ABSTRACT
Curcumin is a phenolic pigment, naturally present in Curcuma longa species. It is a yellow active ingredient and shows a pivotal role in the modulation of biological processes resulting in the prevention of cancer particularly due to its radical scavenging activities. In gastrointestinal cancer cells, curcumin has been shown to induce cell death through apoptosis and to cause cell cycle arrest, down-regulating glycolytic enzyme expressions alongside inhibition of the matrix metalloproteinase-2 (MMP-2) promoter activity and SDF-1α-induced cell invasion. It also activates the expression of cleaved caspase-3, reduces cell viability, regulates the ratio of Bcl-2/Bax, decreases the number of cells in the proliferative G0/G1 phase and increases the number of cells in the S phase. Additionally, curcumin prevents DNA from replication during the S phase. This review discusses the chemo-preventive role of curcumin and its mechanisms against human gastrointestinal cancers to understand its activity and potential utilization as a therapeutic moiety.
1. Introduction
A deadly disease like cancer causes a great deal of death on a global scale (Mohan et al., Citation2020). Cancer is brought on by genetic mutations (Patra et al., Citation2020). These mutations cause dysregulations in the molecular signaling pathways that aid in the genesis and progression of cancer (Tewari et al., Citation2019). Numerous malignant behaviors distinguish cancer cells from healthy cells, including prolonged growth signals, resistance to apoptosis, resistance to anti-growth factors, migration and metastasis into nearby and distant cells and tissues, increased angiogenesis, enhanced proliferative capacity, and genome instability (Banik et al., Citation2019).
Cancer incidence rates continue to rise, endangering the general public’s health. According to epidemiological research, cancer poses a serious hazard to a large number of people worldwide (J. X. Hu et al., Citation2021). For instance, it is projected that there would be 23 million cancer patients by 2035, which is a significant increase from the 14 million cases reported in 2012 (Ferlay et al., Citation2015). The basic causes of cancer formation and their regulation through pharmacological and genetic therapies must therefore be thoroughly researched. It is now pretty evident that anomalies in signaling networks play a major role in the development of cancer thanks to studies conducted in recent decades and cellular approaches employed for genomics screening (Ashrafizadeh et al., Citation2020).
Globally, gastrointestinal (GI) cancers are prevailing at alarming rates and 65% of the deaths from reported cases are being recorded in the Asian region followed by Europe and North America (Arnold et al., Citation2020). To cure malignancy, various approaches are adopted amongst which the treatment from natural compounds is preferred due to their safer nature and fewer or no side effects. The phrase “gastrointestinal (GI) cancers” refers to malignancies that can develop in any part of the gastrointestinal tract, including the oesophagus, pancreas, liver, small intestine, colorectum, and stomach (Thomson et al., Citation2003). With an additional 5.0 million new cases diagnosed in the same year, they are predicted to be responsible for 3.5 million deaths globally in 2020. After lung and breast cancers, colorectal cancer is the most prevalent type of GI cancer. By comparison, gastric, liver, esophageal, and pancreatic cancers are ranked as the fifth, sixth, eighth, and twelfth most frequently diagnosed cancers, respectively (Sung et al., Citation2021).
Turmeric (Curcuma longa) belongs to the ginger family Zingiberaceae. It has many functional and nutraceutical properties and rich source of curcumin. It is a type of herb and is used as a spice to naturally add color and taste to different food items. It has promising beneficial health-promoting perspectives due to the presence of the bioactive compound curcumin having an orange-yellow color and is lipophilic in nature (Kocaadam & Şanlier, Citation2017). It has been reported that tumors can be reduced at different stages of the cell cycle using curcumin. It blocks various enzymes that participate in the growth and development of tumors and may resist tumor treatment. Furthermore, curcumin also modulates cellular progressions, i.e., protein kinase C activity, EGF (epidermal growth factor) receptor intrinsic kinase activity, nuclear factor kappa (NF-kB) activity, nitric oxide synthesize activity, and suppresses lipid peroxidation (Imran et al., Citation2018). Curcumin, a plant-derived polyphenol, has been identified as a therapeutically effective food that exhibits pleiotropic pharmacological effects on a variety of malignancies (Lim, Citation2022). Di-hydrocurcumin, tetra-hydrocurcumin, hexa-hydrocurcumin and octa-hydrocurcumin are most common metabolites of curcumin in a cellular culture. Among these, tetra-hydrocurcumin and hexa-hydrocurcumin are most abundant. Although several studies, both in vitro and in vivo, have explored the metabolic pathways of curcumin’s secondary metabolites, direct relation of these metabolites in amelioration of cancer prevention or treatments has not been made. The most documented functions of these metabolites are anti-oxidative and anti-inflammatory, which could lead to the speculation that they play an anti-cancer role (Aggarwal et al., Citation2014; Pandey et al., Citation2020).
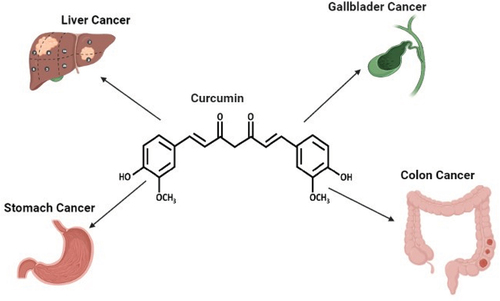
Curcumin has an anti-tumor role in gastric cancer cells via inhibiting invasion and proliferation and inducing apoptotic cell death in experimental subjects (Kwiecien et al., Citation2019). Curcumin has been chosen by the National Cancer Institute as a third-generation cancer chemo preventive drug (Abd El‐Hack et al., Citation2021). In different in vivo and in vitro studies, curcumin has exhibited anticancer effects involving mechanisms such as reduction in the formation of liver tumors, suppression of metastasis of primordial germ cell (PGC), CXCR4 expression, and inhibition of stromal cell-derived factor-1/CXCR4 signaling (Gu et al., Citation2019). Furthermore, curcumin suppresses the p-Akt protein expression, increments in PTEN expression, and reduction in miR-21 levels. It also shows suppression of STAT3 phosphorylation, blocked STAT3-mediated signaling, induction of growth arrest, and apoptosis (Qiang et al., Citation2019). Curcumin has the effects of reducing the dosage, resistance and side effects of chemotherapy drugs, besides a pivotal role in the modulation of biological processes resulting in the prevention of cancer particularly due to its radical scavenging activities and other mechanisms (Zhou et al., Citation2011, Citation2017). The anti-neoplastic effects of curcumin are attained by the suppression of molecular pathways involving proliferation and inflammation and supporting the development of colorectal cancer. In a mouse model of colorectal cancer fed with a diet supplemented with curcumin, there is a reduction in the incidence of cancer, colonic inflammation, and the formation of adenoma/adenocarcinomas (Guo et al., Citation2018). Curcumin has been shown to have positive effects that can reduce tumor volume and chemoresistance in preclinical studies which examine the interaction between curcumin and colorectal cancer chemotherapeutics, such as 5-fluorouracil or oxaliplatin (Hosseini et al., Citation2017). Several studies done by using curcumin in conjunction with various anticancer compounds, as in piperine, selenium, prednisolone and ursolic acid, have shown significant suppression, reduction/inhibition of IL-6, IL-1β, IL-19, TNF-α and COX-2 (Al-Dossari et al., Citation2020; X. Q. Hu et al., Citation2016; Neyrinck et al., Citation2013; Tremmel et al., Citation2019; Yan et al., Citation2019). While flavocoxid in combination with curcumin reduced the transcription factors NF-κB and STAT3 mRNA expression (D’Ascola et al., Citation2019). In similar in vivo studies, boswellic acid and irbesartan in combination with curcumin showed reduction in TNF-α and IL-6 cytokines (Khaled & Mahfouz, Citation2010; Khayyal et al., Citation2018). Reduction in EGFR signaling and glycogen synthase kinase-3 was also observed when paclitaxel was used in conjunction with curcumin (M. Zhou et al., Citation2015). Meanwhile, increased apoptosis and downregulation of XIAP was reported when curcumin was used with resveratrol (Du et al., Citation2013). Since curcumin’s competency to inhibit different cancers is of great importance, the main objective of this review is to summarize specifically its roles in the prevention of gastrointestinal cancers ().
1.1. Chemical structure and source of curcumin
Curcumin is a plant derived phenolic compound naturally present in turmeric. It is also present in mango ginger. It has health endorsing potential due to its antioxidant and anti-inflammatory properties as well as exhibits antiviral, antibacterial, and anticancer activities.
