?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study determined total phenolic content (TPC), 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, α-glucosidase, and nitric oxide (NO) inhibitory activities of black (BR), purple (PR), and red rice (RR) extracted with different polarity solvents and characterized the metabolites by using the 1H NMR-based metabolomics approach. The methanol extract of RR had the highest DPPH scavenging and α-glucosidase inhibitory activities. The water extracts of PR and RR exhibited the highest NO inhibitory activity with IC50 values of 176.28 ± 18.78 and 160.69 ± 14.83 µg/mL, respectively. The metabolites that contribute to radical scavenging and α-glucosidase inhibitory activities of RR methanol extract were chlorogenic acid, protocatechuic acid, and tyrosine, whereas nicotinic acid, γ-aminobutyric acid, and citric acid were the metabolites that contributed to the NO inhibitory activity of RR water extract. The RR extracts are potential sources of bioactive compounds with antioxidant and NO inhibitory properties.
1. Introduction
Rice (Oryza sativa L.) is the most widely consumed staple cereal grain in the world and a significant source of minerals, fibre, vitamins, energy, and other biomolecules. In Asia and developing nations, rice consumption is extraordinarily high. Asian countries produce nearly 95% of the world’s rice, and roughly 50% of the global population consumes it. Rice cultivation ranks third in agricultural production (Priya et al., Citation2019). Pigmented rice has been consumed worldwide, especially in Asian countries. In Thailand, several local pigmented rice varieties with different grain colors have been grown all over the country. Recently, health benefits of pigmented rice products, particularly from black (BR), red (RR), and purple rice (PR) varieties, have been identified and led to an increase in consumption. Pigmented rice extracts have been considered as important sources of antioxidants (Deng et al., Citation2013; Min et al., Citation2014). Phenolic compounds such as phenolic acids and flavonoids are responsible for the health benefits of pigmented rice (Kowsalya et al., Citation2022; Sati & Singh, Citation2019). The biological properties such as free-radical scavenging, anticarcinogenic, antiallergic, anti-inflammatory, antiatherosclerosis, and hypoglycemic activities have been reported in pigmented rice extracts (Min et al., Citation2014; Paiva et al., Citation2014; Sompong et al., Citation2011).
Several techniques have been performed for pigmented rice extraction, in which organic solvent is commonly used. However, this technique is time-consuming and requires a large amount of solvents, and its toxicities need to be considered. Water extraction is a non-chemical method with advantages on food safety and environment-friendly issues (Pingret et al., Citation2012). Nevertheless, as the polarity of extraction solvents can cause changes in the distribution and conformation of components of natural products and can modify the biological activities of the extracts. These extraction parameters can be optimized to preserve those properties of the extracts.
Metabolomics approach has been used as a tool to explain the metabolic state in biological fluids, tissues, and cells via the assessment of metabolite profiles (Kusano et al., Citation2015). Metabolites are profiled and identified using spectroscopy techniques, such as nuclear magnetic resonance (NMR) spectroscopy or mass spectroscopy. Multivariate data analysis (MVDA) is coupled with those tools to simplify and manage the obtained large dataset. Consequently, information on the relationship between metabolites and biological properties can be studied using the metabolomics approach. Application of metabolomics platform in natural plants has been published previously (Abdul-Hamid et al., Citation2015). For pigmented rice, there are few studies that indicate the potential of pigmented rice extracts against inflammation and other chronic diseases. However, little is known on the correlation of biological properties and metabolite profiles of pigmented rice. In addition, research on the effects of extraction conditions on the properties of pigmented rice has been lacking. In the present study, the influences of extraction solvents and varieties of pigmented rice on antioxidant, nitric oxide (NO), and α-glucosidase inhibitory activities were investigated. The profiles of metabolites in the extracts were examined by NMR spectroscopy. The correlation between the identified metabolites and biological properties of pigmented rice was interpreted using the metabolomics approach.
