?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Nutritional composition, functional and antioxidant properties of different parts from grass turtle were investigated. Muscle contained high protein, essential amino acids, and vitamin B6, which were 74.97%, 28363.84, and 14.47 mg/100 g, respectively. Unsaturated fatty acids, vitamin K were significantly higher in the liver which reached 7489.45 and 1591.67 mg/100 g, respectively. Hardshell high content of minerals and vitamin B3 (48998.06 and 17.52 mg/100 g), correspondingly. The molecular weight was obtained in the range of 0.27–87.38% (Mw˂1000 Da). Solubility, emulsifying activity, and emulsion stability index at different pH ranged from 3.89 to 45.89%, 47.53 to 90.22 m2/g, and 65.45 to 89.93%, respectively. Water and oil holding capacities were high in muscle (7.97 and 2.19 mL/g). The highest antioxidant activity was observed in the muscle (88.58% ABTS) and liver (66.71% DPPH and 0.49 FRAP). Muscle, liver, and hard shell properties will provide producers and consumers with useful new information about the nutritional value and health benefits.
RESUMEN
El presente estudio investigó la composición nutricional y las propiedades funcionales y antioxidantes de diferentes partes de la tortuga herbívora. En este sentido se constató que el músculo posee alto contenido de proteínas, aminoácidos esenciales y vitamina B6, registrándose las siguientes proporciones: 74.97%, 28363.84 y 14.47 mg/100g, respectivamente. El contenido de ácidos grasos insaturados y vitamina K es significativamente mayor en el hígado, alcanzando 7489.45 y 1591.67 mg/100g, respectivamente. El caparazón duro contiene altos niveles de minerales y vitamina B3 (48998.06 y 17.52 mg/100g, respectivamente). Se registró un rango de peso molecular que varía entre 0.27-87.38% (Mw˂1000 Da). La solubilidad, la actividad emulsionante y el índice de estabilidad de la emulsión a diferentes pH oscilaron, respectivamente, entre 3.89 y 45.89%, 47.53 y 90.22 m2/g y 65.45 y 89.93%. Se constataron capacidades de retención de agua y aceite elevadas en el músculo (7.97 y 2.19 mL/g). La mayor actividad antioxidante se observó en el músculo (88.58% ABTS) y en el hígado (66.71% DPPH y 0.49 FRAP). Estos hallazgos relativos a las propiedades del músculo, el hígado y el caparazón duro proporcionarán a productores y consumidores nueva información útil sobre el valor nutricional y los beneficios para la salud.
1. Introduction
Grass turtle (Chinemys reevesii) belongs to the aquatic species of the Geoemydidae family. It is particularly native in China, Hong Kong, Korea, Taiwan, and Japan (Dai et al., Citation2012). Turtles have been used as foods and medicines especially in East and Southeast Asia, while China is the largest consumer country in the world. Turtle shells have been utilized as an ingredient for the traditional Chinese medicines (TCM) (T. H. Chen et al., Citation2009). T.-H. Chen et al. (Citation2000) reported that, in 1992–1998, more than 136 metric ton/year of turtle shells were traded in the Taiwan market. Recently, studies have been focussed on the practical utilization of various aquatic species and their by-products (shell, and bone) (Zou et al., Citation2017).
Meat is the major source of high-quality proteins that provide essential amino acids upon digestion, which is closely related to human nutrition. Meat also provides valuable amounts of many essential nutrients, e.g. some polyunsaturated fatty acids, vitamins and minerals (Serpen et al., Citation2012). Zou et al. (Citation2017) reported that the soft-shelled turtle (Pelodiscus sinensis) is the most commercially valuable and delicious aquatic species and also it contains high nutritional (protein and low fat) and medicinal values (such as antioxidation, anticancer). The individual functional properties viz. water and oil holding capacities, solubility, emulsifying activity index (EAI) and emulsion stability index (ESI), which are important to the food industries especially in meat and confectionery industries because it influences the taste of products and contributing to a uniform distribution of dry ingredients in the mixture (Rodrigues Freitas et al., Citation2016). Furthermore, several studies have reported a positive correlation of the increased dietary intake of natural antioxidants with reduced coronary heart disease as well as reduced cancer mortality (Cho et al., Citation2011). In addition, for improving the oxidative stability of meat products (Zhang et al., Citation2015). To the best of our knowledge, the nutritional compositions, molecular weights distribution, functional and antioxidant properties, and nutritional values of grass turtle has not been previously reported. Our hypothesis is that, grass turtles would contain high amount of protein, essential fatty acids, amino acids, and excellent functional properties. This nutrients composition will be value-added to facilitate the processing, utilization, and marketing. Hence, to prove the hypothesis it’s necessary to determine the nutritional compositions and physiochemical properties of the samples. Therefore, the current study aimed to evaluate the chemical compositions, nutritional value, functional and antioxidant properties of different parts of grass turtle.
2. Materials and methods
2.1. Experimental sample
Grass turtle (Chinemys reevesii) is a kind of usual aquatic food in China. And the grass turtle obtained from the breeding products of Guangxi zhongtaikang Technology Industry Co., Ltd., Nanning-530,029, Guangxi, P. R. China. After consultation with relevant Chinese authorities, it was not an experimental animal, and it was unnecessary to issue animal ethics certificate. Grass turtles were rinsed thoroughly with cleaned water and then immediately slaughtered, byproducts were removed and divided into three parts (muscle, liver, and hard shell). Samples were put in the fresh box with ice bag when transported to laboratory (Nutrition and Function Factors Food Research Center). Finally, selected parts were homogenized and packed in vacuum plastic bags and stored at −20 °C for further analysis.
2.2. Chemical reagents
Potassium persulfate, glacial acetic acid, sodium acetate, ferric chloride (FeCl3.6H2O), absolute methanol and ethanol, acetonitrile, NaOH, sodium dodecyl sulfate solution were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and ABTS [2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)]; DPPH [1,1-diphenyl-2-picryl-hydrazyl] were purchased from Aladdin (Shanghai, China). All other chemical reagents and solvents used were pure and analytical grade.
2.3. Proximate analysis
Proximate compositions such as moisture, crude protein, crude lipid, and ash were determined according to a AOAC standard method (AOAC, Citation1995).