Curcumin belongs to the group of curcuminoids, yellow pigment polyphenol. It has been used historically in ayurvedic medicines. It is a combination of a seven-carbon linker and three functional groups: an α, β-unsaturated, β-diketone moiety, and O-methoxy-phenolic group. The O-methoxy-phenolic group is linked by two α, β-unsaturated carbonyl groups shown in (Kita et al., Citation2008). In acidic/neutral aqueous solutions and cell membranes, the keto form is predominant. On the other hand, in an alkaline medium, the enol form of the heptadienone chain predominates. Due to the presence of a highly active, central carbon atom, the keto form of curcumin functions as a very potent H-atom donor in the pH range of 3–7. Because of the delocalization of the unpaired electrons on the nearby oxygens, the C-H bonds of this carbon are particularly weak. The heptadienone moiety of curcumin’s keto-enol-enolate balance controls its physicochemical and antioxidant characteristics (Stanić, Citation2017).
2. Anticancer perspectives
Curcumin shows multispectral anticancer effects and recent studies have explored its mechanism of action to design and develop anticancer therapies. Anticancer role of curcumin against gastrointestinal cancers has been summarized in . The effect of curcumin treatments on various gastrointestinal cancers is discussed below.
Table 1. Anticancer role and mechanisms of curcumin against gastrointestinal cancers.
2.1. Esophagus cancer
In a recent study, curcumin proved inhibitory effect on cell proliferation and invasion stages in combination with 5-fluorouracil (40 μM) in different human ESCC (esophageal squamous cell carcinoma), TE-1, TE-8, and KY-5 (Pendleton et al., Citation2019). In another study, curcumin showed enhanced apoptosis, exerted cell cycle arrest, hindered tumor development and attacked esophageal squamous cell carcinoma in a xenograft model using mice as depicted in (Tung et al., Citation2018).
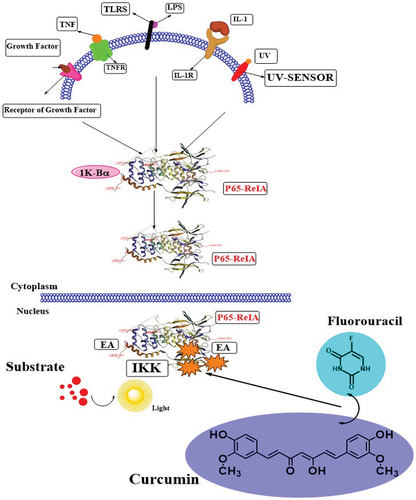
One of the in vivo research projects described that curcumin induces cell death and causes cell cycle arrest in KYSE30 cancer cells at the G1 phase (Alibeiki et al., Citation2017). It was observed that glycolytic enzymes in Ec109 cancer cells are linked with cell invasion and proliferation. The curcumin supplementation in Ec109 cancer cells at the G2/M phase exhibited significant cell cycle seizure and down-regulated glycolytic enzyme expressions in a dose-dependent fashion. Similarly, Li and their team-mates described the in vitro application of different concentrations of curcumin (15–120 µmol/L) in a EC109 human esophageal carcinoma cell line, induced apoptosis, repressed the proliferation, and decreased the level of AKT dose-dependently (X. J. Li et al., Citation2015). It also enhanced the GSK3β, PTEN and caspase-3 expressions and PI3K/AKT signaling pathway (Martins et al., Citation2015). In another in vitro study done by Lin and their colleagues, they determined that nanoparticle-based curcumin has a noteworthy effect on esophageal adenocarcinoma and Barrett’s esophagus cell lines via lowering cell viability (Lin et al., Citation2014). The metastasis is linked with stromal cell-derived factor-1αin in cancer cells, which promotes cell division and invasion. Curcumin has the potential to inhibit the matrix metalloproteinase-2 (MMP-2) promoter activity and SDF-1α-induced cell invasion at lipid rafts and cell surface localization (CXCR4) in esophageal cancer. By preventing the formation of Rac1/PI3K/Akt signaling complexes associated with lipid rafts, curcumin administration greatly reduced the effect of SDF-1 on EC cell invasion. This resulted in the reduction of NF-B-mediated MMP-2 promoter activity and expression (Lin et al., Citation2014). In different human carcinomas cell lines of esophageal (TE-1, TE-8, YES-1, YES-2, KY-5, and KY-10) cells incubated in different concentrations of curcumin (20–80 μM) for 30 hrs exerted anticancer role in a dose-dependent fashion via inhibiting cell proliferation and invasion (Tian et al., Citation2012). A group of researchers and investigators explored the in vivo anticancer role of curcumin in ESCC lines (EC9706 and Eca109) through multiple mechanisms such as inhibition of NF-κB by the suppressing IκBα phosphorylation, induction of apoptosis, reduction in cell viability, tumor growth and volume, down regulation of levels of Bcl-2 cyclinD1, IκBα phosphorylation and suppression of another signaling pathway named NF-Κb (Tian et al., Citation2012). Combined treatment of curcumin along with 5-FU mediates the deactivation of NF-κB activated signaling pathway via p65 expression inhibition () and decrease in cell viability & tumor volume and enhancement in apoptosis cell death in ESCCEC9706 and Eca109 of human xenograft model (Rawat et al., Citation2012).
An in vitro study of OE33 (esophageal cell lines), 50 μM of curcumin supplementation in patients, also prevented DNA damage induced by bile acid and nuclear factor-kappa B (NF-κB) activity, whereas in vivo study showed suppression of IL-8 expression in comparison to the squamous control (Subramaniam et al., Citation2012). A group of researchers and investigators explained the anticancer mechanism of curcumin against esophageal cancer by multiple pathways such as cell growth inhibition, Notch signaling pathway mediation, apoptosis induction, and caspase 3 activation, enhancement in the ratio of Bax to Bcl2 and downregulation of cyclin D1 expressions, respectively. In addition, reduction in number and size of esophagospheres, Notch-1 activation, expression of Jagged-1 and its downstream target Hes-1, along with down-regulation of perilous components of the γ-secretase complex proteins like Nicastrin, and Presenilin 1 was reported after curcumin treatment. Furthermore, Notch-1 specific microRNAs miR-34a and miR-21 expression is also down-regulated whilst tumor suppressor let-7a miRNA is up-regulated. Conclusively, curcumin has proven as a strong esophageal cancer inhibitor due to the release of γ-secretase complex proteins by Notch-1 activation (Schiffman et al., Citation2012). In another study conducted by Bower and their colleagues, they explicated that different doses of curcumin (10–100 micromol/L), a HET-1A (esophageal epithelial cell line) showed minimum inhibition, caused reductions in COX-2 expression and suppression of bile acid induction of COX-2 gene expression (Bower et al., Citation2010).
CUR-LIP polymers improve the stability, bioavailability, and targeting of CUR, according to Feng et al. (Citation2017). However, when CUR-LIP polymers reach the bloodstream, monocytes and macrophages quickly ingest them and focus on tissues with high endothelial content. Its target specificity and the target area’s effective dose are thus decreased. Additionally, it has been shown that the Keap1/Nrf2/kelch-like erythroid cell-derived protein 1 pathway aids in the healing of diabetic wounds (Rabbani et al., Citation2019). In order to determine if CUR-LIP-F127 can influence wound healing by regulating the Nrf2/Keap1 pathway, this work sought to construct a Pluronic F127-liposome-encapsulated CUR. Monocytes and macrophages may easily ingest CUR-LIP polymers when they are injected into the bloodstream, and they then target areas with many endothelial cells. The target area’s effective dosage and target specificity are consequently decreased. Nrf2 promotes corneal epithelial wound healing by speeding up corneal epithelial cells’ ability to migrate (Q. Zhou et al., Citation2022).
2.2. Gallbladder cancer
Apoptosis plays a pivotal role in controlling the growth of cancer cells. Different apoptosis signals are triggered by the Caspase-3; consequently, many vital proteases are inactivated in the cytoskeleton and thus resulted in apoptosis. Cell apoptosis is regulated by different mechanisms such as Bax (apoptosis-promoting proteins), Bcl-2 (anti-apoptotic protein), and increased cleavage of PARP (T. Y. Liu et al., Citation2013). Researchers have investigated that curcumin in GBC-SD cells (gallbladder carcinoma) induced apoptosis, reduced the cell viability, and activated the caspase-3 cleaved, regulated Bcl-2/Bax ratio, substantial rise in the cell numbers in the S phase and decrease in the cell numbers at G0/G1 phase to reduce proliferation. These effects have been seen to be dependent upon the exposure time and the administered doses of the curcumin. Additionally, curcumin can prevent from DNA replication by hindering the cells at S-phase that further suppressing tumor growth (Ono et al., Citation2013).
There are multiple anti-cancer effects of curcumin involved in human gallbladder adenocarcinoma cells (HAG-1) such as induced cell cycle arrest in the G2/M phase, apoptosis, rise in extracellular signal-regulated kinase (ERK1/2) constitutive activity, inhibition of AKT activation, and drop the activities of mTOR (mammalian target of rapamycin) and S6K1 (S6 kinase-1) downstream molecules and elF4E-binding protein-1 in a dose-dependent manner. Besides, curcumin also decreased the Bcl-2 function, increased MAP (mitogen-activated protein) kinase activity, and lowered the levels of phosphorylation of Bcl-2(anti-apoptotic). Furthermore, it has also been recorded that curcumin did not impart any effect on Bax (pro-apoptotic), and NF-κB levels (anti-apoptotic nuclear factor) (Ren et al., Citation2018).
