Citation2017).
Table 2. Molecular weights and polydispersity coefficients of crude TPs. (TP1, hot water extraction. TP2, high-pressure water extraction. TP3, alkali extraction. TP4, acid extraction. TP5, enzyme-assisted extraction).
Tabla 2. Pesos moleculares y coeficientes de polidispersidad de los TP crudos (TP1, extracción con agua caliente; TP2, extracción con agua a alta presión; TP3, extracción con alcalinos; TP4, extracción con ácido; TP5, extracción asistida por enzimas)
3.1.3. UV and FT-IR spectra of crude TPs
The UV spectra of five crude TPs were shown in . They all had no obvious absorption peak at 260 nm and 280 nm, indicating that they do not contain nucleic acid substances and free proteins.
Figure 1. UV spectra of crude TPs (A), UV spectra of crude TPs with I2-KI (B), FT-IR spectra (C) of crude TPs. (TP1, hot water extraction. TP2, high-pressure water extraction. TP3, alkali extraction. TP4, acid extraction. TP5, enzyme-assisted extraction).
Figura 1. Espectros UV de TPs crudos (A), TPs crudos con I2-KI (B), espectros FT-IR (C) de TPs crudos (TP1, extracción con agua caliente; TP2, extracción con agua a alta presión; TP3, extracción con alcalinos; TP4, extracción con ácido; TP5, extracción asistida por enzimas)
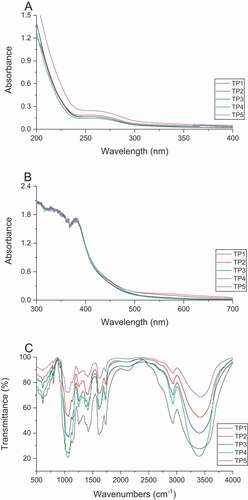
As shown in , no maximum absorption peak at 565 nm appeared in the spectra, and their maximum absorption were under this wavelength. This indicates that TPs may contain longer side chains and more branches. In addition, all the five crude TPs did not turn blue after the reaction with iodine, indicating that they did not contain starch.
FT-IR results show that the peaks of crude TPs were basically the same according to . The absorbance band at 3600–3200 cm-1 and near 2930 cm-1 are the stretching vibration of O-H and C-H (Miao et al., Citation2014). A group of peaks at 1400–1200 cm-1 is C-H variable angle vibration. The absorption peak at 950–1200 cm-1 is caused by the C-O-C stretching vibration and the variable angle vibration of C-O-H. The characteristic peak at 1750–1700 cm-1 is C = O stretching vibration in -COOH, the absorption peak at 1415 cm-1 is -COO symmetric stretching vibration. The above peaks prove the existence of uronic acid. The peak at 800 cm-1 is the characteristic of mannose residue. All the crude TPs have the characteristic absorption peak of saccharides, and their characteristic peak of carboxyl suggests the presence of uronic acid. These results confirm that crude TPs are acidic heteropolysaccharides with mannose as their main chain (Du et al., Citation2009; Gao et al., Citation1996).
3.1.4. Micromorphology of crude TPs
Atomic force microscope (AFM) was used to study the surface structure of solid materials. As shown in , all TPs particles in aqueous solutions showed irregular sphericity. Based on the above UV-visible spectra of the mixtures of TPs and iodine reagent it can be inferred that TPs particles present a spherical structure formed by winding their multiple branches. In addition, the five crude TPs particles showed different aggregation degrees. TP5 particles demonstrated a uniform distribution and their size were consistent, but the other four crude TPs particles showed a non-uniform distribution and inconsistent size. This result conforms to their polydispersity coefficients.
Figure 2. AFM images of crude TPs (5 × 5 μm), (a)plan view, (b) three-dimensional height view. (TP1, hot water extraction. TP2, high-pressure water extraction. TP3, alkali extraction. TP4, acid extraction. TP5, enzyme-assisted extraction).