2.4. Determination of amino acid composition
Amino acids were analyzed by HPLC, following an alkaline hydrolysis for tryptophan and other amino acids were performed by using the guideline of Noman et al. (Citation2018) with some modifications. For tryptophan (total) analysis, 100 mg of sample was hydrolyzed by 8 mL of 5 mol/L NaOH at 120°C for 22 h under nitrogen gas and neutralized by 6.67 mL of 6 M HCl. On the other hand, in other amino acids determination, same amount of samples were taken and hydrolyzed with 8 mL of 6 mol/L HCl under nitrogen gas and incubated in an oven at the same temperature and time and neutralized by 4.8 mL of 10 M NaOH. For the free amino acids evaluation, samples taken 1 g and diluted with 25 mL of 5% TCA then 2 hours stranded and centrifuged at 10,000 g for 10 min. Each hydrolyzed sample (1 μL) was injected into a Zorbax, 80 A C-18 column (column size: 4.0 × 250 mm, 5 μm particle size; Agilent, USA) at 40°C with detection at 338 nm. The mobile phase A was 7.35 mM/L of sodium acetate/triethylamine/tetrahydrofuran (500:0.12:2.5, v/v/v), adjusted to pH 7.2 using acetic acid, while the mobile phase B (pH 7.2) was 7.35 mM/L of sodium acetate/methanol/acetonitrile (1:2:2, v/v/v). The amino acid composition results were expressed as mg/100 g of sample on dry weight basis.
2.5. Analysis of vitamins by HPLC
Fat soluble vitamin A, D, E, and K were analyzed according to a method of (Moreno & Salvado, Citation2000) with slight modification. The raw samples were mashed and taken 0.5 g into 15 mL glass tubes with cap then added 0.5 mL deionized water and 5 mL CH3COOC2H5, placed the capped glass tubes in an ultrasonic bath for 20 min and shakes few min, wait until the layers are completely separated and taken 1 mL of the top layer, putted into 3 mL CH3CN. On the other hand, water-soluble vitamins (B1, B2, B3, B6, B9, and C) were analyzed according to a modified method as described by Moreno and Salvado (Citation2000). Finally, the fat and water-soluble vitamins were analyzed by HPLC (Agilent 1100, USA). The filtrated sample was directly injected (10 μL in fat-soluble vitamins and 5 µL in water-soluble vitamins), Symmetry C18 column (4.6 × 250 mm) was used for the separation of vitamins at temperature 30°C, flow rate at 1.0 mL/min, detector (ultraviolet detector 290 nm). The mobile phase A was consisted CH3OH and phase B was sodium 1-heptanesulfonate (0.05 M) at different time intervals 0, 20, 22, and 26 min of 90% B; 30% B; 30% B; 90% B; 90% B, respectively. The fat and water-soluble vitamins results were expressed as mg/100 g of the sample on wet basis.
2.6. Mineral measurement by HPLC-ICP-MS
The macro and micro minerals were investigated by using a method of Ersoy and Özeren (Citation2009) with some modifications. Freeze-dried samples (1 g) were weighed in porcelain crucible and ash in muffle furnace at 550°C until a complete ash. Then, ash was dissolved into 5 mL of HNO3, HCl, and deionized water at the ratio of 1:2:3 and heated gently on a hot plate until brown fumes disappeared. Afterward, each crucible solution was transferred into a 25 mL volumetric flask and made up with deionized water and filtered by Whatman No. 42 filter paper. Filtered solution was taken 1 mL to volumetric flask and volume made up to 1000 mL with deionized water. Finally, the diluted solution was injected into HPLC (Agilent 1100, USA) coupled with NexION 350D ICP-MS (PerkinElmer, Shanghai, China). The results mentioned as mg/100 g sample on dry weight basis.
2.7. Fatty acid composition analysis
For the determination of fatty acid composition, lipid was extracted from samples as described by Lin and Huang (Citation2007), and prepared to methyl esters of fatty acids (GB/T 17376, ISO5509:2000, IDT) then analyzed by standard method as described by Yang et al. (Citation2011) with minor modifications, using an Agilent 7820A gas chromatograph (GC) (Agilent Technologies, Santa Clara, CA, USA) equipped with a hydrogen flame ionization detector (FID). A Trace TR-FAME capillary column consisted by 0.25 µm, 60 m × 0.25 mm (Thermo Fisher, Waltham, MA, USA). Nitrogen was used as the carrier gas at a flow rate of 1 mL/min. The oven temperature program range was from 80°C for 3 min to 215°C (15°C/min; 20 min). The injection volume was 1 μL at a split ratio (1:100), FID and injector temperature, 250°C. Identified individual fatty acids were accomplished by comparing the retention times of the sample peaks with those of a mixture of FAME standards. Total fatty acid detected with chain lengths of 4–22 carbon atoms. The results were expressed as mg/100 g sample.
2.8. Molecular weight distribution
One hundred milligram sample was diluted with 10 mL deionized water into 15 mL glass tubes and kept 5 min in ultrasonic bath and transferred into centrifuge tubes, centrifuged at 15,000 g for 10 min (SCLOGEX- D3024R, Beijing, China) and collected supernatant. Finally, supernatant was used to molecular weight distribution analyzed by gel permeation chromatography using a HPLC system (Waters-1525, USA) as described by Noman et al. (Citation2018).
2.9. Functional properties
2.9.1. Solubility
The solubility of muscle, liver, and hard shell (dried powder) was estimated according to a procedure described by García‐Moreno et al. (Citation2017) with some modifications. Briefly, the pH of the mixture was adjusted from 2 to 10 by using 0.1 M HCl or 0.1 M NaOH and stirred at 300 rpm and 20°C for 20 min (Blue pard THZ-100, Shanghai, China) and centrifuged at 5000 g for 10 min (SCLOGEX- D3024R, Beijing, China). The solubility result expressed as the percentage of total solids, according to the following equation
where, M1 is the initial mass and M2 is the final mass.
2.9.2. Emulsification capacity
The emulsifying capacity index (EPI) and emulsion stability index (ESI) of grass turtle were measured by the following procedure of Noman et al. (Citation2018) with minor modifications. Briefly, the absorbance of the solutions was analyzed at 500 nm by using UV-1800PC Spectrophotometer (Shanghai Mapada Instruments Co., Ltd., Shanghai, China). EAI and ESI calculated as the following formula:
where, Ab0 and Ab10 is the absorbance at 500 nm at 0 min to 10 time, A500 = Absorption value at 500 nm, S = weight of sample (g), = Oil volume fraction (0.25).