2. Materials and methods
2.1. Chemicals and reagents
Hexane, methanol, acetone, Folin – Ciocalteu reagent, deuterated methanol-d4 (CD3OD), non-deuterated potassium dihydrogen phosphate (KH2PO4), sodium deuterium oxide, trimethylsilyl propionic acid-d4 sodium salt (TSP), deuterium oxide (D2O), dimethyl sulfoxide (DMSO), and sodium hydroxide were supplied from Merck (Darmstadt, Germany). Sodium carbonate, gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), quercetin, p-nitrophenyl-α-d-glucopyranosidase (PNPG), sodium phosphate monobasic monohydrate, sodium phosphate dibasic, α-glucosidase enzyme, curcumin, lipopolysaccharide (LPS), phosphate-buffered saline (PBS), and recombinant murine IFN-γ were purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.). Dulbecco’s Modified Eagle’s Medium (DMEM) containing HEPES and l-glutamine with phenol red as well as that without phenol red, PBS (10×, pH 7.4), penicillin streptomycin, TrypLE Express Enzyme fetal bovine serum (FBS), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Gibco (Eggenstein, Germany).
2.2. Sample preparation
Unpolished rice samples of black, red, and purple pigmented rice were obtained from Nong Sano Agricultural Cooperative, Sam Ngam, Phichit, Thailand, with coordinates of 16.396085°N and 100.063999°E. Six kilograms of each rice sample was collected and then divided into three groups to be subjected to three extractions with different solvents. The samples were ground and sieved to consistent particle size. Then, the samples were vacuum packed and kept at 4°C.
2.3. Extraction of pigmented rice extracts
Crude extracts of pigmented rice samples were prepared according to the method by Gunaratne et al. with some modifications (Gunaratne et al., Citation2013). Each sample was defatted by soaking in hexane overnight. The hexane solution was then removed, and the sample was left under a fume hood to remove the residue of hexane. Six replicates of each sample were prepared. The defatted rice sample of 0.5 g was mixed with 10 mL of methanol, acetone, and deionized water. Then, ultrasonic extraction was conducted using a sonicator for 30 min at a controlled temperature with the addition of ice to avoid heating. After extraction, the mixture was centrifuged at 3,000 rpm for 15 min, and the supernatant was collected. The extract was then evaporated by a rotary evaporator at 40°C, lyophilized, and kept at 4°C prior to further analysis.
2.4. Total phenolic content determination
Total phenolic concentration (TPC) of rice extracts was analyzed using the Folin – Ciocalteu assay (Lee et al., Citation2016). An aliquot (150 µg/mL) of the rice extract (20 µL) was mixed with 10-fold of Folin – Ciocalteu reagent (100 µL) and 7.5% sodium carbonate solution (80 µL). After incubation in the dark for 30 min at room temperature (26°C), the absorbance of the mixture was measured at 760 nm against the reagent blank. TPC was calculated as µg gallic acid equivalent per mg dry sample (µg GAE/mg).
2.5. DPPH radical scavenging activity determination
Radical scavenging activity of rice extracts was determined using the DPPH assay (Lee et al., Citation2019). A serial dilution of 50 µL aliquots of rice extract (2–250 µg/mL) was performed in 96-well plates, and 100 µL DPPH solution in methanol (5.9 mg/100 mL methanol) was added into the wells. Then, the plate was incubated for 30 min in the dark. The absorbance of the solution was measured at 517 nm. Quercetin was used as a positive control. The DPPH scavenging activity (%) of the sample was calculated as follows:
where Ao is the absorbance of the reagent blank and As is the absorbance of the extracts. The results are expressed as IC50 value in µg/mL.
2.6. Anti-inflammatory activity via NO assay
2.6.1. Cell culture
The NO inhibition activity of pigmented rice extracts was determined (Abas et al., Citation2006). The murine macrophage cells (RAW264.7) obtained from the American Type Culture Collection (ATCC, Rockville, MD, U.S.A.) were grown in a plastic culture flask in DMEM with phenol red containing HEPES and l-glutamine supplemented with 10% FBS and 1% antibiotic solution (Gibco/BRL) under controlled atmosphere (37°C, 5% CO2). After 3–4 days, the cells were detached from the flask using TrypLE™ Express Enzyme prior to centrifugation at 1,000 rpm at 4°C for 10 min. The supernatant was then removed, and the cells were suspended in fresh DMEM without phenol red combined with HEPES and l-glutamine supplemented with FBS and antibiotic solution. The standard trypan blue cell counting technique was used for cell count and viability. The concentration of the cells was then adjusted to 1 × 106 cells/mL using the same medium and cultured in 50 µL of the media mentioned above with the triggering agents (200 U/mL of recombinant murine IFN-ϒ and 10 µg/mL LPS) seeded into each well of 96-well culture plates. A serial dilution (16–500 µg/mL) of pigmented rice extract 50 µL was performed in a medium, containing DMSO and yielded a final concentration of DMSO at 0.4% per well. The verification was carried out in six replicates, and the plate was then incubated in a humidified incubator at 37°C with 5% CO2 for 24 h. The cells were cultured in the medium only, and the cells containing triggering agents with 0.4% DMSO were used as controls of the experiment.