2.3. Liver cancer
In different liver cancer cell lines (HepG2TT and HepG2), curcumin significantly suppresses proliferation, invasion, and metastasis. Moreover, it induces apoptosis and decreases TLR4 (toll-like receptor 4) levels, and HSP70, eHSP70 (intracellular heat shock protein 70). The production of TLR4 receptors on the surface of immunological and tumor cells is stimulated by extracellular HSP70. The transcription of inflammatory genes such as cytokines, chemokines, and growth factors is promoted when HSP70 interacts with TLR4. The NF-κB pathway is crucial for the incidence and growth of tumors, and it has been previously documented that curcumin inhibits NF-κB transcription. Because TLR4 is the eHSP70 receptor, blocking this connection may indirectly prevent NF-B activation (Ren et al., Citation2018). A study reported by Chang and their colleagues investigated in vitro anticancer role of curcumin against H22 and HepG2cells tumor-bearing mice through multiple pathways like lowering the cell proliferation and tumor growth, VEGF protein down-regulation, caused tumor microvascular density reduction, and caspases-3 protein up-regulation (Chang et al., Citation2018). In a study conducted by Afrin and their co-workers (Citation2017), curcumin has a preventive role in male mice. The oral gavage curcumin supplementation at a dose rate of 100 mg/kg lowered serum aminotransferases concentrations, pro-inflammatory cytokines, interferon (IFN), the level of steatosis & fibrosis, decreased IFNγ-inducible protein 10, and interleukin-1β, and oxidative stress of hepatic protein expression (Afrin et al., Citation2017). Toll-like receptor 4 was reduced, and HMGB1 (cytoplasmic translocation of high mobility group box 1), ending of vascular endothelial growth factor, weakening nuclear translocation of NF-κB, protein expression of glypican-3 and prothrombin were also reported after curcumin treatment in the NASH liver (Tork et al., Citation2016). The curcumin markedly lowered pro-inflammatory cytokines, α-fetoprotein, and up-regulation of, LC3, Cx43, Q10 mRNA and UCP-3 and Mito in HCC (hepatocellular carcinoma) of female adult albino rats. Additionally, the death of non-apoptotic cells, mitochondrial functions and improvement of C×43 expression were reported to repress the tumor growth after curcumin treatment (Zheng et al., Citation2016).
Curcumin also increases the antitumor activity in HepG2 cells through enhancing the effects of ABT-737 (anti-apoptotic proteins). Synergistically, activation of ROS-ASK1-c-Jun N-terminal kinase pathway is significant effect of ABT-737 and curcumin (Hsu et al., Citation2015). Wang and their co-workers explored the effects of curcumin and found that it is an effective chemo-preventive agent in Hep3B, HepG2 and Huh7-NF-κB-luc2, cells through enhancing the cytotoxicity induced by radiations, depleting MMP, suppressing the NF-κB radiation-induced activity and expressions of downstream NF-κB protein (D. Wang et al., Citation2015). In a recent scientific report, nano-based curcumin lipid carriers showed a minor effect on VEGFR-1 along with down-regulating the levels of VEGF and VEGFR-2 (Qu et al., Citation2018). Overall, curcumin has vital importance for consideration as a therapeutic agent against hepatocellular carcinoma.
2.4. Pancreatic cancer
High glucose has been found to enhance the production rate, migration and invasion of cancer cell. It also exerts activation of ERK and induction of expression of EGF, Akt, and EGFR in BxPC-3 (human pancreatic cancer cells). Curcumin treatment encounters these changes through abrogating the invasion, migration and proliferation of cancer cell stages and also suppresses EGF-modulated initiation of EGFR, Akt and ERK along with inhibiting the level of uPA and E-cadherin, respectively (Arya et al., Citation2018). In an in vitro study, curcumin-based PLGA (poly d,l-lactide-co-glycolide) in metastatic pancreatic cancer increases the anti-invasive, anti-apoptosis and anti-migratory potential (Jalde et al., Citation2018; W. Li et al., Citation2018).
Different studies validated the chemo-preventive role of curcumin for various pancreatic cancer cell e.g. MIA PaCa-2, AsPC-1, and PANC-1 cell lines. It was found that up-regulation in levels of Bax, down-regulating anti-apoptotic proteins (Bcl-2), cleaved caspase 9 and released of cytochrome-c (Le et al., Citation2018; Zhu et al., Citation2018). Curcumin has an inhibitory effect on PANC1 and BxPC3 cell lines growth which markedly suppressed cell proliferation, caused cell cycle seizure at the G2/M phase, induced apoptotic cell death, down-regulated the Bcl2 protein expressions and up-regulated the Bax and LC3II levels (Yoshida et al., Citation2017). In addition, curcumin also sensitizes the cancer cells by suppressing the levels of the Polycomb Repressive Complex 2 (PRC2) subunit Enhancer of Zeste Homolog-2 (EZH2) and its associated lncRNA PVT1, preventing the development of I spheroids, downregulating the self-renewal driving genes, and inhibiting the gemcitabine-resistant tumor growth in a xenograft mouse model of pancreatic ductal adenocarcinoma (D. Yang et al., Citation2017). Su and their colleagues examined the anticancer part of curcumin in pancreatic cancer cells lines through various pathways such as (i) inhibiting the cell growth, migration and invasion, (ii) downregulating NEDD4 (down-regulated protein 4), and (iii) enhancement in PTEN and p73 (J. Su et al., Citation2017). Furthermore, the treatment of PANC-1 and PaCa-2 cells with curcumin dose-dependently improved the resistance to gemcitabine along with the reduction in the accumulation of both gemcitabine and uridine mediated by equilibrative nucleoside transporter 1 (ENT1) (Xu et al., Citation2018).
2.5. Stomach or gastric cancer
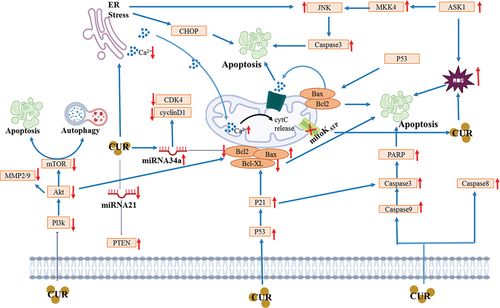
The induction of DMH (dimethylhydrazine) is associated with cancer-causing agent in experimental subjects via enhancing p53 protein expressions along with phosphorylation of p53, significantly increasing the glucose (14C) and 3 H-thymidine uptake, lactate dehydrogenase and alkaline phosphatase activities, and declining the p53 acetylation at residue 382. On the other side, curcumin treatment for the DMH-treated rats normalized these changes to normal levels (Silva et al., Citation2018). Curcumin treated with HGC-27 markedly lowered cell viability, inhibited invasion & migration, negatively regulated the metalloproteinase 2 expression, and induced apoptosisin a dose-dependent way (Dhivya et al., Citation2017; Haghi et al., Citation2017; X. Zhou et al., Citation2016). Likewise, curcumin (25 μm) suppresses cell growth, causes apoptosis, up-regulates the caspase-3, lowers gastrin secretion, enhances gastric pH, lowers gastric secretion, and inhibits gastric cancer progression in gastric cancer cells of mice. Likewise, a different group of researchers reported diverse anticancer mechanisms in MKN-28 and BGC-823 (human gastric cancer cell lines) attributed to curcumin in a dose and time-dependent way. The curcumin potentially suppressed the cell viability, active-caspase-3, caused cell apoptosis, increased the Bax protein, reduced the bcl-2 protein, −9, as well as also enhanced the autophagy-related proteins (Beclin1, Atg5, Atg7 and Atg12), and suppressed the PI3K/Akt/mTOR activation. Moreover, treatment with curcumin also caused the formation of vesicular organelles which are acidic in nature in cytoplasm, alteration of LC3-I into LC3-II (Tung et al., Citation2018; D. Yang et al., Citation2017). Mechanisms of action of curcumin against GI cancers are shown in .