2.9.3. Water and oil holding capacity
Water-holding capacity (WHC) and oil holding capacity (OHC) were evaluated by using a centrifuge assay (Noman et al., Citation2018) with petty modification. Each sample (0.5 g) was dissolved into 10 mL ultrapure water or soybean oil in centrifuge tubes and dispersed by vortex mixer (XW-80A, Zhejiang, China) for 60 sec. The water dispersion was allowed to stand for 7 h and oil left for 20 min at 25°C, and centrifuged (SCLOGEX- D3024R, Beijing, China) at 8000 g for 25 min at 4°C. Finally, supernatants were measured from the weight difference, and the results expressed as mL/g dried sample.
2.10. Antioxidants properties
All antioxidant properties of grass turtle muscle and by-products were evaluated by using a UV-1800PC Spectrophotometer (Shanghai Mapada Instruments Co., Ltd., China). Freeze-dried samples were crushed to powder form by using a mortar and passed through a test sieve (400 µm). All samples were dissolved into ethanol/water mixture (1:1, v/v) and stirred at 300 rpm for 60 min (Blue pard THZ-100, Shanghai, China) at 25°C. The concentration of samples (mg/mL) providing 50% inhibition of ABTS and DPPH, IC50 was calculated from the graph plotted with antioxidant concentration as abscissa and the inhibition percentage as the ordinate. BHT was used as a positive control.
2.10.1. ABTS free radical scavenging activity
2,2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activity was analyzed by the guideline of Hall et al. (Citation2018) with minor modifications. The ABTS+ working solution was prepared by water:ethanol (50:50, v/v) and absorbance was 0.75–0.80 at 734 nm. Then, 1000 μL samples and 3000 μL of ABTS+ working solution were mixed and incubation for 10 min at 25°C, the absorbance was recorded at 734 nm. ABTS free radical cation activity was calculated as the following equation
where, Abscontrol is the absorbance without sample and Abssample is the absorbance with sample.
2.10.2. DPPH Radical scavenging activity
The DPPH free radical scavenging activity was measurement following the procedure described by Serpen et al. (Citation2012) with some modifications. The DPPH working solution was prepared by water: ethanol (50:50, v/v) and absorbance was 0.75–0.80 at 517 nm. Finally, 1 mL samples and 3 mL of DPPH working solution were mixed and incubated for 30 min at 25°C, the absorbance was recorded at 525 nm. The DPPH free radical scavenging capacity was calculated as described in ABTS formula.
2.10.3. Ferric ion reducing antioxidant activity
The reducing power of samples was assessed according to the modified method of Dhanabalan et al. (Citation2017). Increasing absorbance values indicated the increasing reducing power.
2.11. Statistical Analysis
All samples were measured in triplicate. The analysis data were statistically evaluated as the mean ± standard deviation and analyzed by IBM SPSS Statistics for windows, version 22.0 (Corp, I, Citation2013). Significant differences between means were determined by one-way analysis of variance (ANOVA) and Duncan’s Multiple Range Test (p value less than 0.05).
3. Results and discussion
3.1. Proximate composition
Proximate compositions of different parts (muscle, liver, and hard shell) from grass turtle were evaluated. Among different parts, the result showed that, at dry basis, muscle contains the highest amount of crude protein (74.97%) with significant difference (p <.05), followed by hard shell (34.16%), while liver contained the lowest value (25.10%). In this study, muscle content was higher protein than quantities reported in previous studies on Chinese soft-shelled turtle (Pelodiscus sinensis) by Liang et al. (Citation2018) and aquatic turtles Kienzle et al. (Citation2006). On the dry basis, the liver was revealed to contained high fat (9.68%), but no significant difference between muscle (5.84%) and hard shell (5.30%). In general, the grass turtles contained low fat than aquatic turtles (12.0%) (Kienzle et al., Citation2006), which is an advantage for the coronary heart disease. For the moisture, muscle had shown high content (76.73%) than the other parts, which were in agreement with the results obtained by Liang et al. (Citation2018) in Chinese soft-shelled turtle (78.03–80.38%). On the other hand, at dry basis, the hard shell contained the highest amount of ash (49.66%) comparison with muscle (5.79%) and liver (2.169%) with significantly different (p <.05). Ash content of hard shell was in accordance with Kienzle et al. (Citation2006) in aquatic Turtles (60.4–60.8%). The results suggest that the grass turtle contained high protein and low fat, which could be used as food application and nutraceutical.
3.2. Amino acids profiles
Dietary protein source should be considered as individual amino acids. In current study, total of 19 different amino acids (18 protein amino acids and γ-aminobutyric acid) evaluated in the three parts of grass turtle are shown in . As Table showed, the total amino acids were higher in muscle (74599.61 mg/100 g) than the hard shell (33451.37 mg/100 g), while liver (24103.27 mg/100 g) was the lowest amount. The major amino acids in muscle were glutamic acid 12170.46, aspartic acid 8021.44, lysine 5551.44, arginine 5529.43, leucine 5368.93, and γ-aminobutyric acid 676.7 mg/100 g, respectively. On the other hand, proline was higher in hard shell (3808.85 mg/100 g) than other sample studied. Similarly, hydroxyproline was obtained high value in hard shell (2179.10 mg/100 g). Aspartic and glutamic acid are the most important amino acids that contributed to palatability. In addition, alanine, glycine, serine, and threonine deliberated taste sweet, while arginine, leucine, isoleucine, methionine, phenylalanine, histidine, and valine provided bitter taste (Sriket et al., Citation2007), where muscle content of these amino acids was higher than the previous worked on muscle of Chinese soft-shelled turtle by Liang et al. (Citation2018). Grass turtle had the highest concentrations of proline and hydroxyproline, which enhance protein synthesis and regulate the redox state in cells and tissues, these can benefit health and highly contribute to growth, and development of human as a dietary supplementation (Phang et al., Citation2008).
Table 1. Amino acid contents in muscle, liver, and hard shell of grass turtle (mg/100 g sample, on dry weight basis).
