?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
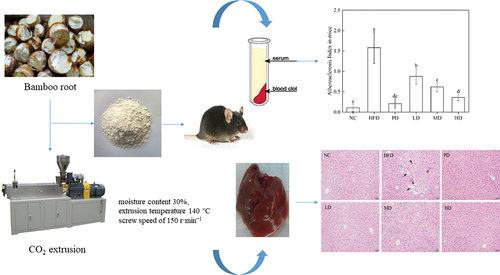
Bamboo root as by-product of bamboo shoot processing is rich in dietary fibre. The protective effects of bamboo root dietary fibre (BRDF) with different intake dose on the hyperlipidaemia mice were investigated. The mice weight in the high dose (HD) group was 31.18 g, lower than that of high fat diet (HFD) group (33.71 g, p < .05), after consuming BRDF. The contents of TC, TG and LDL-C in HD group mice were significantly reduced (p < .05). The mice liver index significantly decreased in the BRDF group compared to the HFD group. TC and TG contents in the liver of HD mice were 0.85 mmol·g−1 and 0.83 mmol·g−1, respectively, significantly lower than 2.25 mmol·g−1 and 1.46 mmol·g−1 in the normal control group (p < .05). H&E staining showed a reduction in inflammatory infiltration. These results suggested that BRDF in the daily diet could reduce blood lipids in vivo and prevent hyperlipidaemia.
HIGHLIGHTS
CO2 extrudedbamboo shoot fiberintervention controlled food-borne obesity in mice.
CO2 extruded bamboo shootfiber, as a food ingredient, hasa good effect on lowering blood lipids.
Reuse bambooshoot processing by-products to save resources and protect the environment.
1. Introduction
High-fat diet may induce a variety of chronic diseases such as hyperlipidaemia, cardiovascular and cerebrovascular symptoms (Yang et al., Citation2019). Hyperlipidaemia usually presents with blood lipids increased such as higher TC, TG and LDL-C content. Excessive intake of fat can increase the risk of hyperlipidaemia. High-fat diet can also destroy the stable flora environment of the intestine, promote inflammation and improve intestinal permeability (Rohr et al., Citation2020). Therefore, cultivating a healthy lifestyle and adjusting dietary structure can ensure health and prevent the occurrence of hyperlipidemia.
The latest epidemiological data confirmed that the increased susceptibility of obesity was closely related to insufficient dietary fibre intake (Chuang et al., Citation2012). Because dietary fibre had good adsorption capacity for unsaturated fatty acid, sodium cholate and cholesterol, etc (Palafox et al., Citation2011). Dietary fibre can not only maintain the health of the human digestive system, but also effectively prevent cardiovascular diseases, diabetes, cancer and other diseases (Yang et al., Citation2018). For example, dietary fibre can promote the release of toxic substances in food, dilute carcinogens, and reduce human blood glucose level and cholesterol level (Rani & Mehta, Citation2015). Some studies have shown that injection of high doses bamboo shell chitin (400 mg·kg−1) could help mice lose weight, reduce serum insulin consumption and blood glucose levels (Zhang et al., Citation2016).
Bamboo root, which is rich in dietary fibre, is an industrial by-product of bamboo shoot processing. Some studies have suggested that soluble dietary fibre (SDF) can promote the growth of beneficial intestines bacteria to produces short-chain fatty acids, and regulate the metabolism of glucose and lipids (Ma and Mu, Citation2016). Insoluble dietary fibre (IDF) can absorb lipids and promote defecation (Weickert & Pfeiffer, Citation2008). Therefore, dietary fibre can improve intestinal health.
Our previous studies showed that the bamboo root dietary fibre treated by CO2 extrusion had good water retention, oil retention, glucose adsorption and cholesterol absorption in a simulated environment (Yang et al., Citation2021a). Therefore, it is necessary to determine the effect of bamboo root dietary fibre (BRDF) on blood lipids in hyperlipidaemia mice through animal experiments.