2.6.2 Measurement of nitrite
The released nitrite (NO2−), the stable NO conversion product, was determined using the Griess reagent (1% sulfanilamide, 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride, and 2.5% H3PO4) to establish the concentration of NO. After 24 h of incubation, 50 µL of aliquots from the supernatant of the cultured cells were removed and incubated with an equal volume of the Griess reagent at room temperature for 10 min. The absorbance was measured at 550 nm using a microplate reader. Curcumin was used as a positive control. The inhibition activity of the extracts was calculated as percentage of inhibition using fresh culture medium as a blank.
2.6.3. Cell viability measurement
The viability of the treated cells was evaluated using the cytotoxicity assay. The media was removed from the wells, and 100 µL of complete DMEM was added into each well. Afterward, 20 µL of MTT solution in PBS (5 mg/mL) was loaded, and the cells were then incubated at 37°C with 5% CO2 for 4 h. The absorbance was measured at 570 nm. The absorbance of formazan in the control, untreated cells was taken as 100% viability. The percentage of dead cells was calculated in comparison to the control.
2.7. α-Glucosidase inhibitory activity
The α-glucosidase inhibitory activity of the pigmented rice extracts was determined following the previously described assay (Lee et al., Citation2014). Serial dilutions of the pigmented rice extracts (concentration range 2–400 µg/mL) were prepared in 96-well microplates and mixed with PNPG dissolved in 50 mM phosphate buffer (pH 6.5). After incubation at room temperature for 5 min, the mixtures were added with 75 µL of PNPG to each well of sample, blank substrate, negative control, and positive control (quercetin). The rest was loaded with 75 µL of 30 mM phosphate buffer. Then, the mixtures were incubated for 15 min at room temperature, and 50 µL of 2 M glycine (pH 10) was added to the sample, blank substrate, and negative control, whereas 50 µL of deionized water was mixed to the others. The absorbance was measured using a spectrophotometer at a wavelength of 405 nm. The percentage (%) of inhibition was calculated as follows:
where an is the difference in absorbance of the negative control and all the blanks, and as is the difference in absorbance of the sample and all the blanks. The result was presented as IC50 value in µg/mL.
2.8. NMR analysis
The preparation procedure was performed following the method by Abdul-Hamid et al. with a slight modification (Abdul-Hamid et al., Citation2016). Rice extract (10 mg) was mixed with 0.375 mL of CD3OD and 0.375 mL of KH2PO4 buffer in D2O (pH 6.0) containing 0.1% TSP in a centrifuge tube (2 mL). The mixture was vortexed for 1 min at room temperature, sonicated for 5 min, and then centrifuged for 10 min at 13,000 rpm. The clear supernatant (0.6 mL) was transferred to an NMR tube and then subjected to 1H NMR analysis. Briefly, the 1H NMR measurements were performed using 500 MHz Varian INOVA NMR spectroscopy (Varian Inc., CA, U.S.A.). The temperature was maintained at 26°C. The required time of each sample was 3.53 min, consisting of 64 scans with an acquisition time, relaxing delay of 1.0 s, and spectral width of 20 ppm. The correction of phasing and baseline was performed manually for all spectra using Chenomx software (v.5.1; Chenomx, Alberta, Canada). The chemical shifts were referenced to the internal standard, TSP = .0 ppm. All spectra were binned and exported into Excel file for MVDA using SIMCA-P+ version 13.0 (Umetrics AB, Umea, Sweden) software. Two-dimensional 1H–1H J-resolved analysis was applied to support the identification of some components. The metabolite identification was done based on examination of metabolite chemical shifts from an online database, Human Metabolome Database (HMDB), peak fitting routine of Chenomx database (v.8.1) and a comparison with a previous study (Pramai et al., Citation2017). Metabolic pathway of the pigmented rice was generated through the enrichment analysis pathway available in MetaboAnalyst online free software version 5.0 (https://www.metaboanalyst.ca/).