Tabla 1. Contenido de aminoácidos del músculo, el hígado y el caparazón duro de la tortuga herbívora (mg/100 g de muestra, en base al peso seco)
On the other hand, free amino acid concentration ranged of muscle 7.81–263.50, liver 1.55–43.32, and hard shell 0.55–29.40 mg/100 g, but γ-aminobutyric acid (GABA) was the most concentrated free amino acid in muscle (263.50 mg/100 g). GABA act as a major inhibitory neurotransmitter, which was widely distributed in foods (Fang et al., Citation2012). In this study, GABA was higher in liver (43.32 mg/100 g) than reported by Kani et al. (Citation2007) obtained 6–19 mg/100 g in liver of four squid species (S. lessoniana, L. edulis, L. bleekeri, and T. pacificus). The essential amino acid of lysine intake is low in many areas of the world, whereas diets were heavily based on cereals. Cereals content of lysine ranges from 26 to 38 mg/g protein, whereas the animal foods value range from 70 to 100 mg/g protein (Nurhasan et al., Citation2010). Grass turtle contained lysine in range of 32 to 74.05 mg/g protein, therefore, it was a good source of lysine especially muscle (74.05 mg/g protein) and liver (62.91 mg/g protein). The standard value of RDI for essential amino acids like leucine 2730 mg/day, isoleucine 1400 mg/day, lysine 2100 mg/day, histidine 700 mg/day, methionine 700 mg/day, phenylalanine 1750 mg/day, threonine 1050 mg/day, tryptophan 280 mg/day, and valine 1820 mg/day daily requirements recommended for adults by W. Joint (Citation2007). As shown in presented the essential amino acids in grass turtle achieved daily requirements recommended for adults. Grass turtles are considered a rich source of essential amino acids specially lysine that could be contributing to the maintenance of human health.
3.3. Vitamins profiles
Vitamins are a broad group of organic compounds that are fat soluble and water soluble. They play important different specific and vital functions in metabolism, and their deficiency leads to specific diseases (Moreno & Salvado, Citation2000). shows that, grass turtle was found high amount of vitamin E (3.16 mg/100 g) in liver followed by muscle (2.40 mg/100 g). The vitamin E content in muscle was higher than the results obtained by Beyza and Akif (Citation2009), who found 0.34 mg/100 g of vitamin E content in aquatic species. In addition, in this study vitamin A was higher than reported result by Ali et al. (Citation2019), who found 4.94 µg/100 g of breast meat. According to RDI standard of vitamin A, D, E, and K were 300, 5, 7.5, and 65 mg/day for ≥19 years male (Joint, F. & Organization, Citation2005). In our study, 100 g sample (wet basis) provided the highest value of vitamin E and K (42.08% and 2448.72% RDI) in liver; vitamin A and D (0.71% and 16.51% RDI) in muscle.
Table 2. Distribution of vitamin contents in grass turtle (mg vitamin/100 g sample, wet weight basis) and Molecular weight distribution area (%).
Tabla 2. Distribución del contenido de vitaminas en la tortuga herbívora (mg de vitamina/100 g de muestra, con base al peso húmedo) y área de distribución del peso molecular (%)
In terms of %RDI, the liver showed high contribution to fat-soluble vitamins specially K found to be 1591.67 mg/100 g (wet basis), which play an important role in maintain ATP production in mitochondrial (Rødbotten et al., Citation2014). For water-soluble vitamins of B1, B2, B6, B9, and C were high in muscle than other parts used, while B3 contained high in hard shell (). The standard value of RDI for water-soluble vitamins B1, B2, B3, B6, B9 and C for male (≥19 years) (Joint, F. & Organization, Citation2005) are displayed in . Based on standard RDI, the 100 g muscle (wet basis) provided the highest value of vitamins B1, B2, B6, B9, and C, while hard shell provided the highest value of vitamin B3. Grass turtles are good sources of vitamins that can be alternative nutrients source for consumers.
3.4. Macro and micro minerals
Thirty one minerals (4 macro and 27 micro) were investigated are presented in . As results showed, hard shell contained the higher amounts of K 2383.06, Ca 18127.37, and Mg 1935.11 mg/100 g than the muscle and liver. Muscle contained the higher than the reported by Sales and Hayes (Citation1996). In this study 100 g sample provided the highest value of Ca 1144.20, Mg 394.01, Na 1088.14, and K 32% RDI in hard shell; whereas, daily requirements intake suggested of Ca, Mg, Na and K for adults (19–30 years) (Health, Citation2016) are presented in .
Table 3. Mineral contents in muscle, liver, and hard shell of grass turtle (mg/100 g sample, on dry weight basis).
Tabla 3. Contenido de minerales del músculo, el hígado y el caparazón duro de la tortuga herbívora (mg/100 g de muestra, con base al peso seco)
On the other hand, in muscle content (mg/100 g) micro minerals as followed Zn> Fe> B>Al> Ba> Mn> Cu> Ti> Pb> Ni> Se> Cr> Mo> As> V>Sb> Sn> Co and in liver Fe> Zn> Al> B>Cu> Mn> Ba> Pb> Ti> Se> Ni> Cr> V>Sb> As> Sn> Mo> Co. Trace elements such as iron, copper, zinc and selenium were the highest in hard shell than other parts. Results of this study achieved higher amount of Cu 2.92 and Zn 25.40 mg/100 g in liver; Fe 42.71, Cu 6.83 and Zn 254.08 mg/100 g in hard shell than reported result by Kienzle et al. (Citation2006). Mir-Marqués et al. (Citation2016) reported, zinc is a co-factor of many enzymes that contribute to synthesis and degradation of protein, lipid, and carbohydrate. On the other hand, iron deficiency is the most common nutritional disorder (anemia) in the world specially in nurture countries (Nurhasan et al., Citation2010). According to standard RDI of Zn, Fe, and Se for 19–30 years adults requirements daily intake (Health, Citation2016) as presented in . In this study, 100 g sample provided the highest value of Zn 2004.72, Fe 149.77, and Se 6916.80% RDI in hard shell. The authors concluded that grass turtles are the rich source of minerals especially in hard shell that can contribute to specific functional health benefits.