Figura 2. Imágenes AFM de TPs crudos (5 × 5 μm), (a) vista en plano, (b) vista tridimensional en altura (TP1, extracción con agua caliente; TP2, extracción con agua a alta presión; TP3, extracción con alcalinos; TP4, extracción con ácido; TP5, extracción asistida por enzimas)
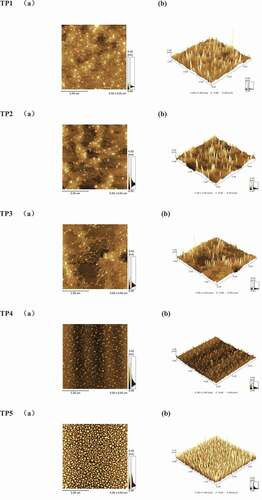
The surface characteristic values of five crude TPs are shown in . TP5 particles had the largest diameter and height followed by TP2, TP1, TP3 and TP4. This order is consistent with that of their relative molecular weights.
Table 3. Surface eigenvalues of crude TPs. (TP1, hot water extraction. TP2, high-pressure water extraction. TP3, alkali extraction. TP4, acid extraction. TP5, enzyme-assisted extraction).
Tabla 3. Valores propios de superficie de los TP crudos (TP1, extracción con agua caliente; TP2, extracción con agua a alta presión; TP3, extracción con alcalinos; TP4, extracción con ácido; TP5, extracción asistida por enzimas)
The differences in the surface features of TPs molecules may be related to their branching degrees. TP5 particles showed the highest aggregation degree and size but with a smaller gap. This may contribute to that mild enzyme-assisted extraction condition is helpful to maintain the main chain and branch chain structures of the polysaccharides in a relatively complete way. TP4 particles were the smallest, indicating that acidic condition can hydrolyze the polysaccharide and thus can significantly reduce the degree of its molecular aggregation. Therefore, different extraction methods can affect the main chain and branch chains of the polysaccharides to different degrees, resulting in five crude TPs with different aggregation degrees.
3.2. Static rheological properties of crude TPs solutions
3.2.1. Effect of shear rate and concentration of TPs solutions on their viscosity
The viscosity curves of three concentrations of TPs solutions at different shear rates are shown in . Except for TP4, the viscosity of TP1, TP2, TP3 and TP5 solutions at 1% decreased with the increase of shear rate, demonstrating the characteristics of a shear-thinning non-Newtonian fluid (Pongsawatmanit et al., Citation2006). After the shear rate exceeded 100 s-1, the viscosity of TP1 and TP3 solutions changed very little, showing the characteristic of a Newtonian fluid (Lu et al., Citation2007). However, TP2 and TP5 solutions presented the characteristic of a Newtonian fluid after the shear rate was over 400 s-1. When the shear rate is small enough, polysaccharide molecules are entangled with each other, and the entanglement structure is constantly disintegrated and reconstructed. At this time, the speed of disintegration is equal to that of reconstruction, and the viscosity of polysaccharide solution is the highest. With the increase of shear rate, the entanglement structure between polysaccharide molecular chains is accelerated to be destroyed, and the disintegration is over the reconstruction, so the viscosity of the solution decreases continuously, showing a pseudoplastic characteristic of non-Newtonian fluid. When the shear rate continues to increase, the entanglement structure of polysaccharide molecules is completely destroyed, and there is no time to rebuild. The viscosity of the system reaches the minimum and remains constant, showing the characteristic of a Newtonian fluid. TP5 and TP2 solutions had the largest decrease in their viscosity values, and they exhibited an obvious pseudo-plastic characteristic. TP4 solution had a small decrease in its viscosity and presented an obvious Newtonian fluid characteristic. This may be related to their molecular weights. The greater the molecular weights of a polysaccharide, the greater the flexibility of its chain and the higher the degree of its pseudo-plasticity.
Figure 3. Rheological curves of crude TPs solutions at different concentrations. (TP1, hot water extraction. TP2, high-pressure water extraction. TP3, alkali extraction. TP4, acid extraction. TP5, enzyme-assisted extraction).