2.9. Statistical analysis
Analysis of variance (ANOVA) and Duncan test were carried out to evaluate the significance between groups (p < .05) using SPSS Statistics for Windows, version 17.0 software (SPSS Inc., Chicago, IL, U.S.A.). All the experiments were performed in six replicates, and the results are expressed as mean ± standard deviation. A p value <0.05 was considered statistically significant.
3. Results
3.1. TPC and biological activities of pigmented rice extracts
shows the TPC determination of different varieties of pigmented rice extracts, BR, PR, and RR, obtained from different extraction solvents. Difference in TPC was observed among the extracts due to the effects of extraction solvents and varieties of the samples. In general, the TPC values ranged from 16 to 1,143 mg GAE/100 g sample. The RR extracts were significantly higher in TPC (934.06–1,142.77 mg GAE/100 g sample) than the BR (64.10–430.68 mg GAE/100 g sample) and PR extracts (16.24–194.60 mg GAE/100 g sample). For BR, the acetone and methanol extracts were higher in TPC than the aqueous extracts. The highest TPC of PR sample was observed when using methanol as solvent for extraction. On the other hand, the TPC of water extract of RR was found to be greater than that of other extracts (p < .05).
Table 1. Biological activities of different varieties of pigmented rice extracted with different solvents.
The DPPH scavenging activity of pigmented rice extracts was determined to indicate their antioxidant activity (). The activity was affected by varieties of pigmented rice and extraction solvents. The RR extracts showed higher scavenging activity, with the IC50 values ranging from 2.95 to 7.25 µg/mL, than the BR and PR extracts. Considering the extraction solvents on the activity, the methanol extracts of RR and PR exhibited lower IC50 values than the water and acetone extracts. On the other hand, the acetone extracts of BR and PR indicated that the percentage inhibition was lower than 50% at 250 µg/mL. The lowest IC50 value was found in the methanol extract of RR that represented the highest scavenging activity.
The α-glucosidase enzyme is a key enzyme for digestion of dietary carbohydrate that breaks down oligosaccharides and disaccharides into absorbable monomers for intestinal absorption. Inhibition of this enzyme can effectively reduce postprandial blood glucose levels, especially in patients with type 2 diabetes. shows the α-glucosidase inhibitory activity of pigmented rice extracts expressed as IC50 value. Among different varieties of pigmented rice, the RR extracts exhibited greater inhibition with IC50 values ranging from 0.43 to 2.90 µg/mL than the BR and PR extracts. The extracts from RR, containing higher TPC than those from BR and PR, showed greater α-glucosidase inhibitory activity. The extraction solvents showed similar impacts on the inhibitory activity of BR and PR extracts. The methanol extract of RR indicated higher activity than the acetone and water extracts with IC50 value of 0.43 ± 0.07 µg/mL. However, no significant difference in the inhibitory activity was observed (p > .05) for acetone and water extracts.
While NO is one of the inflammatory mediators that relates to oxidative stress disorder in body tissues. shows the anti-inflammatory activity based on NO inhibitory activity of the pigmented rice extracts. The effectiveness of the inhibitory activity toward NO was as follows: RR > PR > BR. RR extracts exhibited greater inhibition with IC50 values ranging from 160.69 to 225.91 µg/mL than PR and BR extracts. For the extraction solvent effects, the water extract of RR showed the highest NO inhibitory activity with an IC50 value of 160.69 µg/mL followed by the water extract of PR with an IC50 value of 176.28 µg/mL.
3.2. 1H NMR profiles of different pigmented rice extracts
The representative 1H NMR spectra were obtained from the extracts of pigmented rice extracted with different extraction solvents (). The spectra represented differences in peaks and peak intensities in sugar (δ 3.0–5.5), aliphatic (δ 0.5–3.0), and aromatic regions (δ 5.5–9.0). Different pigmented rice varieties also indicated different patterns of spectra and peak intensities particularly in aliphatic and aromatic regions.
Figure 1. 1H NMR spectra of different pigmented rice; black (BR), purple (PR) and red (RR) extracted using different extraction solvents.