In this study, the effects of BRDF on food intake, body weight, liver coefficient, blood lipid, arteriosclerosis index, contents of liver cholesterol and triglyceride in mice were to investigated. This study provided a more comprehensive understanding of the lipid lowering mechanism of BRDF, which was important for preventing hyperlipaemia and developing functional foods.
2. Materials and methods
2.1. Materials
BRDF was prepared based on our previous research (Yang et al., Citation2021a), and stored in a dryer in room temperature until use. The chemical composition of BRDF was 7.32%water, 8.17% ash, 10.61% protein, 58.54% IDF and 11.05% SDF.
The basic feed for mice was purchased from Jiangsu Synergy Medical Bioengineering Co., Ltd. (the implementation standard is GB14924.3–2010). It is composed of corn, flour, fish meal, soybean meal, yeast powder, soybean oil, etc.
Cholestyramine, purchased from Housheng Pharmaceutical Co., Ltd. (Nanjing, China). Total cholesterol (TC), total triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) kits were obtained from Jiancheng Institute of Bioengineering (Nanjing, China). Other reagents were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China).
2.2. Animal groups and feeding
Forty-eight C57BL/6 male mice (20 ± 2 g, 6-week-old) were purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd (Beijing, China). This experiment (No. njcjdx20180526) was conducted at Nanjing OG Pharmaceutical Technology Service Co., Ltd. in strict accordance with the Chinese national standard General Requirements for Experimental Animal Experiments (GBT35823–2018). After 7-days of administration with normal diet, the mice were randomly divided into 6 groups: NC (Normal Control), HFD (High Fat Diet), PD (Positive Drug, cholestyramine), LD (Low Dietary Fibre), MD (Medium Dietary Fibre), and HD (High Dietary Fibre) (8 mice per group). Each group of mice were fed for 8 weeks. Feeding methods of the experimental mice is shown in .
Table 1. Comparison of animal feeding.
After the experiment, all mice were starved for 8 hours. Eyeball blood of mice was collected. After taking blood, the mice were decapitated and killed. The liver was obtained immediately, and frozen in liquid nitrogen and store it at −80°C.
2.3. Determination of mice body weight and food intake
Accurately recorded the food intake of each group of mice every 3 days, weigh and record the weight every 3 days.
2.4. Biochemical assays
The mouse blood was centrifuged at 4°C and 3000 r·min−1 for 15 min to obtain serum, which was used to measure the serum lipid level. The contents of TC, TG, HDL-C and LDL-C were determined according to the instructions of the kits.
2.5. Determination of atherosclerosis index (AI) in mice
According to the measurement results of TC and HDL-C in serum, AI is calculated by the following formula (Wu et al., Citation2023)
2.6. Liver index of mice
After liver removal, surface blood was immediately removed. The liver weight was weighed. The liver index (%) was the ratio of liver weight to mouse weight.
2.7. Determination of contents of TC and TG in mice liver
Washed the liver with normal saline twice, weighed 1 g of liver into a glass homogenizer, added 9 mL of normal saline, rotated and ground it up and down for 10 times. Fully ground it to homogenize the tissue, and prepared 10% liver homogenate. The liver homogenate was obtained by shearing and dispersing at a rotation speed of 10,000 r·min−1 for 1 min, and then centrifuged at 2500 r·min−1 for 10 min to obtain supernatant for detection. The contents of TC and TG were determined according to the instructions of Nanjing Jiancheng kit (Nanjing, China).
2.8. H&E staining of mice liver
The histological observation of the mice liver was performed according to Yang’s method (Yang et al., Citation2021b) with slight modifications, 4% paraformaldehyde replaced 10% neutral formaldehyde.
2.9. Statistical analysis
Statistical analysis was conducted using SPSS (20.0, U.S.A.). The data were expressed in X±S, and the significance test was conducted using One-way ANOVA. The significant level of the difference was p < .05.