Figura 3. Curvas reológicas de las soluciones de TPs crudas a diferentes concentraciones. (TP1, extracción con agua caliente. TP2, extracción con agua a alta presión. TP3, extracción con alcalinos. TP4, extracción con ácido. TP5, extracción asistida por enzimas)
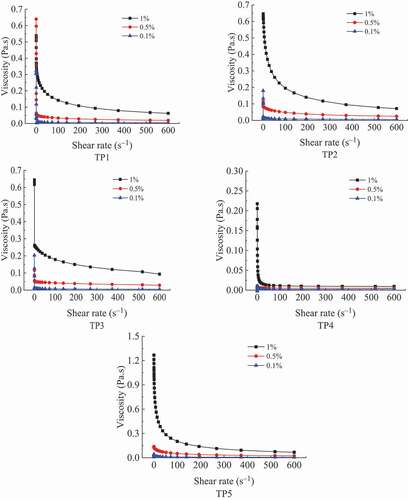
Crude TPs solutions showed a shear-thinning of non-Newtonian fluid characteristic at high concentrations but they demonstrated a Newtonian fluid characteristic at low concentrations. This is because the viscosity of TPs solutions at low concentrations is very small and its change is not obvious. In addition, there is a critical concentration. When TPs concentration is lower than this concentration, its solution shows Newtonian; when TPs concentration is higher than this concentration, its solution presents non-Newtonian. The higher the concentration of TPs solution is, the higher its pseudoplasticity (Barrangou et al., Citation2006; Che et al., Citation2008; Michèle et al., Citation2001). J. Xu et al. (Citation2013) found that higher concentrations of the polysaccharide solution had a stronger shear-thinning ability and TPs solutions were pseudoplastic fluid. In addition, with the increase of mass concentration of TPs solutions, molecules begin to overlap, forming intermolecular junctions or “junction regions”. This behavior limits the movement and stretching of polymer chains in the aqueous system, leading to a significant increase in viscosity with the increase of mass concentration (Chronakis et al., Citation1996).
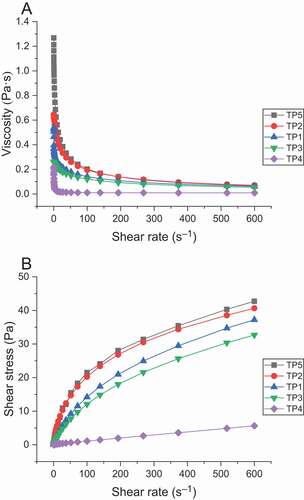
The effect of shear rate on viscosity of TPs solutions with the same mass concentration were shown in . At 1%, TP5 solution had the highest viscosity followed by TP2, TP1, TP3 and TP4 at the same shear rate. This order is in accordance with that of their molecular weights. During the flow process, the polysaccharide molecular chains entangle with each other to form a network structure. This mutual entanglement effect is strengthened as the molecular weight and the branching degree of a polysaccharide increase, thus leading to higher flow resistance and viscosity of the system.
Figure 4. Apparent viscosity versus shear rate plot (A) and shear stress-shear rate curves(B) of crude TPs solutions. (TP1, hot water extraction. TP2, high-pressure water extraction. TP3, alkali extraction. TP4, acid extraction. TP5, enzyme-assisted extraction).
Figura 4. Gráfico de viscosidad aparente versus la velocidad de cizallamiento (A) y curvas de tensión de cizallamiento-velocidad de cizallamiento (B) de las soluciones de TPs crudas. (TP1, extracción con agua caliente. TP2, extracción con agua a alta presión. TP3, extracción con alcalinos. TP4, extracción con ácido. TP5, extracción asistida por enzimas)
3.2.2. Effect of shear rate on shear stress of TPs solutions
The relationship between shear stress and shear rate of TPs solutions at 1% were shown in . It can be seen that their curves do not pass through the original point and they belong to the pseudo-plastic fluid in the power-law model (Diego et al., Citation2008). At the same shear rate, TP5 solution had the biggest shear stress followed by TP2, TP1, TP3 and TP4 solution. This order is consistent with their molecular weights, suggesting that the larger the molecular weight, the greater the internal force to cope with deformation, and the tighter the intermolecular entanglement of the polysaccharides, the less deformation. The shear stress of TP5 and TP2 solutions changed the fastest and their pseudo-plastic characteristic was the most obvious, indicating that the larger the molecular weight is, the better pseudo-plasticity of the fluid can be reflected.