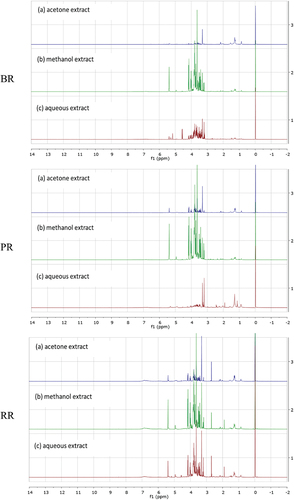
The spectra of the representative pigmented rice extracts were characterized. shows the identified compounds. Sugars (UDP-glucose, sucrose, maltose, glucose, fructose), amino acids (histidine, τ-methylhistidine, tyrosine, choline, alanine, valine, isoleucine), organic acids (nicotinic acid, γ-aminobutyric acid (GABA), 2-oxoglutaric acid, citric acid, succinic acid, pyruvic acid, acetic acid, 2-hydroxyisovalerate), fatty acid derivatives, phenolic compounds (chlorogenic acid, vanillic acid, protocatechuic acid), and some organic compounds (trigonelline, propylene glycol) were identified metabolites in the pigmented rice extracts.
Table 2. 1H-NMR characteristic signals of pigmented rice extracts.
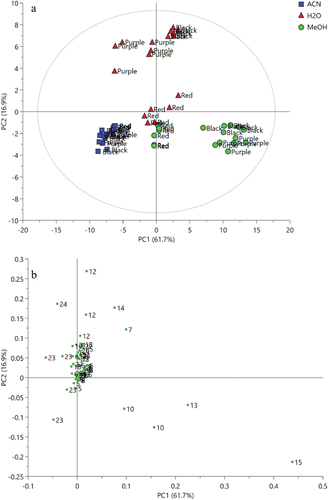
3.3. Discrimination of the pigmented rice extracts by principal component analysis
shows the principal component analysis (PCA) score plot of the 1H NMR data of the BR, PR, and RR extracts obtained from different extraction solvents. The score plot revealed the relationship and variation of samples by clusters of data. According to the score plot, the separation of metabolites distributed in each extract was observed. It is clearly shown that acetone extracts of all varieties of pigmented rice were well separated from other extracts. The methanol extracts were situated at the lower right of the PCA score plot; however, the methanol extracts of RR were slightly far from the other varieties. In addition, the water extracts of RR were close to the methanol extracts of RR indicating that they have similar metabolite features. All water extracts except for the RR variety were situated at the upper quadrant of the PCA score plot. The findings suggested that discrimination and metabolite variation of the samples were dominated by the solvent types instead of varieties of pigmented rice. The first two PCA components accounted for 78.8% (PC1 contributed to 61.9% and PC2 contributed to 16.9%, respectively) resulting in well separation of the clusters. The R2 (0.989) and Q2 (0.958) of the model showed a good fitness and high predictability. shows that the loading plot suggested the metabolites that contributed to the discrimination of clusters. The loading plot suggested that fructose, sucrose, vanillic acid, and propylene glycol were the characteristic metabolites of the water extracts of BR and PR that were the least to be found in the acetone extracts of all varieties. In contrast, fatty acid derivatives were found to be involved in the discrimination of all the acetone extracts from other extracts. The metabolites that related to the separation of the methanol extracts of BR and PR were sucrose, GABA, and 2-dimethylamine, which were located further away from the plot origin. Furthermore, the metabolites including chlorogenic acid, protocatechuic acid, organic acid, and amino acid contributed to the separation of water and methanol extracts of RR from the rest of the samples.