3. Results and discussion
3.1. Effect of BRDF on body weight and food intake in mice
The weight gain of mice during the experiment is shown in . At the beginning of the test, there was no significant difference in the weight of mice in each group. After 30 days, the weight of mice in the HFD group was 15.22% higher than that in the NC group. shows the changes in body weight during the experiment. After being fed for 21 days, the weight of HD group (29.69 ± 0.44 g) was significantly lower than that of HFD group (31.14 ± 0.29 g) (p < .05). On the 27th day of feeding, the mice weight in LD group (32.28 ± 0.57 g) was significantly lower than that in HFD group (32.93 ± 0.52 g) (p < .05). High content of potato skin dietary fibre can significantly reduce blood lipid and triglyceride in mice (Salem et al., Citation2018). It was showed that dietary fibre had the effect of assisting in weight loss. Dietary fibre had a strong absorption capacity of cholesterol, removing cholesterol from liver circulation and excreting it through feces (Dai et al., Citation2019).
Table 2. Body weight changes in the modeled mice.
Table 3. Effect of BRDF on the body weight of mice.
It could be seen from that there was no significant difference in the food intake of all mice during the feeding period (p > .05). The calorie intake of mice can be seen in the supplementary material (Table S1). The results indicated that BRDF did not affect the taste and appetite of feed. Dietary fibre did not inhibit the appetite of mice and did not affect their food intake (Isken et al., Citation2010). During the experiment, the mice showed lively, bright smooth fur, and active feeding.
Table 4. Effect of BRDF on the intake of mice.
3.2. Effect of BRDF on the levels of serum lipids in mice
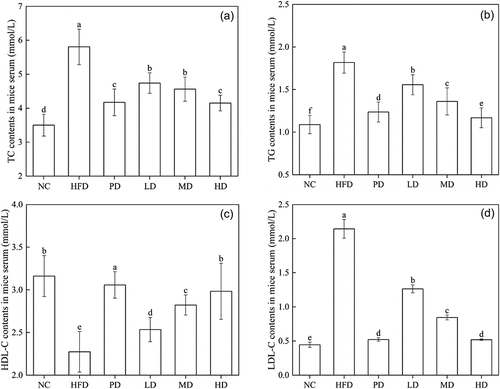
reflects the changes of serum TC content in hyperlipidaemia mice after BRDF intervention. Compared to HFD, BRDF and PD can effectively reduce the TC content in mouse serum, indicating that BRDF can regulate the liver’s ability to metabolize lipids and inhibit the accumulation of fat in the liver. The serum TC content of NC, PD, MD and HD group was 3.5 mmol·L−1, 4.17 mmol·L−1, 4.56 mmol·L−1 ,and 4.15 mmol·L−1, respectively. TC content of HD group was 21.5% lower than HFD (5.81 mmol·L−1). The TC content of mice fed by BRDF was lower than that of the HFD group. BRDF had strong oil holding and cholesterol adsorption abilities. Daily intake of BRDF can help reduce the absorption of cholesterol in the diet. shows the changes of serum TG content in hyperlipidaemia mice after BRDF intervention. Compared with HFD group (1.82 mmol·L−1), the serum TG of mice in the high, medium, and low-dose groups decreased by 35.7%, 25.3% and 14.3%, respectively.
Blood lipid index is the main criterion to judge hyperlipidaemia. Long-term high-fat diet can lead to hyperlipidaemia (Foschia et al., Citation2013). Relevant studies have shown that dietary fibre can reduce the risk of diseases such as hyperlipidaemia and atherosclerosis (Brostow et al., Citation2012).
The lipid peroxide of LDL-C can lead to the formation of atherosclerotic plaques (Vassalle et al., Citation2002). The levels of LDL-C () in LD group (1.26 mmol·L−1), MD group (0.84 mmol·L−1) and HD group (0.61 mmol·L−1) were 41.1%, 60.7% and 71.5%lower than that in HFD group (2.14 mmol·L−1), respectively. Compared with the HFD group (2.27 mmol·L−1), HDL-C () of mice in the LD, MD and HD groups increased by 11.45%, 24.22%, and 31.28%, respectively. The serum HDL-C (2.98 mmol·L−1) of HD group was lower than that of the PD group (3.05 mmol·L−1). The results showed that BRDF can effectively regulate the lipid metabolism of hyperlipidaemia mice in dose-dependent manner. Our results were similar with the Wood’s study that oat dietary fibre can also significantly reduce the TC level of hyperlipidaemia mice, significantly reduce the serum LDL-C value and AI value of normal mice, and increase the HDL-C value (Wood, Citation1994).