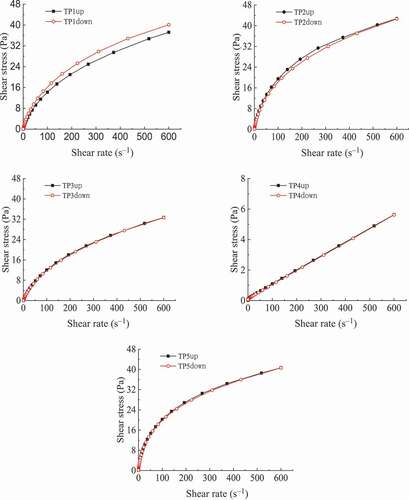
3.2.3. Thixotropy of crude TPs solutions
The thixotropy curves of TPs solutions were shown in . TP1 solution had the largest thixotropic ring followed by TP2 and TP5 solution while TP3 and TP4 solutions hardly form a thixotropic ring. This indicates that TP3, TP4 and TP5 solutions have good deformation resistance and can be quickly recovered after external force action while TP1 and TP2 solutions do not so. So TP3, TP4 and TP5 are suitable for food products with good fluidity and require shape retention, and TP1 and TP2 can be applied to foods that do not require shape retention.
Figure 5. Thixotropy of crude TPs solutions. (TP1, hot water extraction. TP2, high-pressure water extraction. TP3, alkali extraction. TP4, acid extraction. TP5, enzyme-assisted extraction).
Figura 5. Tixotropía de las soluciones crudas de TPs. (TP1, extracción con agua caliente. TP2, extracción con agua a alta presión. TP3, extracción con alcalinos. TP4, extracción con ácido. TP5, extracción asistida por enzimas)
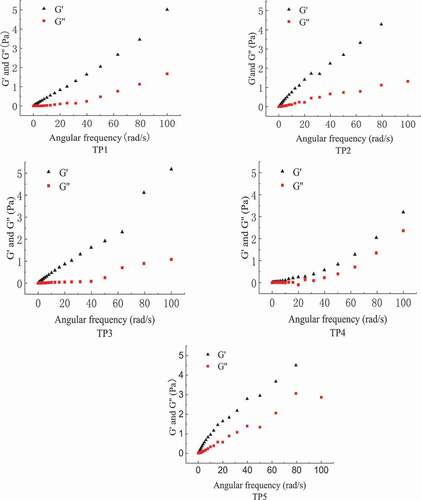
3.3. Dynamic rheological properties of crude TPs solution
The dynamic viscoelastic curves of crude TPs solutions were shown in . They all exhibit viscoelastic properties of macromolecules. At low frequency, all TPs solutions had a higher storage modulus (G’) and a lower loss modulus (G′’), that is, G’ < G’’, TPs solutions were mainly viscous and showed a liquid flow state. With a rise in vibration frequency, their G’ and G’’ gradually increased, and the amplitude of G’ increase was larger than that of G’’. When the vibration frequency applied to TPs solutions reached a certain degree, G’ exceeded G’’. At that time, the system mainly presented an elastic characteristic similar to a solid and weak gelation. Under low frequency, TPs solutions had a long stress relaxation time and slow deformation, most of the energy in the system was lost due to viscous flow, and the molecular energy was low, thus resulting in G’ < G’’. As vibration frequency increased, the relaxation time between TPs molecular chains decreased and they gradually entangled into a solid-like network structure. This structure could help the system to store energy until G’ > G’’, and the solutions began to show an elastic characteristic. TP5 solution first exhibited an elastic characteristic. This may be because of the higher the molecular weight, the more entanglement between the chains and the ability to start storing energy at a lower frequency. All TPs solutions had their own gel spot, indicating that they had an ability to form gel. This is consistent with that Tremella fuciformis polysaccharide exhibited properties of pseudo-plastic fluids in distilled water and could form a weak gel in high frequency at 25°C (Y. K. Zhang et al., Citation2017). TPs has a linear chemical structure, which turns into a winding structure in aqueous solution, and the degree of winding increases with the increase of shear rate (Liu et al., Citation2016). Huangshan mushroom polysaccharide also has the similar phenomenon (J. L. Xu et al., Citation2016).
Figure 6. Dynamic rheological curves of crude TPs solutions. (TP1, hot water extraction. TP2, high-pressure water extraction. TP3, alkali extraction. TP4, acid extraction. TP5, enzyme-assisted extraction).