3.5. Fatty acid profiles
The fatty acid profiles of three parts of grass turtle are displayed in . The results showed that total unsaturated fatty acids were higher than saturated fatty acids in all samples. The liver contained the highest concentration of MUFAs (6270.94 mg/100 g) and SFAs (2186.59 mg/100 g) than the muscle and hard shell, whereas, the highest PUFAs concentration was found in muscle 1342.75 mg/100 g. Unsaturated fatty acids was higher than those mentioned by Liang et al. (Citation2018) in Chinese soft-shelled turtle. With regard to MUFAs, the liver was a rich in oleic acid (5152.88 mg/100 g) and palmitoleic acid (924.16 mg/100 g) than other parts. On the other hand, muscle contained the high concentration of arachidonic acid (C20:4 n-6) (96.84 mg/100 g), which is in agreement with result reported by Liang et al. (Citation2018) and Ozaki et al. (Citation2008) . In general, the result had shown that the most abundant essential fatty acids was linoleic acid and the most predominant was docosahexaenoic acid.
Table 4. Fatty acid contents of three parts of grass turtle (mg/100 g sample).
Tabla 4. Contenido de ácidos grasos de tres partes de la tortuga herbívora (mg/100 g de muestra)
The highest content of essential fatty acid was achieved in muscle (linoleic acid 868.49 and α-linolenic acid 42.38 mg/100 g), followed by hard shell (linoleic acid 397.92 and α-linolenic acid 9.27 mg/100 g), these results were higher than the published data (linoleic acid 243.08–335.02 and α-linolenic acid 17.29–31.28 mg/100 g in muscle; linoleic acid 76.17–201.49 and α-linolenic acid 4.29–7.99 mg/100 g in hard shell) (Liang et al., Citation2018). On the other hand, the total content of conditionally essential fatty acid [Eicosapentaenoic acid (EPA)+ Docosapentaenoic acid (DPA)+ Docosahexaenoic acid (DHA)] were 335.05 mg/100 g in muscle and EPA 55.55 mg/100 g in hard shell, these findings were higher than reported by Liang et al. (Citation2018). Linoleic acid content in liver and α-linolenic acid in muscle achieved the daily requirements recommended by standard RDI (Panel, Citation2002) for adults (19–30 years), whereas 100 g of our sample provided the highest value of linoleic acid 5.67% RDI in liver and α-linolenic acid 2.65% RDI in muscle. In addition, daily requirements of highly polyunsaturated fatty acids recommended by standard RDI for male (19–30 years) (Listed, Citation2008) are presented in . Therefore, the dietary intake of grass turtle can be preventing chronic diseases and brain development.
3.6. Molecular weight distribution
The molecular weight (MW) distribution of freeze-dried powder of three samples are presented in . The results showed that MW ˂1000 Da fractions were >87% in liver and 85% in muscle, but only 3.31% in hard shell. MW <180 Da in muscle and liver were contained 24.59% and 58.95%, respectively, whereas hard shell was not detected, which are presented in . May be this reasoned that muscle and liver both were contained excellent amount of essential amino acids. It has been reported that the dietary proteins rich in low molecular weight peptides have more available nutritionally (Noman et al., Citation2018). Muscle and liver contained lower MW peptides, which can be used in food products to improve the nutritional value.
3.7. Functional properties
3.7.1. Solubility
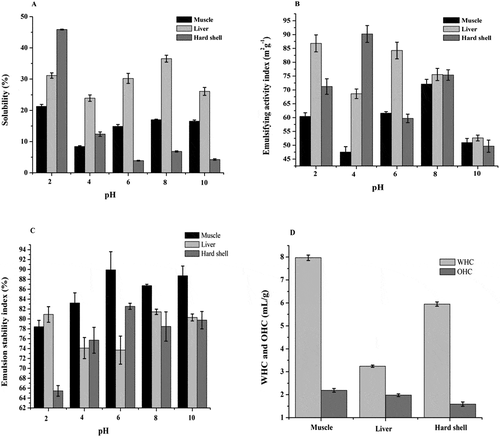
The solubility of dried powder samples in the pH range of 2–10 are presented in . As general trend, it can be observed that solubility was affected by pH. The results showed that, the liver had the highest solubility in alkaline condition (36.56% at pH 8), but muscle (21.29%) and hard shell (45.89%) had high solubility in acidic condition (pH 2). Our result was lower than reported by Sorapukdee and Narunatsopanon (Citation2017). Binsi et al. (Citation2016) reported that smaller peptides have high proportion of exposed polar residues, thereby showing enhanced solubility, in our work liver and muscle contained smaller peptides <1000 Da (87.38% and 85%). Decreased solubility of hard shell may be caused to physical and chemical properties. Overall, liver and muscle were a good solubility than hard shell, which can contribute to improve of appearance of the products and offer a smooth taste in foods.
Figure 2. The functional properties of various parts of grass turtle including (A) Solubility (%); (B) Emulsifying activity index (m2g−1); (C) Emulsion stability index (%); (D) Water and oil holding capacity (mL/g); Each bar indicates mean values of sample. Vertical bars are standard deviation (n = 3, p < 0.05).
Figura 2. Propiedades funcionales de varias partes de la tortuga herbívora, incluyendo (a) solubilidad (%); (b) índice de actividad emulsionante (m2g−1); (c) índice de estabilidad de la emulsión (%); (d) capacidad de retención de agua y aceite (mL/g). Cada barra indica los valores medios de la muestra. Las barras verticales representan la desviación estándar (n = 3, p < 0.05)
3.7.2. Emulsification capacity and stability
The grass turtles are a surface-active material that contained both hydrophilic and hydrophobic groups; hence, it encouraged the formation of an oil-in-water emulsion. The EAI and ESI values of muscle, liver, and hard shell are presented in under the different pH levels. As shown in , the higher emulsification capacity index obtained in hard shell (90.22 m2/g; pH 4), followed by liver (86.81 m2/g; pH 2) and muscle (72.10 m2/g; pH 8), these differences may be due to the acidic condition and low molecular weight of peptides (<1000 Da).
On the other hand, reveals that all ESI results were above 74% (except hard shell at pH 2) during the experimental time. Where the maximum value was observed at pH 6 and 10 and the minimum value at pH 2 and 4, may be this result was closely associated with more hydrophobic peptides contribute to the stability of the emulsion and others factors (Vilailak et al., Citation2007). At neutral and alkaline pH, ESI of all the measures were higher than that of the acidic condition, and the values were comparable with each other. These results suggest that raw samples were decreased the emulsifying activity in the pH range of 2–4 (except muscle), while the emulsion stability to be improved at pH 6 and above.