Another study on time and dose levels of curcumin has following anticancer mechanisms in BGC-823, SGC-7901, and MKN-28 (human gastric cancer cell lines) such as (i) reducing of cell viability, (ii) initiation of apoptosis, (iii) induction of autophagy, and (iv) inhibition of activation signaling pathway of PI3K/Akt/mTOR. In an in vivo study conducted by Wang and their colleagues, they explored that curcumin in gastric cancer of nude mice models markedly exhibited inhibition on cell growth and proliferation, regulation of cellular redox homeostasis, and disruption of mitochondrial homeostasis. In addition, it also lowered oxygen consumption by mitochondria and glycolysis (aerobic) via decreasing DNA polymerase γ (POLG) and mtDNA content (Xu et al., Citation2018). The decrease in cell production and initiation of apoptosis in BGC-823 and SGC-7901 (human gastric cancer cell lines) were also reported in vitro study after treatment with 5–40 μmol/L of curcumin (Silva et al., Citation2018). Multiple anticancer approaches are involved after combining the effect of curcumin with 5-FU (5-fluorouracil) and oxaliplatinhas in BGC-823 (gastric cancer cell line). Both compounds increased the level caspase 3, 8, and 9 and Bax, down-regulated the expression of Bcl-2 protein and mRNA and induced apoptosis (Dhivya et al., Citation2017; X. Zhou et al., Citation2016). A study conducted by a group of researchers on the supplementation of curcumin (IC50 40.3 μM) exhibited anticancer potential in a dose-dependent manner in gastric cancer cell line through increasing the cell death rate, downregulating survivin expression, and pSTAT3 levels (Haghi et al., Citation2017). Likewise, another study explicated that curcumin in combination with quercetin markedly inhibited proliferation of cells along with cytochrome c release, reduction in the membrane potential of mitochondria, ERK and AKT reduction in phosphorylation (J. Y. Zhang et al., Citation2015).
In a scientific investigation, gastric cancer was induced in mice by tobacco deactivated the ERK1/2 (extracellular protein kinases 1 and 2), activator AP-1 (protein 1), ERK5 MAPK pathways, the JNK (Jun N-terminal kinase), p38, caused an enhancement in protein and mRNA levels of the epithelial markers (ZO-1 and E-cadherin) and N-cadherin reduction in protein and mRNA levels. Conversely, curcumin treatment significantly reverted these changes in gastric cell lines cancer. Furthermore, curcumin also abrogated JNK MAPK pathways, activation of ERK1/2 caused by TS, changes in EMT (Z. Liang et al., Citation2015). Another research conducted by Liang et al. evaluated the different doses of curcumin (80 to 160 mg/kg/day) on gastric cancer cell line (SGC-7901) and found loweredProx-1 (Prospero homeobox 1), VEGFR- (3 vascular endothelial growth factor receptor 3), and LVD (podoplanin levels, lymphatic vessel density) (Da et al., Citation2015). Dose-dependent induction of MMP damage and enhancement in the rate of cell apoptosis were reported after curcumin treatment in SGC-7901 (gastric cancer cell line). Likewise, curcumin with diazoxide impaired the MMP damage (Cao et al., Citation2015). In another study by Ji and their colleagues, they reported that curcumin has inhibitory effect on β-catenin and STAT3 pathway in mouse model of gastric cancer (Ji et al., Citation2014).
Kruppel-like factor 4 (KLF4) is a transcription factor that promotes development and progression in different types of carcinomas. Curcumin inhibits apoptosis in human BGC-823 gastric carcinoma cells by inhibiting cell invasion (Z. Liang et al., Citation2015). Curcumin has strong anticancer potential in BGC-823 (human gastric cancer cells) through various processes such as activation of ASK1, suppression of reactive oxygen species, induction and up-regulation of ASK1-MKK4-JNK signaling and their protein expressions (Wu et al., Citation2019).
2.6. Intestinal cancer (small intestine, colon and rectum)
In a recent study, curcumin momentously lowered the colonic cytokines, tumor multiplicity and burden, colon mucosa cells abnormal proliferation, suppressed the activation of PI3K/Akt/mTOR/NF-κB/Wnt in colorectal cancer cell lines of high-fat diets (HFDs) mice (Moradi-Marjaneh et al., Citation2018). Long circulating liposomes doxorubicin encapsulated curcumin showed anti-tumor role in a xenograft mouse model of C26 cells via inhibiting oxidative stress, suppressing angiogenesis, the dysregulation of Th1/Th2 cell, and modulating VEGF signaling regulatory miRNAs (Sesarman et al., Citation2019). A peer group of researchers determined that encapsulated curcumin in an in vitro study has an inhibitory effect on invasion and dysregulation in C26 murine colon cancer cells (Ravindranathan et al., Citation2018; C. Zhang et al., Citation2018). Stopping cell proliferation, up-regulation of cell autophagy and suppression of YAP expressions have also been reported after curcumin treatment in colon cancer cells (Yoshida et al., Citation2017). Curcumin in combination with oligomeric proanthocyanidins (OPCs) has a significant effect on modulation of glutathione and porphyrin metabolisms, protein export as well as HMOX1, SEC61B, HSPA5, PDE3B, and G6PD. This combined treatment also suppressed colorectal carcinogenesis and modulated the genes (H. H. Liang et al., Citation2018). On the other hand, curcumin also works as anti-cancer agent in HSP27-KD cells via G2/M phase cell cycle arrest, lowering the Bcl-2, Akt, p-Bad, and p-Akt proteins, inducing autophagy in cells, neutralizing or scavenging the effects of free radicals, and increasing the Bad and c-PARP levels (Alibolandi et al., Citation2018; P. Su et al., Citation2018). In a recent study reported by Lee and their colleagues, and they determined that curcumin as the bioactive compound in LoVo/CPT11 cells has been found to attenuate CPT11 chemoresistance and lower the identification markers of CSC (Lee et al., Citation2018). The reduction in SIRT1 protein level, induction of SIRT1 and proteasomal degradation in colon cancer was reported after treatment with curcumin (Collett et al., Citation2001). In a study, it was found that curcumin inhibits the 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) in the Apc (min) proximal small intestine that results in increased PhIP-induced apoptosis and tumorigenesis (Shen et al., Citation2006). In rodent cancer cell models, curcumin showed preventive role to inhibit cancerous tumorigenesis. Single curcumin dose of 1,000 mg/kg in Nrf2 knockout C57BL/6J/Nrf2(-/-) mice in Wild-type C57BL/6J and 154 induced and 68 suppressed in small intestine (Song et al., Citation2016). Furthermore, the reduction of tumor growth rate, induction of apoptosis, and downregulation of LIG4-overexpression were reported after curcumin treatment in human rectal cancer cells HT-29 (Pendleton et al., Citation2019). Synergistic anti-cancer effects of curcumin with other chemical compounds were presented in .
Table 2. Synergistic anti-cancer effects of curcumin with other chemical compounds.
By increasing miR-130a, curcumin inhibited the Wnt/beta-catenin pathway to suppress colon cancer cell proliferation. Through the enhancement of miR-222-3p, which targets SOX10, our study found that curcumin plays a critical role in the inhibition of melanoma cell proliferation, migration, and invasion. Furthermore, curcumin’s impact on melanoma cells proliferation, migration, and invasion was significantly inhibited by miR-222-3p inhibitor. Together, these findings suggested that curcumin’s anticancer molecular processes relied heavily on the control of miRNAs (K. Wang et al., Citation2018).
3. Conclusion
Despite curcumin’s potential in treating gastrointestinal carcinomas, a number of gaps between studies have been found. The use of in vitro and preclinical studies, which could not accurately reflect the complicated nature of actual gastrointestinal cancers, constitutes a particular restriction. Clinical trials should be prioritized in future research to confirm curcumin’s effectiveness in actual patient populations. Furthermore, curcumin has been the subject of the majority of trials as a stand-alone therapy. The absence of set outcome measures and biomarkers to evaluate therapy response is yet another challenge in curcumin research. To give more reliable data on the efficacy of curcumin, future studies should include solid endpoints such overall survival, disease-free survival, and quality of life indicators. Likewise, personalized medicine strategies would benefit from the discovery and confirmation of particular biomarkers that might forecast the response to curcumin treatment. Future research should examine the interactions of curcumin with currently used treatment modalities, such as chemotherapy, radiation therapy, and targeted therapies, considering the multifactorial character of gastrointestinal cancers. Another challenge in curcumin research is the lack of standardized outcome measures and biomarkers to assess treatment response. Future studies should incorporate robust endpoints, such as overall survival, disease-free survival, and quality of life measures, to provide more reliable data on curcumin’s effectiveness. Furthermore, the identification and validation of specific biomarkers that can predict the response to curcumin treatment would be valuable in personalized medicine approaches.
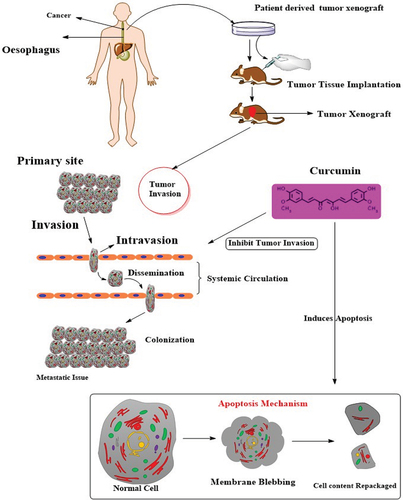
Curcumin possesses anticancer effects against different human gastrointestinal cancer cell lines through various mechanisms. It suppresses the cell proliferation, invasion and propagation stages in different human cancer cell lines. Furthermore, its sole and co-administration exhibit different effects on cancer cells that make it an important compound to be used as a therapeutic agent in different nutraceutical and pharmaceutical formulations. However, the safety of its different analogues and derivatives needs to be considered before administration to human subjects. In the near future, curcumin is anticipated to be proven as a novel drug to cure and treat several human gastrointestinal cancer cell lines.