Figura 6. Curvas reológicas dinámicas de las soluciones crudas de TPs. (TP1, extracción con agua caliente. TP2, extracción con agua a alta presión. TP3, extracción con alcalinos. TP4, extracción con ácido. TP5, extracción asistida por enzimas)
4. Conclusion
Five crude polysaccharides from Tremella fuciformis were prepared by hot-water extraction (TP1), high-pressure water extraction (TP2), alkali extraction (TP3), acid extraction (TP4) and enzyme-assisted extraction(TP5), and their chemical composition, molecular weights, micromorphological structure and rheological properties were studied. The weight average and number average molecular weights of TP5 and TP2 were significantly higher than those of TP1, TP3 and TP4. TP5 had the largest molecular weight, the smallest polydispersity coefficient, the most concentrated molecular weight distribution, the largest diameter and height. They were acidic polysaccharides with uronic acid and being mannose as their main chain. Their particles were present in an irregular sphere. They were all pseudoplastic fluid with a shear-thinning characteristic and their viscosity decreased with the increase of shear rate. At the same mass concentration, the apparent viscosity of their solution in descending order was TP5, TP2, TP1, TP3 and TP4. All the crude polysaccharides had similar dynamic viscoelasticity. They showed a viscosity characteristic at low frequency, an elastic characteristic at high frequency and a weak gel property.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bao, H. M. (2015). Study on extraction technology and stability of Tremella polysaccharide by enzymatic hydrolysis. Food Research and Development, 36(11), 17–21. https://doi.org/https://doi.org/10.3969/j.issn.1005-6521.2015.11.004
- Barrangou, L. M., Drake, M. A., Daubert, C. R., & Foegeding, E. A. (2006). Textural properties of agarose gels. ii. relationships between rheological properties and sensory texture. Food Hydrocolloids, 20(2/3), 196–203. https://doi.org/https://doi.org/10.1016/j.foodhyd.2005.03.013
- Bradford, M. M. A. (1976). A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 25(1), 248–256. https://doi.org/https://doi.org/10.1016/0003-2697(76)90527-3
- Che, L. M., Li, D., Wang, L. J., ÖZkan, N., Chen, X. D., & Mao, Z. H. (2008). Rheological properties of dilute aqueous solutions of cassava starch. Carbohydrate Polymers, 74(3), 385–389. https://doi.org/https://doi.org/10.1016/j.carbpol.2008.03.007
- Chen, B. (2010). Optimization of extraction of tremella fuciformis polysaccharides and its antioxidant and antitumour activities in vitro. Carbohydrate Polymers, 81(2), 420–424. https://doi.org/https://doi.org/10.1016/j.carbpol.2010.02.039
- Chen, G., Bu, F., Chen, X., Li, C., Wang, S., & Kan, J. (2018). Ultrasonic extraction, structural characterization, physicochemical properties and antioxidant activities of polysaccharides from bamboo shoots (chimonobambusa quadrangularis) processing by-products. International Journal of Biological Macromolecules, 112, 656–666. https://doi.org/https://doi.org/10.1016/j.ijbiomac.2018.02.013
- Cho, H. M., & Yoo, B. (2015). Rheological characteristics of cold thickened beverages containing xanthan gum-based food thickeners used for dysphagia diets. Journal of the Academy of Nutrition & Dietetics, 115(1), 106–111. https://doi.org/https://doi.org/10.1016/j.jand.2014.08.028
- Chronakis, I. S., Piculell, L., & Borgstroem, J. (1996). Rheology of kappa-carrageenan in mixtures of sodium and cesium iodide: Two types of gels. Carbohydrate Polymers, 31(4), 215–225. https://doi.org/https://doi.org/10.1016/S0144-8617(96)00117-8
- Diego, G. D., Navaza, J. M., & Quintáns-Riveiro, L. C. (2008). Influence of mixing and temperature on the rheological properties of carboxymethyl cellulose/κ-carrageenan mixtures. European Food Research and Technology, 227(5), 1397–1402. https://doi.org/https://doi.org/10.1007/s00217-008-0858-2