3.7.3. Water and oil holding capability
The WHC and OHC both were affected by molecular weights, where high molecular weight leads to increase in the absorption of water and oil. As shown in , the values of WHC was the highest in muscle (7.97 mL/g), followed by hard shell (5.95 mL/g), while the liver was the lowest values (3.24 mL/g). In addition, the food industry especially in meat and confectionery industries is an important parameter (oil holding capacity) of functional characterization of the ingredients used in the foods, because it influences the taste of products and also contributes to an uniform distribution of dry ingredients in the mixture (Rodrigues Freitas et al., Citation2016). The results revealed that the OHC was the lowest value in hard shell (1.59 mL/g) than liver (1.98 mL/g) and muscle (2.19 mL/g). The water and oil absorbed variation may be attributed to the hydrophilic polar side chains (Noman et al., Citation2018), in our work hydrophilic amino acids value in muscle was higher than the other parts ().
3.8. Antioxidants properties
3.8.1. ABTS free radical scavenging activity
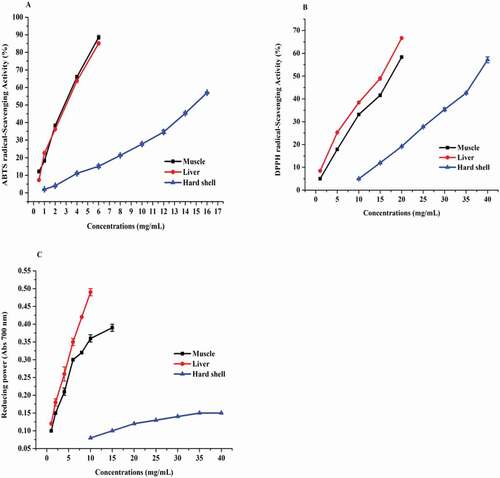
The total ABTS•+ scavenging activity of the dried muscle, liver, and hard shell are presented in . The total radical scavenging activity was in range of 57.02–88.58%. Comparing the ABTS•+ results of the samples, the total antioxidant activity of muscle was significantly higher than the liver and hard shell. Binsi et al. (Citation2016) reported that some amino acids such as His, Tyr, Met, Lys, and Trp contribute to high antioxidant activity. Additionally, branched chain amino acids (Val, Leu, and Ile) contained good antioxidant activity. Muscle contained the highest amount of above mention amino acids () than the other parts. And also shows that our study was higher than the previous published data (Serpen et al., Citation2012), would be due to muscle and liver have low molecular weight peptides (<1000 Da). Stefanović et al. (Citation2014) finding that low molecular mass peptides <1000 Da could have a higher ABTS radical scavenging activity than other molecular mass peptides. Their IC50 value in the muscle sample was highly active (3.08 ± 0.05 mg/mL) than liver (3.21 ± 0.05 mg/mL), while hard shell low active (15.12.99 ± 0.25 mg/mL). However, ABTS of grass turtle was significantly lower than that of the standard BHT (IC50 = 0.18 ± 0.01 mg/mL).
Figure 3. The antioxidant activities of muscle, liver, and hard shell of grass turtle including (A) ABTS free radical scavenging activity (%); (B) DPPH free radical scavenging activity (%); and (C) Reducing power at 700 nm. Each bar indicates mean ± SD values of sample.
Figura 3. Actividades antioxidantes del músculo, el hígado y el caparazón duro de la tortuga herbívora, incluyendo (A) actividad de eliminación de radicales libres ABTS (%); (B) actividad de eliminación de radicales libres DPPH (%); y (C) poder reductor a 700 nm. Cada barra indica los valores medios ± DE de la muestra
3.8.2. DPPH radical scavenging activity
DPPH free radical scavenging activity of three parts is revealed in at various concentrations. The result shows the highest DPPH scavenging activity was 66.71% in liver, followed by muscle (58.33%) and lowest value obtained in hard shell 19.20% at the concentration (20 mg/mL). These values may be related to molecular weight (˂1000 Da). The activities of test materials to scavenge DPPH inveterate in the terms of their IC50 values, which the concentration of test sample required to scavenge 50% of DPPH free radical. Their IC50 values for DPPH radical of liver, muscle, and hard shell were 14.47 ± 0.14, 17.13 ± 0.15, and 37.90 ± 0.49 mg/mL, respectively, which are lower in antioxidant activity than commercial standard BHT (IC50 = 0.23 ± 0.00 mg/mL). These results may be attributed to the content of small molecular weights in muscle and liver (Binsi et al., Citation2016). Therefore, grass turtles contained a good source of antioxidants, which can be used as functional foods.
3.8.3. Reducing power
The reducing power was indicated to increase the absorbance at 700 nm. shows that, liver was the higher reducing powder (0.494 unit) at concentration of 10 mg/mL than muscle (0.364 unit), and hard shell (0.078 unit). Hard shell was low reducing powder, which achieved absorbance 0.141 at high concentration (40 mg/mL). Although, our results were lower than standard BHT (0.641 unit at concentration of 0.5 mg/mL), Binsi et al. (Citation2016) discover that MW<5000 Da were mainly responsible to increase of antioxidant activity, which can be retarded the oxidation reaction of volatile compounds in food storage (Saiga et al., Citation2003). This finding is closely associated with result obtained of molecular weight <5000 Da.
4. Conclusions
Our findings revealed that there were significant differences (p <.05) in the nutritional parameters and physiochemical properties between the individual parts of the grass turtle. The samples contained the well-balanced essential amino acid especially in muscle and liver. DHA and DPA was the highest in muscle, but EPA was high in liver. Liver contained the highest value of vitamin E and K, whereas vitamin B3 in hard shell and vitamin A in muscle. The functional properties affected by pH, where observed that the muscle and liver had a high functional properties. Among the three antioxidant assays evaluated, the ABTS free radical activity achieved to be significantly highest in muscle, whereas, FRAP and DPPH free radical activity were high in the liver, which could be a help to health-benefiting biological activities. Therefore, further studies should extract protein and evaluate the specific novel antioxidant peptides, which are highly needed to develop the functional foods.