Dietary fibre can inhibit the absorption of TC in the intestines, hinder the reabsorption of bile acids into the hepatointestinal circulation, and promote the excretion of bile acids and TC (Ghaffarzadegan et al., Citation2018). TC, TG and LDL-C are risk factors for coronary heart disease and atherosclerosis (Woo et al., Citation2008), while HDL-C can transport cholesterol and TG from surrounding tissues to the liver for metabolism (Irudayaraj et al., Citation2013). The role of LDL is to transport TC from the liver to the outside of the cell, while the role of HDL is to transport TC back to the liver for metabolism, reduce serum TC and LDL-C content, and increase HDL-C content (Maninder & Baojun, Citation2018).
3.3. Effect of BRDF on the levels of AI in mice
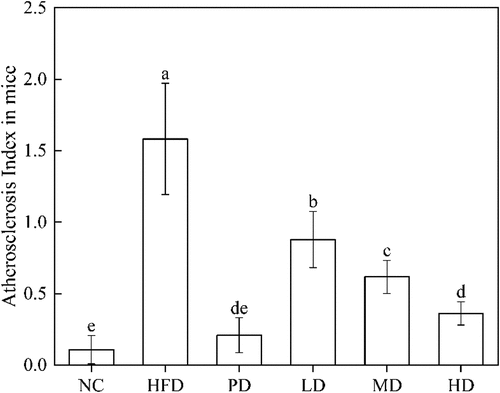
The Atherosclerosis Index (AI) is an important indicator used to determine atherosclerosis. AI value is positively correlated with atherosclerosis, and the risk of cardiovascular and cerebrovascular diseases increases with the increase of AI value. Elevated serum TC, TG and LDL-C levels can all be diagnosed as hyperlipidaemia (Yang et al., Citation2019). Hyperlipidaemia is related to heredity, diet, nutrition, drugs and other factors, it is the primary cause of atherosclerosis. The high AI value means a high risk of developing cardiovascular and cerebrovascular diseases (Wu et al., Citation2023). It can be seen from that the AI value of HFD group is 1.58, much higher than that of NC group (0.11) (p < .01). After 30 days of dietary intervention, the AI values of mice fed with low (0.88), medium (0.62) and high (0.36) doses of BRDF were lower than the HFD group, and the AI values decreased with the increase of dietary fibre intake. This is similar to the results of Galisteo et al. (Citation2010), the addition of methyl cellulose and plantain husk fibre can reduce serum TG and TC in obese rats induced by high fat. The main reason that BRDF reduces blood lipid is its good adsorption and embedding properties. In our previous study, BRDF had a smaller particle size (average particle size of 220.5 μm) and a larger specific surface area (Yang et al., Citation2021a). Larger specific surface area making bile more easily absorbed by dietary fibre in the small intestine, and bile acid cannot be absorbed by the intestinal wall of the small intestine and then return to the liver. The liver will absorb cholesterol from the blood by supplementation of consumed bile acids to meet the normal metabolic requirements, thus effectively regulating the levels of HDL-C, TC, LDL-C and AI in mice serum. Higher consumption of dietary fibre contributes to reduce the risk of hyperlipidaemia caused by high fat diet (Han et al., Citation2015).
BRDF played an important role in reducing the risk of atherosclerosis. Li et al. (Citation2021) extracted IDF (61.30%) and SDF (7.51%) from bamboo shoots, found that they had good hydrophilic effects. BRDF contains 58.54% IDF and 11.05% SDF, this should be the main reason that BRDF had a relieving and inhibitory effect on preventing hyperlipidaemia in mice.
3.4. Effect of BRDF on the liver weight and liver index in mice
The liver index can reflect the degree of fat accumulation in the liver to a certain extent. When consuming too much high fat diet, fat was difficult to metabolize, and it was accumulated in the liver, resulting in a high liver index (Zhao et al., Citation2020).