3.4. Correlation between the metabolites and biological activities of the pigmented rice extracts
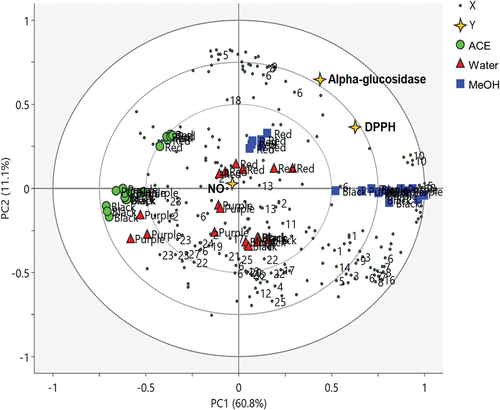
To describe the correlation between the metabolites and bioactivities of the pigmented rice extracts, partial least squares (PLS) discriminant analysis was performed as supervised MVDA. The PLS biplot of 1H NMR metabolites of the pigmented rice extracts obtained from three different varieties is shown in , and the clusters of the extracts were exhibited. According to the biplot, the acetone extracts of all pigmented rice varieties that have the least DPPH free-radical scavenging and α-glucosidase inhibitory activities were well separated from the methanol extracts by PC1, whereas the RR extracts were separated from the BR and PR extracts by PC2. The metabolites of RR methanol extract were found to be positively correlated with DPPH scavenging and α-glucosidase inhibitory activities, whereas the metabolites of RR water extract were strongly correlated to NO inhibitory activity.
Figure 3. PLS biplot of 1H NMR spectra of different varieties of the pigmented rice extracts (black, purple, and red) from different extraction solvents (ACE: acetone, MeOH: methanol, H2O: water) describing the relationship between metabolites and bioactivities of pigmented rice extracts (1: trigonelline, 2: Nicotinic acid, 3: Histidine, 4: T-methylhistidine, 5: UDP glucose, 6: Chlorogenic acid, 7: Vanillic acid, 8: Protocatechuic acid, 9: Tyrosine, 10: Sucrose, 11: Maltose, 12: Glucose, 13: 4-Aminobutyric acid (GABA), 14: Fructose, 15: 2-Dimethylamine, 16: Choline, 17: 2-Oxoglutaric acid, 18: citric acid, 19: Succinic acid, 20: Pyruvic acid, 21: Acetic acid, 22: Alanine, 23: Fatty acid derivatives, 24: Propylene glycol, 25: Valine, 26: Isoleucine, 27: 2-Hydroxyisovaleric acid).
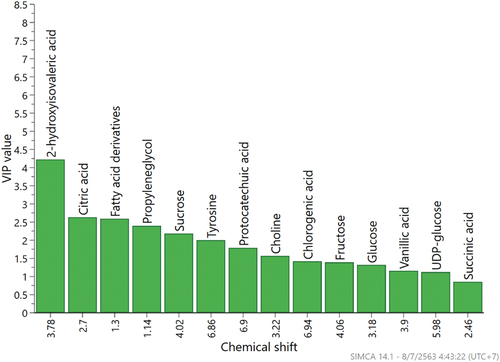
The contribution power of the characterized metabolites to the activities was examined by variable importance in the projection (VIP) and the value achieved ≥0.7 (Maulidiani et al., Citation2013), representing the significant contribution of the metabolites toward the observed activities (). According to the VIP column plot, 2-hydroxyisovaleric acid, citric acid, fatty acid derivatives, propylene glycol, sucrose, tyrosine, protocatechuic acid, choline, chlorogenic acid, fructose, glucose, vanillic acid, UDP-glucose, and succinic acid were found to be the important metabolites that contributed to the separation of pigmented rice extracts in the PLS model. The metabolites such as chlorogenic acid, protocatechuic acid, tyrosine, and citric acid are positively correlated to its biological activities, in contrast to 2-hydroxyisovaleric acid, fatty acid derivatives, and propylene glycol that are negatively correlated with its biological activities. Notwithstanding, the VIP value for the NO inhibitory activity of different pigmented rice extracts could not be performed due to the number of model dimension that was zero.
Figure 4. The variable importance in the projections (VIP) values of major contributing metabolites based on PLS model of pigmented rice from different cultivars and solvents.
The PLS model validation was performed by internal cross-validation and permutation test. The cross-validation provided the R2 of 0.859 and Q2 of 0.778, representing a good fitness and predictive ability of the model (Mediani et al., Citation2012). The permutation test using 100 random permutations exhibited the Y-axis intercepts of R2 and Q2 in the range of 0.04–0.09 and −0.26 to −0.09, respectively (Supplementary Figure S1). The values were within the limits of R2 <0.3 and Q2 <0.05, confirming that the model is valid and not over fitted (Wei et al., Citation2019). Hence, the present model demonstrated a valid and reliable model.