- Du, X. J., Zhang, J. S., Yang, Y., Ye, L. B., Tang, Q. J., Jia, W., Liu, Y., Zhou, S., Hao, R., Gong, C., & Pan, Y. (2009). Structural elucidation and immuno-stimulating activity of an acidic heteropolysaccharide (tapa1) from Tremella aurantialba. Carbohydrate Research, 344(5), 672–678. https://doi.org/https://doi.org/10.1016/j.carres.2009.01.021
- Duan, C., & Wang, C. T. (2016). Optimization of extracting Tremella fuciformis polysaccharide and detection of its toxicity to cells of human body. China Surfactant Detergent & Cosmetics, 46(2), 101–105. https://doi.org/https://doi.org/10.13218/j.cnki.csdc.2016.02.009
- Fischer, P., Pollard, M., Erni, P., Marti, I., & Padar, S. (2009). Rheological approaches to food systems. Comptes Rendus Physique, 10(8), 740–750. https://doi.org/https://doi.org/10.1016/j.crhy.2009.10.016
- Florina, D., Paulina, M., Margarida, M., & Vitor, D. (2015). Novel mango bars using gellan gum as gelling agent: Rheological and microstructural studies. LWT - Food Science and Technology, 62(1), 576–583. https://doi.org/https://doi.org/10.1016/j.lwt.2014.09.037
- Gao, Q. P., Chen, H. Q., Jiang, R. Z., & Seljelid, R. (1996). Characterisation of acidic heteroglycans from tremella fuciformis berk with cytokine stimulating activity. Carbohydrate Research, 288(1), 135–142. https://doi.org/https://doi.org/10.1016/S0008-6215(96)90789-2
- García, M. C., Alfaro, M. C., & Muñoz, J. (2015). Influence of the ratio of amphiphilic copolymers used as emulsifiers on the microstructure, physical stability and rheology of α-pinene emulsions stabilized with gellan gum. Colloids and Surfaces B: Biointerfaces, 135, 465–471. https://doi.org/https://doi.org/10.1016/j.colsurfb.2015.07.060
- Geng, S. N., Chen, J., & Xu, X. (2009). Determination of lentinan content and its molecular weight by GPC. Modern Food Science and Technology, 25(4), 458–460.
- Hou, Y., Wang, J., Jin, W., Zhang, H., & Zhang, Q. (2012). Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydrate Polymers, 87(1), 153–159. https://doi.org/https://doi.org/10.1016/j.carbpol.2011.07.031
- Jiang, C. X., Zhao, L., Li, S. L., Zhao, X. L., Zhang, Q. H., & Xiong, Q. P. (2013). Preliminary characterization and immunostimulatory activity of polysaccharides from Glossaulax didyma. Food & Chemical Toxicology, 62, 226–230. https://doi.org/https://doi.org/10.1016/j.fct.2013.08.079
- Lai, J. X., He, C. F., Zhao, J., & Dong, Y. M. (2010). Optimization of extraction technology of polysaccharide from Tremella fuciformis on commercialized basis and its function in skin care cosmetics. China Surfactant Detergent & Cosmetics, 40(4), 259–262. https://doi.org/https://doi.org/10.13218/j.cnki.csdc.2010.04.008
- Liu, J., Meng, C. G., Yan, Y. H., Shan, Y. N., Kan, J., & Jin, C. H. (2016). Structure, physical property and antioxidant activity of catechin grafted tremella fuciformis polysaccharide. International Journal of Biological Macromolecules, 82, 719–724. https://doi.org/https://doi.org/10.1016/j.ijbiomac.2015.11.027
- Lu, Z. H., Yuan, M. L., Sasaki, T., Li, L. T., & Kohyama, K. (2007). Rheological properties of fermented rice flour gel. Cereal Chemistry, 84(6), 620–625. https://doi.org/https://doi.org/10.1094/CCHEM-84-6-0620
- Miao, M., Bai, A., Jiang, B., Song, Y., Cui, S. W., & Zhang, T. (2014). Characterisation of a novel water-soluble polysaccharide from leuconostoc citreum sk24.002. Food Hydrocolloids, 36, 265–272. https://doi.org/https://doi.org/10.1016/j.foodhyd.2013.10.014
- Michèle, M., Hoshahili, A. R. T., & Ramaswamy, H. S. (2001). Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Research International, 34(8), 695–703. https://doi.org/https://doi.org/10.1016/S0963-9969(01)00091-6