Ethical statement
This research article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgments
The authors would like to thank the National first-class discipline program of Food Science and Technology (JUFSTR20180204) for financially supported, and China Scholarship Council (CSC), Beijing, China (CSC No. 2017GXZ017968). Also thanks to Guangxi zhongtaikang Technology Industry Co., Ltd., Guangxi, P. R. China for providing the samples to carry out experiments.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Ali, M., Lee, S.-Y., Park, J.-Y., Jung, S., Jo, C., & Nam, K.-C. (2019). Comparison of functional compounds and micronutrients of chicken breast meat by breeds. Food Science of Animal Resources, 39(4), 632. https://doi.org/https://doi.org/10.5851/kosfa.2019.e54
- AOAC. (1995). Official methods of analysis of AOAC International (16th ed.). Association of Officiation of Official Analytical Chemists.
- Beyza, E., & Akif, O. Z. (2009). The effect of cooking methods on mineral and vitamin contents of African catfish. Food Chemistry, 115(2), 419–422. https://doi.org/https://doi.org/10.1016/j.foodchem.2008.12.018
- Binsi, P. K., Viji, P., Panda, S. K., Mathew, S., Zynudheen, A. A., & Ravishankar, C. N. (2016). Characterisation of hydrolysates prepared from engraved catfish (Nemapteryx caelata) roe by serial hydrolysis. Journal of Food Science and Technology, 53(1), 158–170. https://doi.org/https://doi.org/10.1007/s13197-015-1998-6
- Chen, T. H., Chang, H. C., & Kuangyang, L. (2009). Unregulated trade in turtle shells for Chinese traditional medicine in East and Southeast Asia: The case of Taiwan. Chelonian Conservation & Biology, 8(May2009), 11–18. https://doi.org/https://doi.org/10.2744/CCB-0747.1
- Chen, T.-H., Lin, H.-C., & Chang, H.-C. (2000). Current status and utilization of chelonians in Taiwan. Chelonian Research Monographs, 2, 45–51.
- Cho, M., Lee, H. S., Kang, I. J., Won, M. H., & You, S. (2011). Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chemistry, 127(3), 999–1006. https://doi.org/https://doi.org/10.1016/j.foodchem.2011.01.072
- Copper, I. (2001). Dietary reference intakes for vitamin A vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. The National Academies Press.
- Corp, I. (2013). IBM SPSS statistics for windows. version 22.0. IBM Corp.
- Dai, S., Ota, H., Oh, H. S., & Hikida, T. (2012). Origin of Japanese populations of reeves’ pond turtle, Mauremys reevesii (Reptilia: Geoemydidae), as inferred by a molecular approach. Chelonian Conservation & Biology, 10(Dec2011), 237–249. https://doi.org/https://doi.org/10.2744/CCB-0885.1
- Dhanabalan, V., Xavier, M., Kannuchamy, N., Asha, K. K., Singh, C. B., & Balange, A. (2017). Effect of processing conditions on degree of hydrolysis, ACE inhibition, and antioxidant activities of protein hydrolysate from Acetes indicus. Environmental Science and Pollution Research, 24(26), 21222–21232. https://doi.org/https://doi.org/10.1007/s11356-017-9671-4
- Ersoy, B., & Özeren, A. (2009). The effect of cooking methods on mineral and vitamin contents of African catfish. Food Chemistry, 115(2), 419–422. https://doi.org/https://doi.org/10.1016/j.foodchem.2008.12.018
- Fang, Z., Ou, J., Huang, Y., Qian, L., Xu, G., Liu, Z., & Yang, S. (2012). Determination of 21 free amino acids in fruit juices by HPLC using a modification of the 6-Aminoquinolyl- N -hydroxysuccinimidyl Carbamate (AQC) method. Food Analytical Methods, 8(2), 1–10. https://doi.org/https://doi.org/10.1007/s12161-014-9905-8
- García‐Moreno, P. J., Pérez‐Gálvez, R., Espejo‐Carpio, F. J., Ruiz‐Quesada, C., Pérez‐Morilla, A. I., Martínez‐Agustín, O., … Guadix, E. M. (2017). Functional, bioactive and antigenicity properties of blue whiting protein hydrolysates: Effect of enzymatic treatment and degree of hydrolysis. Journal of the Science of Food and Agriculture, 97(1), 299–308. https://doi.org/https://doi.org/10.1002/jsfa.7731
- Hall, F., Johnson, P. E., & Liceaga, A. (2018). Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chemistry, 262, 39–47. https://doi.org/https://doi.org/10.1016/j.foodchem.2018.04.058
- Health, N. I. O. (2016). Nutrient recommendations: Dietary reference intakes (DRI). In Food and Nutrition Board, National Academy of Sciences.
- Joint, W. (2007). Protein and amino acid requirements in human nutrition. World Health Organization Technical Report Series, (935), 1.
- Joint, F., & Organization, W. H. (2005). Vitamin and mineral requirements in human nutrition. World Health Organization, 341.
- Kani, Y., Yoshikawa, N., Okada, S., & Abe, H. (2007). Comparison of extractive components in muscle and liver of three Loliginidae squids with those of one Ommastrephidae species. Fisheries Science, 73(4), 940–949. https://doi.org/https://doi.org/10.1111/j.1444-2906.2007.01417.x
- Kienzle, E., Kopsch, G., Koelle, P., & Clauss, M. (2006). Chemical composition of turtles and tortoises. Journal of Nutrition, 136(7Suppl), 2053S–2054S. https://doi.org/https://doi.org/10.1093/jn/136.7.2053S
- Liang, H., Tong, M., Cao, L., Li, X., Li, Z., & Zou, G. (2018). Amino acid and fatty acid composition of three strains of chinese soft-shelled turtle (Pelodiscus sinensis). Pakistan Journal of Zoology, 50(3), 1061–1069. https://doi.org/https://doi.org/10.17582/journal.pjz/2018.50.3.1061.1069
- Lin, W.-Y., & Huang, C.-H. (2007). Fatty acid composition and lipid peroxidation of soft-shelled turtle, Pelodiscus sinensis, fed different dietary lipid sources. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 144(4), 327–333. https://doi.org/https://doi.org/10.1016/j.cbpc.2006.10.006
- Listed, N. (2008). Fats and fatty acids in human nutrition. Report of an expert consultation. Fao Food Nutr Pap, 91(91), 1–166.