Declaration
The work described has not been published before (except in the form of an abstract, a published lecture or academic thesis). It is not under consideration for publication elsewhere.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abd El‐Hack, M. E., El‐Saadony, M. T., Swelum, A. A., Arif, M., Abo Ghanima, M. M., Shukry, M., El‐Tarabily, A. E., El‐Tarabily, K. A., & Noreldin, K. A. (2021). Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. Journal of the Science of Food and Agriculture, 101(14), 5747–5762. https://doi.org/10.1002/jsfa.11372
- Afrin, R., Arumugam, S., Rahman, A., Wahed, M. I. I., Karuppagounder, V., Harima, M., Suzuki, H., Miyashita, S., Suzuki, K., Yoneyama, H., Ueno, K., & Watanabe, K. (2017). Curcumin ameliorates liver damage and progression of NASH in NASH-HCC mouse model possibly by modulating HMGB1-NF-κB translocation. International Immunopharmacology, 44, 174–182. https://doi.org/10.1016/j.intimp.2017.01.016
- Aggarwal, B. B., Deb, L., & Prasad, S. (2014). Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules, 20(1), 185–205. https://doi.org/10.3390/molecules20010185
- Al-Dossari, M. H., Fadda, L. M., Attia, H. A., Hasan, I. H., & Mahmoud, A. M. (2020). Curcumin and selenium prevent lipopolysaccharide/diclofenac-induced liver injury by suppressing inflammation and oxidative stress. Biological Trace Element Research, 196(1), 173–183. https://doi.org/10.1007/s12011-019-01910-4
- Alibeiki, F., Jafari, N., Karimi, M., & Peeri Dogaheh, H. (2017). Potent anti-cancer effects of less polar curcumin analogues on gastric adenocarcinoma and esophageal squamous cell carcinoma cells. Scientific Reports, 7(1), 1–9. https://doi.org/10.1038/s41598-017-02666-4
- Alibolandi, M., Hoseini, F., Mohammadi, M., Ramezani, P., Einafshar, E., Taghdisi, S. M., Ramezani, M., & Abnous, K. (2018). Curcumin-entrapped MUC-1 aptamer targeted dendrimer-gold hybrid nanostructure as a theranostic system for colon adenocarcinoma. International Journal of Pharmaceutics, 549(1–2), 67–75. https://doi.org/10.1016/j.ijpharm.2018.07.052
- Arnold, M., Abnet, C. C., Neale, R. E., Vignat, J., Giovannucci, E. L., McGlynn, K. A., & Bray, F. (2020). Global burden of 5 major types of gastrointestinal cancer. Gastroenterology, 159(1), 335–349. https://doi.org/10.1053/j.gastro.2020.02.068
- Arya, G., Das, M., & Sahoo, S. K. (2018). Evaluation of curcumin loaded chitosan/PEG blended PLGA nanoparticles for effective treatment of pancreatic cancer. Biomedicine & Pharmacotherapy, 102, 555–566. https://doi.org/10.1016/j.biopha.2018.03.101
- Ashrafizadeh, M., Zarrabi, A., Hashemipour, M., Vosough, M., Najafi, M., Shahinozzaman, M., Hushmandi, K., Khan, H., & Mirzaei, H. (2020). Sensing the scent of death: Modulation of microRnas by curcumin in gastrointestinal cancers. Pharmacological Research, 160, 105199. https://doi.org/10.1016/j.phrs.2020.105199
- Banik, K., Ranaware, A. M., Deshpande, V., Nalawade, S. P., Padmavathi, G., Bordoloi, D., Sailo, B. L., Shanmugam, M. K., Fan, L., Arfuso, F., Sethi, G., & Kunnumakkara, A. B. (2019). Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacological Research, 144, 192–209. https://doi.org/10.1016/j.phrs.2019.04.004
- Bimonte, S., Barbieri, A., Palma, G., Luciano, A., Rea, D., & Arra, C. (2013). Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. BioMed Research International, 2013, 810423. https://doi.org/10.1155/2013/810423
- Bower, M. R., Aiyer, H. S., Li, Y., & Martin, R. C. (2010). Chemoprotective effects of curcumin in esophageal epithelial cells exposed to bile acids. World Journal of Gastroenterology: WJG, 16(33), 4152. https://doi.org/10.3748/wjg.v16.i33.4152
- Cao, A. L., Tang, Q. F., Zhou, W. C., Qiu, Y. Y., Hu, S. J., & Yin, P. H. (2015). Ras/ERK signaling pathway is involved in curcumin-induced cell cycle arrest and apoptosis in human gastric carcinoma AGS cells. Journal of Asian Natural Products Research, 17(1), 56–63. https://doi.org/10.1080/10286020.2014.951923
- Chang, M., Wu, M., & Li, H. (2018). Antitumor activities of novel glycyrrhetinic acid-modified curcumin-loaded cationic liposomes in vitro and in H22 tumor-bearing mice. Drug Delivery, 25(1), 1984–1995. https://doi.org/10.1080/10717544.2018.1526227
- Collett, G. P., Robson, C. N., Mathers, J. C., & Campbell, F. C. (2001). Curcumin modifies apcmin apoptosis resistance and inhibits 2-amino 1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) induced tumour formation in apcmin mice. Carcinogenesis, 22(5), 821–825. https://doi.org/10.1093/carcin/22.5.821
- D’Ascola, A., Irrera, N., Ettari, R., Bitto, A., Pallio, G., Mannino, F., Atteritano, M., Campo, G. M., Minutoli, L., Arcoraci, V., Squadrito, V., Picciolo, G., Squadrito, F., & Altavilla, D. (2019). Exploiting curcumin synergy with natural products using quantitative analysis of dose-effect relationships in an experimental in vitro model of osteoarthritis. Frontiers in Pharmacology, 10(1347), 1–9. https://doi.org/10.3389/fphar.2019.01347
- Da, W., Zhu, J., Wang, L., & Sun, Q. (2015). Curcumin suppresses lymphatic vessel density in an in vivo human gastric cancer model. Tumor Biology, 36(7), 5215–5223. https://doi.org/10.1007/s13277-015-3178-8
- Dhivya, R., Ranjani, J., Bowen, P. K., Rajendhran, J., Mayandi, J., & Annaraj, J. (2017). Biocompatible curcumin loaded PMMA-PEG/ZnO nanocomposite induce apoptosis and cytotoxicity in human gastric cancer cells. Materials Science and Engineering: C, 80, 59–68. https://doi.org/10.1016/j.msec.2017.05.128
- Du, Q., Hu, B., An, H. M., Shen, K. P., Xu, L., Deng, S., & Wei, M. M. (2013). Synergistic anticancer effects of curcumin and resveratrol in Hepa1-6 hepatocellular carcinoma cells. Oncology Reports, 29(5), 1851–1858. https://doi.org/10.3892/or.2013.2310
- Feng, T., Wei, Y., Lee, R. J., & Zhao, L. (2017). Liposomal curcumin and its application in cancer. International Journal of Nanomedicine, 12, 6027. https://doi.org/10.2147/IJN.S132434
- Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D. M., Forman, D., & Bray, F. (2015). Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer, 136(5), E359–E386. https://doi.org/10.1002/ijc.29210
- Gheibi, S., Gouvarchin Ghaleh, H. E., Motlagh, B. M., Azarbayjani, A. F., & Zarei, L. (2019). Therapeutic effects of curcumin and ursodexycholic acid on nonalcoholic fatty liver disease. Biomedicine & Pharmacotherapy, 115(108938), 1–8. https://doi.org/10.1016/j.biopha.2019.108938
- Guo, Y., Wu R., Gaspar J. M., Sargsyan D., Su Z. Y., Zhang C., Gao L., Cheng D., Li W., Wang C., & Yin R. (2018). DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis, 39 (5), 669–680.