The liver weight of experimental mice after diet intervention is shown in . The liver weight of NC group (1.13 ± 0.07 g) was the lowest. The liver weight of HFD group (1.80 ± 0.06 g) was the highest, indicating that the high-fat diet caused fat accumulation around the liver of the mice and increased liver weight. The average liver weight of mice in the HD group (1.42 ± 0.03 g) was close to that of the PD group (1.35 ± 0.04 g). The liver index of the mice in the HD group was 4.56 ± 0.15, which was much lower than that of HFD group (5.33 ± 0.21). It showed that BRDF can achieve the effect of lowering the liver weight of mice on high-fat diet. BRDF can effectively reduce liver weight and antagonize disorder of liver lipid metabolism.
Table 5. Effect of BRDF on liver weight and liver index of mice.
3.5. Effect of BRDF on the TC and TG content in mice liver
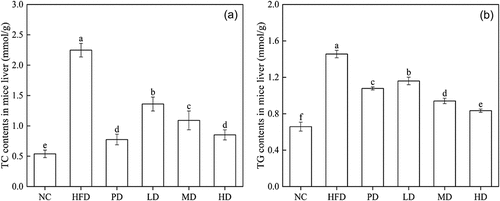
The liver is one of the places where cholesterol is catabolized. In order to explore the effect of BRDF on hyperlipidaemia mice, the contents of TC () and TG () in the liver of mice were analysed. The TC (0.77 ± 0.08 mmol·g−1) and TG (1.08 ± 0.02 mmol·g−1) content of PD were significantly lower than that of the HFD group. The liver TC content of the HD group was 0.85 mmol·g−1, which was 62.2% lower than in HFD group. TG contents of MD group and HD group were 0.94 mmol·g−1 and 0.83 mmol·g−1, lower than that of HFD group.
High-fat diet caused an increase in cholesterol and triglycerides in the liver of experimental mice. The levels of TC and TG in the liver of the three groups of mice fed with BRDF were lower than those of the HFD group. The decrease degree of TC and TG in the liver was positively correlated with the intake of BRDF, which indicated that BRDF had an effect on the liver lipid levels of mice. The liver lipid adjustment was dose-dependent on BRDF. This was similar to the research that barley dietary fibre can effectively reduce liver TG and TC levels in high-fat obese mice (Choi et al., Citation2010). Quinoa dietary fibre can promote the excretion of TC and bile acids, thereby effectively reducing liver TC and TG levels (Noratto Giuliana et al., Citation2019). After consuming dietary fibre, the absorption of lipids in the digestive system will be affected. Dietary fibre can promote the conversion of excess cholesterol in the liver into bile acids, thereby reducing the levels of TC and LDL-C in serum and liver (Niu et al., Citation2013). Some scholars believe that gut microbiota can ferment dietary fibre that cannot be directly absorbed by the host body into short chain fatty acids, which may promote the excretion of lipids, which is often associated with lowering serum cholesterol levels in experimental animals (Neyrinck et al., Citation2012).
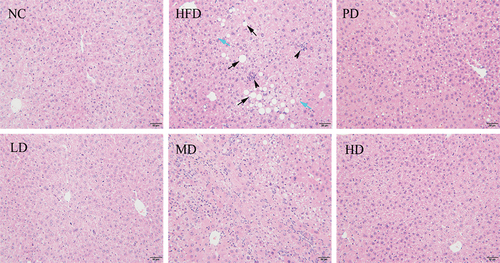
3.6. Liver histological image of mice
In the HFD group (), the liver tissue cells of mice were arranged in disorder. Lipid droplets of varying sizes, circles, and tension appeared in the cytoplasm of liver cells. The liver tissue was damaged to varying degrees. The degree of hepatocyte pathological changes of PD group was reduced, and the lipid content was reduced. Hepatocytes were mildly fatty and there was no necrosis. The degree of hepatocellular lesion in MD group was higher than that in NC group, and no necrosis was observed. Mice hepatocytes were mild steatosis and the inflammatory cell in the liver. The cellular structure of liver tissue in HD group was similar to that in NC group, with complete structure, orderly arrangement of hepatic cords, clear hepatic sinuses, uniform cytoplasm, clear and visible nuclei, and no degeneration or necrosis. The Liver histological results were similar to the results of Li et al. (Citation2021). The results of histopathological analysis showed that BRDF can reduce liver fat deposition, improve liver cell lipid change, repair liver damage, and have no toxic side effects on the liver and the body. In addition, BRDF reduced TC and TG levels in blood and livers. BRDF improved the degree of steatosis in liver cells of hyperlipidaemia mice, and had the effect of anti-atherosclerosis and liver protection.