- Mahdi, A. A., Al-Ansi, W., Ahmed, M. I., Xiaoyun, C., Mohammed, J. K., Sulieman, A. A., Mushtaq, B. S., Harimana, Y., & Wang, H. (2020). Microwave assisted extraction of the bioactive compounds from peel/pulp of Citrus medica L. var. sarcodactylis swingle along with its nutritional profiling. Journal of Food Measurement and Characterization, 14(1), 283–292. https://doi.org/https://doi.org/10.1007/s11694-019-00290-6
- Mir-Marqués, A., Cervera, M. L., & Guardia, M. D. L. (2016). Mineral analysis of human diets by spectrometry methods. Trends in Analytical Chemistry, 82, 457–467. https://doi.org/https://doi.org/10.1016/j.trac.2016.07.007
- Moreno, P., & Salvado, V. (2000). Determination of eight water-and fat-soluble vitamins in multi-vitamin pharmaceutical formulations by high-performance liquid chromatography. Journal of Chromatography A, 870(1–2), 207–215. https://doi.org/https://doi.org/10.1016/S0021-9673(99)01021-3
- Noman, A., Xu, Y., AL-Bukhaiti, W. Q., Abed, S. M., Ali, A. H., Ramadhan, A. H., & Xia, W. (2018). Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme. Process Biochemistry, 67, 19–28. https://doi.org/https://doi.org/10.1016/j.procbio.2018.01.009
- Nurhasan, M., Maehre, H. K., Malde, M. K., Stormo, S. K., Halwart, M., James, D., & Elvevoll, E. O. (2010). Nutritional composition of aquatic species in Laotian rice field ecosystems. Journal of Food Composition & Analysis, 23(3), 205–213. https://doi.org/https://doi.org/10.1016/j.jfca.2009.12.001
- Ozaki, Y., Koga, H., Takahashi, T., Adachi, S., & Yamauchi, K. (2008). Lipid content and fatty acid composition of muscle, liver, ovary and eggs of captive-reared and wild silver Japanese eel Anguilla japonica during artificial maturation. Fisheries Science, 74(2), 362–371. https://doi.org/https://doi.org/10.1111/j.1444-2906.2008.01525.x
- Panel, O. M. (2002). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). National Academics Press.
- Phang, J. M., Donald, S. P., Pandhare, J., & Liu, Y. (2008). The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids, 35(4), 681–690. https://doi.org/https://doi.org/10.1007/s00726-008-0063-4
- Rødbotten, R., Gundersen, T., Vermeer, C., & Kirkhus, B. (2014). Vitamin K2 in different bovine muscles and breeds. Meat Science, 97(1), 49–53. https://doi.org/https://doi.org/10.1016/j.meatsci.2014.01.005
- Rodrigues Freitas, I., Cortez-Vega, W. R., & Prentice, C. (2016). Physicochemical and functional properties of protein recovered from fish waste. Journal of Aquatic Food Product Technology, 25(7), 1034–1044. https://doi.org/https://doi.org/10.1080/10498850.2015.1008714
- Saiga, A., Tanabe, S., & Nishimura, T. (2003). Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. Journal of Agricultural and Food Chemistry, 51(12), 3661–3667. https://doi.org/https://doi.org/10.1021/jf021156g
- Sales, J., & Hayes, J. P. (1996). Proximate, amino acid and mineral composition of ostrich meat. Journal Food Chemistry, 56(2), 167–170. https://doi.org/https://doi.org/10.1016/0308-8146(95)00201-4
- Serpen, A., Gökmen, V., & Fogliano, V. (2012). Total antioxidant capacities of raw and cooked meats. Meat Science, 90(1), 60–65. https://doi.org/https://doi.org/10.1016/j.meatsci.2011.05.027
- Sorapukdee, S., & Narunatsopanon, S. (2017). Comparative study on compositions and functional properties of porcine, chicken and duck blood. Korean Journal for Food Science of Animal Resources, 37(2), 228. https://doi.org/https://doi.org/10.5851/kosfa.2017.37.2.228
- Sriket, P., Benjakul, S., Visessanguan, W., & Kijroongrojana, K. (2007). Comparative studies on chemical composition and thermal properties of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) meats. Food Chemistry, 103(4), 1199–1207. https://doi.org/https://doi.org/10.1016/j.foodchem.2006.10.039
- Stefanović, A., Jovanović, B., Jelena, R., Grbavčić, Sanja, Ž., & Verica, B. (2014). Impact of ultrasound on egg white proteins as a pretreatment for functional hydrolysates production. European Food Research & Technology, 239(6), 979–993. https://doi.org/https://doi.org/10.1007/s00217-014-2295-8
- Vilailak, K., Soottawat, B., Duangporn, K., & Fereidoon, S. (2007). Antioxidative activity and functional properties of protein hydrolysate of yellow Stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chemistry, 102(4), 1317–1327. https://doi.org/https://doi.org/10.1016/j.foodchem.2006.07.016
- Yang, M., Liu, C., Huang, F., Zheng, C., & Zhou, Q. (2011). Effect of dehulling treatment on the oxidative stability of cold‐pressed low erucic acid rapeseed oil. Journal of the American Oil Chemists’ Society, 88(10), 1633–1639. https://doi.org/https://doi.org/10.1007/s11746-011-1822-z
- Zhang, Y., Luo, H., Liu, K., Jia, H., Chen, Y., & Wang, Z. (2015). Antioxidant effects of liquorice (Glycyrrhiza uralensis) extract during aging of longissimus thoracis muscle in Tan sheep. Meat Science, 105, 38–45. https://doi.org/https://doi.org/10.1016/j.meatsci.2015.03.002
- Zou, Y., Xu, P., Li, P., Cai, P., Zhang, M., Sun, Z., Sun, C., Xu, W., & Wang, D. (2017). Effect of ultrasound pre-treatment on the characterization and properties of collagen extracted from soft-shelled turtle (Pelodiscus sinensis). LWT - Food Science and Technology, 82, 72–81. https://doi.org/https://doi.org/10.1016/j.lwt.2017.04.024