- Gu, X., Zhang, Q., Zhang, W., & Zhu, L. (2019). Curcumin inhibits liver metastasis of gastric cancer through reducing circulating tumor cells. Aging (Albany NY), 11(5), 1501–1509. https://doi.org/10.18632/aging.101848
- Haghi, A., Azimi, H., & Rahimi, R. (2017). A comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin, and allicin, in the treatment of gastric cancer. Journal of Gastrointestinal Cancer, 48(4), 314–320. https://doi.org/10.1007/s12029-017-9997-7
- Hosseini, M., Hassanian, S. M., Mohammadzadeh, E., ShahidSales, S., Maftouh, M., Fayazbakhsh, H., Khazaei, M., & Avan, A. (2017). Therapeutic potential of curcumin in treatment of pancreatic cancer: Current status and future perspectives. Journal of Cellular Biochemistry, 118(7), 1634–1638. https://doi.org/10.1002/jcb.25897
- Hsu, F. T., Liu, Y. C., Liu, T. T., & Hwang, J. J. (2015). Curcumin sensitizes hepatocellular carcinoma cells to radiation via suppression of radiation-induced NF-κB activity. BioMed Research International, 2015, 1–7. https://doi.org/10.1155/2015/363671
- Huang, M., Xiong, H., Luo, D., Xu, B., & Liu, H. (2020). CSN5 upregulates glycolysis to promote hepatocellular carcinoma metastasis via stabilizing the HK2 protein. Experimental Cell Research, 388(2), 111876. https://doi.org/10.1016/j.yexcr.2020.111876
- Hu, X. Q., Sun, Y., Lau, E., Zhao, M., & Su, S. B. (2016). Advances in synergistic combinations of Chinese herbal medicine for the treatment of cancer. Current Cancer Drug Targets, 16(4), 346–356. https://doi.org/10.2174/1568009616666151207105851
- Hu, J. X., Zhao, C. F., Chen, W. B., Liu, Q. C., Li, Q. W., Lin, Y. Y., & Gao, F. (2021). Pancreatic cancer: A review of epidemiology, trend, and risk factors. World Journal of Gastroenterology, 27(27), 4298. https://doi.org/10.3748/wjg.v27.i27.4298
- Imran, M., Ullah, A., Saeed, F., Nadeem, M., Arshad, M. U., & Suleria, H. A. R. (2018). Cucurmin, anticancer, & antitumor perspectives: A comprehensive review. Critical Reviews in Food Science and Nutrition, 58(8), 1271–1293. https://doi.org/10.1080/10408398.2016.1252711
- Jalde, S. S., Chauhan, A. K., Lee, J. H., Chaturvedi, P. K., Park, J. S., & Kim, Y. W. (2018). Synthesis of novel Chlorin e6-curcumin conjugates as photosensitizers for photodynamic therapy against pancreatic carcinoma. European Journal of Medicinal Chemistry, 147, 66–76. https://doi.org/10.1016/j.ejmech.2018.01.099
- Ji, J., Wang, H. S., Gao, Y. Y., Sang, L. M., & Zhang, L. (2014). Synergistic anti-tumor effect of KLF4 and curcumin in human gastric carcinoma cell line. Asian Pacific Journal of Cancer Prevention, 15(18), 7747–7752. https://doi.org/10.7314/APJCP.2014.15.18.7747
- Khaled, M., & Mahfouz, M. (2010). Curcumin/Irbesartan combination improves insulin sensitivity and ameliorates serum pro-inflammatory cytokines levels in diabetes rat model. The Journal of American Science, 6(11), 1051–1059.
- Khayyal, M. T., El-Hazek, R. M., El-Sabbagh, W. A., Frank, J., Behnam, D., & Abdel-Tawab, M. (2018). Micellar solubilisation enhances the antiinflammatory activities of curcumin and boswellic acids in rats with adjuvant-induced arthritis. Nutrition, 54, 189–196. https://doi.org/10.1016/j.nut.2018.03.055
- Kita, T., Imai, S., Sawada, H., Kumagai, H., & Seto, H. (2008). The biosynthetic pathway of curcuminoid in turmeric (curcuma longa) as revealed by 13C-labeled precursors. Bioscience, Biotechnology, and Biochemistry, 72(7), 1789–1798. https://doi.org/10.1271/bbb.80075
- Kocaadam, B., & Şanlier, N. (2017). Curcumin, an active component of turmeric (curcuma longa), and its effects on health. Critical Reviews in Food Science and Nutrition, 57(13), 2889–2895. https://doi.org/10.1080/10408398.2015.1077195
- Kwiecien, S., Magierowski, M., Majka, J., Ptak-Belowska, A., Wojcik, D., Sliwowski, Z., Magierowska, K., & Brzozowski, T. (2019). Curcumin: A potent protectant against esophageal and gastric disorders. International Journal of Molecular Sciences, 20(6), 1477. https://doi.org/10.3390/ijms20061477
- Lee, Y. H., Song, N. Y., Suh, J., Kim, D. H., Kim, W., Ann, J., Lee, J., Baek, J.-H., Na, H.-K., & Surh, Y. J. (2018). Curcumin suppresses oncogenicity of human colon cancer cells by covalently modifying the cysteine 67 residue of SIRT1. Cancer Letters, 431, 219–229. https://doi.org/10.1016/j.canlet.2018.05.036
- Le, U. M., Hartman, A., & Pillai, G. (2018). Enhanced selective cellular uptake and cytotoxicity of epidermal growth factor-conjugated liposomes containing curcumin on EGFR-overexpressed pancreatic cancer cells. Journal of Drug Targeting, 26(8), 676–683. https://doi.org/10.1080/1061186X.2017.1408114
- Liang, H. H., Huang, C. Y., Chou, C. W., Makondi, P. T., Huang, M. T., Wei, P. L., & Chang, Y. J. (2018). Heat shock protein 27 influences the anti-cancer effect of curcumin in colon cancer cells through ROS production and autophagy activation. Life Sciences, 209, 43–51. https://doi.org/10.1016/j.lfs.2018.07.047
- Liang, Z., Wu, R., Xie, W., Geng, H., Zhao, L., Xie, C., Wu, J., Geng, S., Li, X., Zhu, M., Zhu, W., Zhu, J., Huang, C., Ma, X., Zhong, C., & Han, H. (2015). Curcumin suppresses MAPK pathways to reverse tobacco smoke‐induced gastric epithelial–mesenchymal transition in mice. Phytotherapy Research, 29(10), 1665–1671. https://doi.org/10.1002/ptr.5398
- Li, W., Jiang, Z., Xiao, X., Wang, Z., Wu, Z., Ma, Q., & Cao, L. (2018). Curcumin inhibits superoxide dismutase-induced epithelial-to-mesenchymal transition via the PI3K/Akt/NF-κB pathway in pancreatic cancer cells. International Journal of Oncology, 52(5), 1593–1602. https://doi.org/10.3892/ijo.2018.4295
- Li, X. J., Li, Y. Z., Jin, C. T., Fan, J., & Li, H. J. (2015). Curcumin induces apoptosis by PTEN/PI3K/AKT pathway in EC109 cells. Zhongguo ying yong sheng li xue za zhi= Zhaongguo yingyong shenglixue zazhi= Chinese Journal of Applied Physiology, 31(2), 174–177.
- Lim, D. J. (2022). 3-O-Ethyl-L-Ascorbic acid doped enteric-coated gelatin capsules towards the advanced oral curcumin delivery for cancers. Polymers, 14(11), 2207. https://doi.org/10.3390/polym14112207
- Lin, M. L., Lu, Y. C., Chen, H. Y., Lee, C. C., Chung, J. G., & Chen, S. S. (2014). Suppressing the formation of lipid raft‐associated Rac1/PI3K/Akt signaling complexes by curcumin inhibits SDF‐1α‐induced invasion of human esophageal carcinoma cells. Molecular Carcinogenesis, 53(5), 360–379. https://doi.org/10.1002/mc.21984
- Li, H., Shi, B., Li, Y., & Yin, F. (2017). Polydatin inhibits cell proliferation and induces apoptosis in laryngeal cancer and HeLa cells via suppression of the PDGF/AKT signaling pathway. Journal of Biochemical and Molecular Toxicology, 31(7), e21900.
- Liu, T. Y., Tan, Z. J., Jiang, L., Gu, J. F., Wu, X. S., Cao, Y., Liu, T.-Y., Li, M.-L., Wu, K.-J., & Liu, Y. B. (2013). Curcumin induces apoptosis in gallbladder carcinoma cell line GBC-SD cells. Cancer Cell International, 13(1), 1–9. https://doi.org/10.1186/1475-2867-13-64
- Liu, Y., Wang, X., Zeng, S., Zhang, X., Zhao, J., Zhang, X., Chen X., Yang W., Yang Y., Dong Z., & Xu, X. (2018). The natural polyphenol curcumin induces apoptosis by suppressing STAT3 signaling in esophageal squamous cell carcinoma. Journal of Experimental & Clinical Cancer Research, 37(1), 303. https://doi.org/10.1186/s13046-018-0959-0
- Martins, C. A., Leyhausen, G., Volk, J., & Geurtsen, W. (2015). Curcumin in combination with piperine suppresses osteoclastogenesis in vitro. Journal of Endodontia, 41(10), 1638–1645. https://doi.org/10.1016/j.joen.2015.05.009
- Mohan, A., Garg, A., Gupta, A., Sahu, S., Choudhari, C., Vashistha, V., & Guleria, R. (2020). Clinical profile of lung cancer in North India: A 10-year analysis of 1862 patients from a tertiary care center. Lung India: official organ of Indian Chest Society, 37(3), 190.