4. Conclusion
Bamboo root dietary fibre can effectively reduce blood lipids and lose weight. The body weights of experimental mice in the HD (8.0%), MD (4.0%) and LD (2.0%) groups were lower than that of the HFD group after 30 days BRDF intervention, indicating that BRDF had the potential to prevent hyperlipaemia. Although BRDF had the effect of reducing blood lipids, BRDF was not as effective as PD. BRDF was difficult to restore blood lipids to normal levels in mice. The intervention of high-dose BRDF significantly reduced TC, TG and LDL-C level of high-fat-diet mice, and increased the value of HDL-C. The atherosclerosis index (0.36) of HD group mice was significantly lower than HFD group (1.58), which reduced the risk of atherosclerosis in mice. In addition, the intervention of BRDF reduced the liver index, TC and TG levels in the liver. H&E staining showed a reduction in inflammatory infiltration. The results suggested that BRDF in the diet can effectively prevent hyperlipaemia. However, the regulatory mechanisms of BRDF on blood lipid levels in the body are complex, further metabolomics related research is needed to understand the regulatory mechanisms of BRDF.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19476337.2023.2274371.
Additional information
Funding
References
- Brostow, D. P., Hirsch, A. T., Collins, T. C., & Kurzer, M. S. (2012). The role of nutrition and body composition in peripheral arterial disease. Nature Reviews Cardiology, 9(11), 634–643. https://doi.org/10.1038/nrcardio.2012.117
- Choi, J. S., Kim, H., Jung, M. H., Hong, S., & Song, J. (2010). Consumption of barley β-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Molecular Nutrition & Food Research, 54(7), 1004–1013. https://doi.org/10.1002/mnfr.200900127
- Chuang, S. C., Norat, T., Murphy, N., Olsen, A., Tjonneland, A., Overvad, K., Boutron-Ruault, M. C., Perquier, F., Dartois, L., Kaaks, R., Teucher, B., Bergmann, M. M., Boeing, H., Trichopoulou, A., Lagiou, P., Trichopoulos, D., Grioni, S., Sacerdote, C. … Riboli, E. (2012). Fiber intake and total and cause-specific mortality in the European prospective investigation into cancer and nutrition cohort. American Journal of Clinical Nutrition, 96(1), 164–174. https://doi.org/10.3945/ajcn.111.028415
- Dai, B., Huang, S., & Deng, Y. (2019). Modified insoluble dietary fibers in okara affect body composition, serum metabolic properties, and fatty acid profiles in mice fed high-fat diets: An NMR investigation. Food Research International, 116, 1239–1246. https://doi.org/10.1016/j.foodres.2018.10.011
- Foschia, M., Peressini, D., Sensidoni, A., & Brennan, C. S. (2013). The effects of dietary fibre addition on the quality of common cereal products. Journal of Cereal Science, 58(2), 216–227. https://doi.org/10.1016/j.jcs.2013.05.010
- Galisteo, M., MoRón, R., RiveraI, L., Romero, R., Anguera, A., & Zarzuelo, A. (2010). Plantago ovata husks-supplemented diet ameliorates metabolic alterations in obese Zucker rats through activation of AMP-activated protein kinase. Comparative study with other dietary fibers. Clinical Nutrition, 29(2), 261–267. https://doi.org/10.1016/j.clnu.2009.08.011