- Pongsawatmanit, R., Temsiripong, T., Ikeda, S., & Nishinari, K. (2006). Influence of tamarind seed xyloglucan on rheological properties and thermal stability of tapioca starch. Journal of Food Engineering, 77(1), 41–50. https://doi.org/https://doi.org/10.1016/j.jfoodeng.2005.06.017
- Tao, R. X., Jia, D. Y., Yao, K., & Chi, Y. L. (2015). Extraction of polysaccharide from Tremella fuciformis under high pressure. Food Science and Technology, 40(6), 229–233. https://doi.org/https://doi.org/10.13684/j.cnki.spkj.2015.06.053
- Vierhuis, E., Maurizio, S., Baldioli, M., Schols, H. A., & Montedoro, G. F. (2001). Effect of enzyme treatment during mechanical extraction of olive oil on phenolic compounds and polysaccharides. Journal of Agricultural and Food Chemistry, 49(3), 1218–1223. https://doi.org/https://doi.org/10.1021/jf000578s
- Wang, X., Zhang, Z., & Zhao, M. (2015). Carboxymethylation of polysaccharides from Tremella fuciformis for antioxidant and moisture-preserving activities. International Journal of Biological Macromolecules, 72, 526–530. https://doi.org/https://doi.org/10.1016/j.ijbiomac.2014.08.045
- Wu, Y. J., Wei, Z. X., Zhang, F. M., Linhardt, R. J., Sun, P. L., & Zhang, A. Q. (2019). Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: A review. Biological Macromolecules, 121(8), 1005–1010. https://doi.org/https://doi.org/10.1016/j.ijbiomac.2018.10.117
- Xu, J., Inglett, G. E., Chen, D., & Liu, S. X. (2013). Viscoelastic properties of oat β-glucan-rich aqueous dispersions. Food Chemistry, 138(1), 186–191. https://doi.org/https://doi.org/10.1016/j.foodchem.2012.10.049
- Xu, J. L., Zhang, J. C., Liu, Y., Sun, H. J., & Wang], J. H. (2016). Rheological properties of a polysaccharide from floral mushrooms cultivated in huangshan mountain. Carbohydrate Polymers, 139, 43–49. https://doi.org/https://doi.org/10.1016/j.carbpol.2015.12.011
- Xu, Y., Cai, F., Yu, Z., Zhang, L., Li, X., Yang, Y., & Liu, G. (2016). Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity. Food Chemistry, 194, 650–658. https://doi.org/https://doi.org/10.1016/j.foodchem.2015.08.061
- Yao, Y., Xiang, H., You, L., Cui, C., & Zhao, M. (2017). Hypolipidaemic and antioxidant capacities of polysaccharides obtained from Laminaria japonica by different extraction media in diet-induced mouse model. International Journal of Food Science & Technology, 52(10), 2274–2281. https://doi.org/https://doi.org/10.1111/ijfs.13508
- Zhang, L., & Wang, M. (2016). Polyethylene glycol-based ultrasound-assisted extraction and ultrafiltration separation of polysaccharides from Tremella fuciformis (snow fungus). Food & Bioproducts Processing, 100, 464–468. https://doi.org/https://doi.org/10.1016/j.fbp.2016.09.007
- Zhang, N., Chen, H., Ma, L., & Zhang, Y. (2013). Physical modifications of polysaccharide from Inonotus obliquus and the antioxidant properties. International Journal of Biological Macromolecules, 54, 209–215. https://doi.org/https://doi.org/10.1016/j.ijbiomac.2012.12.030
- Zhang, N., Chen, H. X., Zhang, Y., Xing, L. S., Li, S. Q., Wang, X. M., & Sun, Z. (2015). Chemical composition and antioxidant properties of five edible Hymenomycetes mushrooms. International Journal of Food Science & Technology, 50(2), 465–471. https://doi.org/https://doi.org/10.1111/ijfs.12642
- Zhang, W. H., Wu, J., Weng, L. Y., Zhang, H. J., Zhang, J., & Wu, A. B. (2020). An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydrate Polymers, 227, 115332. https://doi.org/https://doi.org/10.1016/j.carbpol.2019.115332
- Zhang, Y. K., Zhang, Q., Lu, J., Xu, J. L., Zhang, H., & Wang, J. H. (2017). Physicochemical properties of Tremella fuciformis polysaccharide and its interactions with myofibrillar protein. Bioactive Carbohydrates and Dietary Fibre, 11, 18–25. https://doi.org/https://doi.org/10.1016/j.carbpol.2019.115332