- Moradi-Marjaneh, R., Hassanian, S. M., Rahmani, F., Aghaee-Bakhtiari, S. H., Avan, A., & Khazaei, M. (2018). Phytosomal curcumin elicits anti-tumor properties through suppression of angiogenesis, cell proliferation and induction of oxidative stress in colorectal cancer. Current Pharmaceutical Design, 24(39), 4626–4638. https://doi.org/10.2174/1381612825666190110145151
- Neyrinck, A. M., Alligier, M., Memvanga, P. B., Névraumont, E., Larondelle, Y., Préat, V., Cani, P. D., Delzenne, N. M., & Sanz, Y. (2013). Curcuma longa extract associated with white pepper lessens high fat diet-induced inflammation in subcutaneous adipose tissue. PLoS One, 8(11), 1–10. https://doi.org/10.1371/journal.pone.0081252
- Ning, X., Du, Y., Ben, Q., Huang, L., He, X., Gong, Y., Gao J., Wu H., Man X., Jin J., & Li, Z. (2016). Bulk pancreatic cancer cells can convert into cancer stem cells (CSCs) in vitro and 2 compounds can target these CSCs. Cell Cycle, 15(3), 403–412. https://doi.org/10.1080/15384101.2015.1127471
- Ono, M., Higuchi, T., Takeshima, M., Chen, C., & Nakano, S. (2013). Antiproliferative and apoptosis-inducing activity of curcumin against human gallbladder adenocarcinoma cells. Anticancer Research, 33(5), 1861–1866.
- Pandey, A., Chaturvedi, M., Mishra, S., Kumar, P., Somvanshi, P., & Chaturvedi, R. (2020). Reductive metabolites of curcumin and their therapeutic effects. Heliyon, 6(11), e05469. https://doi.org/10.1016/j.heliyon.2020.e05469
- Patra, S., Mishra, S. R., Behera, B. P., Mahapatra, K. K., Panigrahi, D. P., Bhol, C. S., Praharaj P. P., Sethi G., Patra S. K., & Bhutia, S. K. (2020, May). Autophagy-modulating phytochemicals in cancer therapeutics: Current evidences and future perspectives. In Seminars in cancer biology (pp. 205–217). Academic Press. https://doi.org/10.1016/j.semcancer.2020.05.008
- Pendleton, E. G., Jamasbi, R. J., & Geusz, M. E. (2019). Tetrahydrocurcumin, curcumin, and 5-fluorouracil effects on human esophageal carcinoma cells. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 19(8), 1012–1020. https://doi.org/10.2174/1871520619666190116141448
- Qiang, Z., Meng, L., Yi, C., Yu, L., Chen, W., & Sha, W. (2019). Curcumin regulates the miR-21/PTEN/Akt pathway and acts in synergy with PD98059 to induce apoptosis of human gastric cancer MGC-803 cells. Journal of International Medical Research, 47(3), 1288–1297. https://doi.org/10.1177/0300060518822213
- Qin, Y., Zhou, Y., Ge, A., Chang, L., Shi, H., Fu, Y., & Luo, Q. (2019). Overexpression of SNORA21 suppresses tumorgenesis of gallbladder cancer in vitro and in vivo. Biomedicine & Pharmacotherapy, 118, 109266. https://doi.org/10.1016/j.biopha.2019.109266
- Qu, C., Wang, Q., Meng, Z., & Wang, P. (2018). Cancer-associated fibroblasts in pancreatic cancer: Should they be deleted or reeducated?.Reeducated? Integrative Cancer Therapies, 17(4), 1016–1019. https://doi.org/10.1177/1534735418794884
- Rabbani, P. S., Soares, M. A., Hameedi, S. G., Kadle, R. L., Mubasher, A., Kowzun, M., & Ceradini, D. J. (2019). Dysregulation of Nrf2/Keap1 redox pathway in diabetes affects multipotency of stromal cells. Diabetes, 68(1), 141–155. https://doi.org/10.2337/db18-0232
- Ravindranathan, P., Pasham, D., Balaji, U., Cardenas, J., Gu, J., Toden, S., & Goel, A. (2018). A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer. Scientific Reports, 8(1), 1–12. https://doi.org/10.1038/s41598-018-32267-8
- Rawat, N., Alhamdani, A., McAdam, E., Cronin, J., Eltahir, Z., Lewis, P., Griffiths, P., Baxter, J. N., & Jenkins, G. J. (2012). Curcumin abrogates bile-induced NF-κB activity and DNA damage in vitro and suppresses NF-κB activity whilst promoting apoptosis in vivo, suggesting chemopreventative potential in barrett’s oesophagus. Clinical and Translational Oncology, 14(4), 302–311. https://doi.org/10.1007/s12094-012-0799-x
- Ren, B., Luo, S., Tian, X., Jiang, Z., Zou, G., Xu, F., Yin, T., Huang, Y., & Liu, J. (2018). Curcumin inhibits liver cancer by inhibiting DAMP molecule HSP70 and TLR4 signaling. Oncology Reports, 40(2), 895–901. https://doi.org/10.3892/or.2018.6485
- Schiffman, S. C., Li, Y., & Martin, R. C. (2012). The association of manganese superoxide dismutase expression in barrett’s esophageal progression with MnTBAP and curcumin oil therapy. Journal of Surgical Research, 176(2), 535–541. https://doi.org/10.1016/j.jss.2011.11.1013
- Sesarman, A., Tefas, L., Sylvester, B., Licarete, E., Rauca, V., Luput, L., Patras, L., Porav, S., Banciu, M., & Porfire, A. (2019). Co-delivery of curcumin and doxorubicin in PEGylated liposomes favored the antineoplastic C26 murine colon carcinoma microenvironment. Drug Delivery and Translational Research, 9(1), 260–272. https://doi.org/10.1007/s13346-018-00598-8
- Shen, G., Xu, C., Hu, R., Jain, M. R., Gopalkrishnan, A., Nair, S., Huang, M.-T., Chan, J. Y., & Kong, A. N. T. (2006). Modulation of nuclear factor E2-related factor 2–mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Molecular Cancer Therapeutics, 5(1), 39–51. https://doi.org/10.1158/1535-7163.MCT-05-0293
- Silva, G., Teixeira Lima, F., Seba, V., Mendes Lourenco, A. L., Lucas, T. G., De Andrade, B. V., Torrezan, G., Polaquini, C., Garcia, M., Couto, L., Bestetti, R., de Castro França, S., Fachin, A., Regasini, L., & Marins, M. (2018). Curcumin analog CH-5 suppresses the proliferation, migration, and invasion of the human gastric cancer cell line HGC-27. Molecules, 23(2), 279. https://doi.org/10.3390/molecules23020279
- Song, Y., Yang, G., Qiu, J., Wang, D., & Wang, X. (2016). Study of the effect of LIG4 on the radiosensitivity enhancement of rectal cancer cells by curcumin. Zhonghua wei chang wai ke za zhi= Chinese Journal of Gastrointestinal Surgery, 19(12), 1395–1399.
- Stanić, Z. (2017). Curcumin, a compound from natural sources, a true scientific challenge–A review. Plant Foods for Human Nutrition, 72(1), 1–12. https://doi.org/10.1007/s11130-016-0590-1
- Subramaniam, D., Ponnurangam, S., Ramamoorthy, P., Standing, D., Battafarano, R. J., Anant, S., Sharma, P., & Chandra, D. (2012). Curcumin induces cell death in esophageal cancer cells through modulating notch signaling. PloS One, 7(2), e30590. https://doi.org/10.1371/journal.pone.0030590
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
- Su, P., Yang, Y., Wang, G., Chen, X., & Ju, Y. (2018). Curcumin attenuates resistance to irinotecan via induction of apoptosis of cancer stem cells in chemoresistant colon cancer cells. International Journal of Oncology, 53(3), 1343–1353. https://doi.org/10.3892/ijo.2018.4461
- Su, J., Zhou, X., Yin, X., Wang, L., Zhao, Z., Hou, Y., Wang, L., Zheng, N., Xia, J., & Wang, Z. (2017). The effects of curcumin on proliferation, apoptosis, invasion, and NEDD4 expression in pancreatic cancer. Biochemical Pharmacology, 140, 28–40. https://doi.org/10.1016/j.bcp.2017.05.014
- Tang, D., Zhang, S., Shi, X., Wu, J., Yin, G., Tan, X., Liu F., Wu X., & Du, X. (2019). Combination of astragali polysaccharide and curcumin improves the morphological structure of tumor vessels and induces tumor vascular normalization to inhibit the growth of hepatocellular carcinoma. Integrative Cancer Therapies, 18, 15347. https://doi.org/10.1177/1534735418824408
- Tewari, D., Patni, P., Bishayee, A., Sah, A. N., & Bishayee, A. (2019, December). Natural products targeting the PI3K-Akt-Mtor signaling pathway in cancer: A novel therapeutic strategy. In Seminars in cancer biology (pp. 1–17). Academic Press. https://doi.org/10.1016/j.semcancer.2019.12.008
- Thomson, C. A., LeWinn, K., Newton, T. R., Alberts, D. S., & Martinez, M. E. (2003). Nutrition and diet in the development of gastrointestinal cancer. Current Oncology Reports, 5(3), 192–202.