- Ghaffarzadegan, T., Zhong, Y., Llenius, F., & Nyman, M. (2018). Effects of barley variety, dietary fiber and β-glucan content on bile acid composition in cecum of rats fed low- and high-fat diets. The Journal of Nutritional Biochemistry, 53, 104–110. https://doi.org/10.1016/j.jnutbio.2017.10.008
- Han, S., Jiao, J., Zhang, W., Xu, J., Wan, Z., Zhang, W., Gao, X., & Qin, L. (2015). Dietary fiber prevents obesity-related liver lipotoxicity by modulating sterol-regulatory element binding protein pathway in C57BL/6J mice fed a high-fat/cholesterol diet. Scientific Reports, 5(1), 15256. https://doi.org/10.1038/srep15256
- Irudayaraj, S. S., Sunil, C., Duraipandiyan, V., & Ignacimuthu, S. (2013). In vitro antioxidant and antihyperlipidemic activities of toddalia asiatica (l) lam. leaves in triton wr-1339 and high fat diet induced hyperlipidemic rats. Food & Chemical Toxicology, 60(10), 135–140. https://doi.org/10.1016/j.fct.2013.07.035
- Isken, F., Klaus, S., Osterhoff, M., Pfeiffer, A. F. H., & Weickert, M. O. (2010). Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. The Journal of Nutritional Biochemistry, 21(4), 278–284. https://doi.org/10.1016/j.jnutbio.2008.12.012
- Li, Q., Fang, X., Chen, H., Han, Y., Liu, R., Wu, W., & Gao, H. (2021). Retarding effect of dietary fibers from bamboo shoot (phyllostachys edulis) in hyperlipidemic rats induced by a high-fat diet. Food & Function, 12(10), 4696. https://doi.org/10.1039/D0FO02407D
- Ma, M., & Mu, T. (2016). Anti-diabetic effects of soluble and insoluble dietary fibre from deoiled cumin in low-dose streptozotocin and high glucose-fat diet-induced type 2 diabetic rats. Journal of Functional Foods, 25, 186–196. https://doi.org/10.1016/j.jff.2016.05.011
- Maninder, M., & Baojun, X. (2018). A critical review on anti-diabetic and anti-obesity effects of dietary resistant starch. Critical Reviews in Food Science and Nutrition, 59(3), 1–39. https://doi.org/10.1080/10408398.2018.1481360
- Neyrinck, A. M., Possemiers, S., Verstraete, W., De Backer, F., Cani, P. D., & Delzenne, N. M. (2012). Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. Journal of Nutritional Biochemistry, 23(1), 51–59. https://doi.org/10.1016/j.jnutbio.2010.10.008
- Niu, Y., Xie, Z., Zhang, H., Sheng, Y., & Yu, L. (2013). Effects of structural modifications onphysicochemical and bile acid-binding properties of psyllium. Journal of Agricultural and Food Chemistry, 61(3), 596–601. https://doi.org/10.1021/jf3043117
- Noratto Giuliana, D., Murphy, K., & Chew Boon, P. (2019). Quinoa intake reduces plasma and liver cholesterol, lessens obesity-associated inflammation, and helps to prevent hepatic steatosis in obese db/db mouse. Food Chemistry, 287, 107–114. https://doi.org/10.1016/j.foodchem.2019.02.061
- Palafox-Carlos, H., Ayala-Zavala, J. F., & Gonzaiez-Aguilar, G. A. (2011). The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. Journal of Food Science, 76(1). https://doi.org/10.1111/j.1750-3841.2010.01957.x
- Rani, M., & Mehta, B. (2015). A review on health benefits of dietary fiber. Annals of Agri Bio Research, 20(1), 139–142.