- Tian, F., Fan, T., Zhang, Y., Jiang, Y., & Zhang, X. (2012). Curcumin potentiates the antitumor effects of 5-FU in treatment of esophageal squamous carcinoma cells through downregulating the activation of NF-κB signaling pathway in vitro and in vivo. Acta biochimica et biophysica Sinica, 44(10), 847–855. https://doi.org/10.1093/abbs/gms074
- Tork, O. M., Khaleel, E. F., & Abdelmaqsoud, O. M. (2016). Altered cell to cell communication, autophagy and mitochondrial dysfunction in a model of hepatocellular carcinoma: Potential protective effects of curcumin and stem cell therapy. Asian Pacific Journal of Cancer Prevention, 16(18), 8271–8279. https://doi.org/10.7314/APJCP.2015.16.18.8271
- Tremmel, L., Rho, O., Slaga, T. J., & DiGiovanni, J. (2019). Inhibition of skin tumor promotion by TPA using a combination of topically applied ursolic acid and curcumin. Molecular Carcinogenesis, 58(2), 185–195. https://doi.org/10.1002/mc.22918
- Tung, L. N., Song, S., Chan, K. T., Choi, M. Y., Lam, H. Y., Chan, C. M., Chen, Z., Wang, H. K., Leung, H. T., Law, S., Huang, Y., Song, H., & Lee, N. P. (2018). Preclinical study of novel curcumin analogue SSC-5 using orthotopic tumor xenograft model for esophageal squamous cell carcinoma. Cancer Research and Treatment: Official Journal of Korean Cancer Association, 50(4), 1362–1377. https://doi.org/10.4143/crt.2017.353
- Wang, D., Qiu, J., Yang, G., Song, Y., Deng, Q., & Zhang, X. (2015). Mechanism of radiotherapy sensitization of curcumin on rectal cancer cells. Zhonghua wei chang wai ke za zhi= Chinese Journal of Gastrointestinal Surgery, 18(6), 602–605.
- Wang, Q., Qu, C., Xie, F., Chen, L., Liu, L., Liang, X., Wu X., Wang P., & Meng, Z. (2017). Curcumin suppresses epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells by inhibiting cancer-associated fibroblasts. American Journal of Cancer Research, 7(1), LB–125. https://doi.org/10.1158/1538-7445.AM2017-LB-125
- Wang, K., Tan, S. L., Lu, Q., Xu, R., Cao, J., Wu, S. Q., Wang, Y.-H., Zhao, X.-K., & Zhong, Z. H. (2018). Curcumin suppresses microRNA-7641-mediated regulation of p16 expression in bladder cancer. The American Journal of Chinese Medicine, 46(6), 1357–1368. https://doi.org/10.1142/S0192415X18500714
- Wu, X., Koh, G. Y., Huang, Y., Crott, J. W., Bronson, R. T., & Mason, J. B. (2019). The combination of curcumin and salsalate is superior to either agent alone in suppressing pro-cancerous molecular pathways and colorectal tumorigenesis in obese mice. Molecular Nutrition & Food Research, 63(8), e1801097. https://doi.org/10.1002//mnfr.201801097
- Xu, H., Yu, W. B., Gao, Y., Zhang, M. J., Malhotra, A., & Yu, W. H. (2018). Modulatory potential of curcumin and resveratrol on p53 post-translational modifications during gastric cancer. Journal of Environmental Pathology, Toxicology and Oncology, 37(2), 93–101. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2018025547
- Yang, H., Huang, S., Wei, Y., Cao, S., Pi, C., Feng, T., Liang J., Zhao L., & Ren, G. (2017). Curcumin enhances the anticancer effect of 5-fluorouracil against gastric cancer through down-regulation of COX-2 and NF- κB signaling pathways. Journal of Cancer, 8(18), 3697–3706. https://doi.org/10.7150/jca.20196
- Yang, D., Li, Y., & Zhao, D. (2017). Curcumin induces apoptotic cell death in human pancreatic cancer cells via the miR-340/XIAP signaling pathway. Oncology Letters, 14(2), 1811–1816. https://doi.org/10.3892/ol.2017.6321
- Yan, F., Li, H., Zhong, Z., Zhou, M., Lin, Y., Tang, C., & Li, C. (2019). Co-delivery of prednisolone and curcumin in human serum albumin nanoparticles for effective treatment of rheumatoid arthritis. International Journal of Nanomedicine, 14, 9113–9125. https://doi.org/10.2147/IJN.S219413
- Yin, J., Wang, L., Wang, Y., Shen, H., Wang, X., & Wu, L. (2019). Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-beta/Smad2/3 signaling pathway. Oncotargets and Therapy, 12, 3893–3903. https://doi.org/10.2147/OTT.S199601
- Yoshida, K., Toden, S., Ravindranathan, P., Han, H., & Goel, A. (2017). Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis, 38(10), 1036–1046. https://doi.org/10.1093/carcin/bgx065
- Zhang, C., He, L. J., Ye, H. Z., Liu, D. F., Zhu, Y. B., Miao, D. D., Liu, D., Zhang, S., Chen, Y., Jia, Y., Shen, J., & Liu, X. P. (2018). Nrf2 is a key factor in the reversal effect of curcumin on multidrug resistance in the HCT‑8/5‑Fu human colorectal cancer cell line. Molecular Medicine Reports, 18(6), 5409–5416. https://doi.org/10.3892/mmr.2018.9589
- Zhang, J. Y., Lin, M. T., Zhou, M. J., Yi, T., Tang, Y. N., Tang, S. L., Yang, Z.-J., Zhao, Z.-Z., & Chen, H. B. (2015). Combinational treatment of curcumin and quercetin against gastric cancer MGC-803 cells in vitro. Molecules, 20(6), 11524–11534. https://doi.org/10.3390/molecules200611524
- Zhao, Z., Malhotra, A., & Seng, W. Y. (2019). Curcumin modulates hepatocellular carcinoma by reducing UNC119 expression. Journal of Environmental Pathology, Toxicology and Oncology, 38(3), 195–203. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2019029549
- Zheng, R., You, Z., Jia, J., Lin, S., Han, S., Liu, A., LONG, H., & Wang, S. (2016). Curcumin enhances the antitumor effect of ABT-737 via activation of the ROS-ASK1-JNK pathway in hepatocellular carcinoma cells. Molecular Medicine Reports, 13(2), 1570–1576. https://doi.org/10.3892/mmr.2015.4715
- Zhou, Q., Cai, X., Huang, Y., & Zhou, Y. (2022). Pluronic F127-liposome-encapsulated curcumin activates Nrf2/Keap1 signaling pathway to promote cell migration of HaCaT cells. Molecular and Cellular Biochemistry, 478(2), 1–7. https://doi.org/10.1007/s11010-022-04481-6
- Zhou, M., Li, Z., Han, Z., & Tian, N. (2015). Paclitaxel-sensitization enhanced by curcumin involves down-regulation of nuclear factor- κB and Lin28 in Hep3B cells. Journal of Receptor and Signal Transduction Research, 35(6), 1–8. https://doi.org/10.3109/10799893.2015.1041644
- Zhou, Q. M., Sun, Y., Lu, Y. Y., Zhang, H., Chen, Q. L., & Su, S. B. (2017). Curcumin reduces mitomycin C resistance in breast cancer stem cells by regulating Bcl-2 family-mediated apoptosis. Cancer Cell International, 17(1), 1–13. https://doi.org/10.1186/s12935-017-0453-3
- Zhou, Q. M., Wang, X. F., Liu, X. J., Zhang, H., Lu, Y. Y., Huang, S., & Su, S. B. (2011). Curcumin improves MMC-based chemotherapy by simultaneously sensitising cancer cells to MMC and reducing MMC-associated side-effects. European Journal of Cancer, 47(14), 2240–2247. https://doi.org/10.1016/j.ejca.2011.04.032
- Zhou, X., Wang, W., Li, P., Zheng, Z., Tu, Y., Zhang, Y., & You, T. (2016). Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncology Research, 23(1–2), 29. https://doi.org/10.3727/096504015X14452563486011
- Zhu, J., Zhao, B., Xiong, P., Wang, C., Zhang, J., Tian, X., & Huang, Y. (2018). Curcumin induces autophagy via inhibition of yes-associated protein (YAP) in human colon cancer cells. Medical Science Monitor: International Medical Journal of Experimental & Clinical Research, 24, 7035. https://doi.org/10.12659/MSM.910650