- Rohr, M. W., Narasimhulu, C. A., Rudeski-Rohr, T. A., & Parthasarathy, S. (2020). Negative effects of a high-fat diet on intestinal permeability: A review. Advances in Nutrition, 11(1), 77–91. https://doi.org/10.1093/advances/nmz061
- Salem, E., Bartley, G. E., Yokoyama, W. H., & Mendel, F. (2018). Dietary supplementation of potato peel powders prepared from conventional and organic russet and non-organic gold and red potatoes reduces weight gain in mice on a high-fat diet. Journal of Agricultural and Food Chemistry, 66(24), 6064–6072. https://doi.org/10.1021/acs.jafc.8b01987
- Vassalle, C., Lubrano, V., Labbate, A., & Clerico, A. (2002). Determination of nitrite plus nitrate and malondialdehyde in human plasma: Analytical performance and the effect of smoking and exercise. Clinical Chemistry and Laboratory Medicine, 40(8), 802–809. https://doi.org/10.1515/CCLM.2002.139
- Weickert, M. O., & Pfeiffer, A. F. (2008). Metabolic effects of dietary fiber consumption and prevention of diabetes. The Journal of Nutrition, 138(3), 439–442. https://doi.org/10.1093/jn/138.3.439
- Woo, M. N., Bok, S. H., Lee, M. K., Kim, H. J., Jeon, S. M., & Do, G. M., Shin, S K., Ha, T Y., Choi, M-S. (2008). Anti-obesity and hypolipidemic effects of a proprietary herb and fiber combination (S&S PWH) in rats fed high-fat diets. Journal of Medicinal Food, 11(1), 169–178. https://doi.org/10.1089/jmf.2007.082
- Wood, P. J. (1994). Evaluation of oat bran as a soluble fibre source, characterization of oat β-glucan and its effects on glycaemic response. Carbohydrate Polymers, 25(4), 331–336. https://doi.org/10.1016/0144-8617(94)90059-0
- Wu, X., Gao, Y., Wang, M., Peng, H., Zhang, D., Qin, B., Pan, L., & Zhu, G. (2023). Atherosclerosis indexes and incident t2dm in middle-aged and older adults: Evidence from a cohort study. Diabetology & Metabolic Syndrome, 15(1), 23. https://doi.org/10.1186/s13098-023-00992-4
- Yang, L., Li, Z., Song, Y., Liu, Y., Zhao, H., Liu, Y., Zhang, T., Yuan, Y., Cai, X., Wang, S., Wang, P., Gao, S., Li, L., Li, Y., & Yu, C. (2019). Study on urine metabolic profiling and pathogenesis of hyperlipidemia. Clinica Chimica Acta; International Journal of Clinical Chemistry, 495(1), 365–373. https://doi.org/10.1016/j.cca.2019.05.001
- Yang, M., Wu, L., Cao, C., Wang, S., & Zhang, D. (2018). Improved function of bamboo shoot fibre by high-speed shear dispersing combined with enzyme treatment. International Journal of Food Science & Technology, 54(3), 844–853. https://doi.org/10.1111/ijfs.14004
- Yang, M., Wu, L., Cao, C., Wang, S., & Zhang, D. (2021a). Extrusion improved the physical and chemical properties of dietary fibre from bamboo shoot by-products. International Journal of Food Science & Technology, 56(2), 847–856. https://doi.org/10.1111/ijfs.14728
- Yang, M., Zheng, J., Zong, X., Yang, X., Zhang, Y., Man, C., & Jiang, Y. (2021b). Preventive effect and molecular mechanism of lactobacillus rhamnosus JL1 on food-borne obesity in mice. Nutrients, 13(11), 3989. https://doi.org/10.3390/nu13113989
- Zhang, S., Wang, Q., Lu, X., Lin, L., Tian, Y., Xiao, J., & Zheng, B. (2016). Characterization and hypoglycemic activity of a β-pyran polysaccharides from bamboo shoot (leleba oldhami nakal) shells. Carbohydrate Polymers, 144, 438–446. https://doi.org/10.1016/j.carbpol.2016.02.073
- Zhao, Z., Chen, L., Zhao, Y., Wang, C., Duan, C., Yang, G., Niu, C., & Li, S. (2020). Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Applied Microbiology and Biotechnology, 104(12), 5273–5282. https://doi.org/10.1007/s00253-020-10633-9