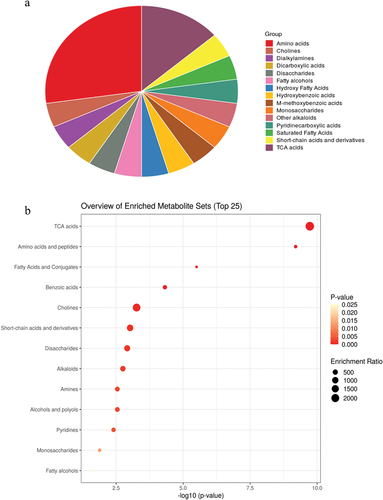
3.5. Metabolic pathway involved in pigmented rice
In addition to the 1H NMR metabolomics, the metabolic pathway involved in pigmented rice is proposed via the Kyoto Encyclopedia of Genes and Genomes (https://www.kegg.jp/kegg/pathway.html) guideline. According to the over-representation analysis (ORA), tricarboxylic acid (TCA) cycle, amino acids, fatty acids and conjugates, benzoic acids, choline, short-chain acids and derivatives, disaccharides, alkaloids, amines, alcohols and polyols, pyridines, monosaccharides, and fatty alcohols are the significant (p < .05) pathways involved in pigmented rice ( and Supplementary Table S1). ORA was conducted based on the hypergeometric test to evaluate whether a particular metabolite set is represented more than expected by chance within the provided compound list that was subjected to the analysis. In addition, the results implied that TCA and amino acids are the most important pathways in pigmented rice.
4. Discussion
4.1. TPC and biological activities of pigmented rice extracts
The TPC result were in agreement with a previous study where the extracts of RR from different solvents showed a higher TPC amount than the extracts of BR (Muntana & Prasong, Citation2010). Phenolic compounds are secondary metabolites found in pigmented rice mainly in the bran layer of the grain (Chen et al., Citation2012). These bioactive compounds can be extracted by solvents extraction according to their polarities (like-dissolve-like behavior). Extraction solvent is known as an important parameter of phenolic compounds extraction. The difference in polarity of extraction solvent is the result in variation in phenolic compounds content. Sonication is an alternative technique that has been applied in plants and food products extraction with its advantages of being less time-consuming and safety (Das et al., Citation2017). The rupture of cell walls occurs during the process and enhances contact with solvent with extractable cell materials. Therefore, higher yields of extracted components are obtained (Pingret et al., Citation2012). As for the DPPH scavenging activity, RR extracts had a high amount of phenolic content and displayed the lowest IC50 values, which indicates that the phenolic content present in RR extracts may contribute directly to their antioxidant activity. However, the RR water extract containing the highest TPC did not exhibit the highest radical scavenging activity. Instead, the highest activity was obtained from the RR methanol extract that has slightly lower TPC than that from the RR water extract. This might indicate that besides phenolic, there are some other compounds have participated in the DPPH scavenging activity of the RR water extract. Oki et al. reported a high DPPH scavenging activity in the acetone extract of RR, whereas no activity was detected in the acetone extracts from white rice and BR (Oki et al., Citation2002). The RR extract, containing proanthocyanidins as major antioxidants and has a stronger antioxidant activity than the BR extract (Gunaratne et al., Citation2013). As for the α-glucosidase inhibitory and anti-inflammatory activities of pigmented rice and rice bran extracts had been studied by some researchers. An opposite finding was observed by Yao et al. in which the BR extract showed higher α-glucosidase inhibitory activity than the RR and PR extracts (Yao et al., Citation2010). Different results may be observed due to differences in genetics as well as climate and cultivation factors of the samples used. Those factors can cause variation of metabolites and bioactive components in the samples. Therefore, the biological activities of pigmented rice from different sources can be varied. The anti-inflammatory potential of the pigmented rice was assessed by measuring its ability to inhibit NO production. NO serves as a crucial mediator in the activation of inflammation in leukocyte and macrophage cells. Overproduction of NO production during the host’s immune response can lead to alterations in DNA, proteins, lipids, transcription factors, and enzyme functions. These changes contribute to vasodilation and vasoconstriction, which are responsible for the characteristic redness and swelling associated with inflammation (Tripathi et al., Citation2007). In this study, NO was synthesized in LPS and IFN-γ stimulated RAW 264.7 cells and subsequently quantified using the Griess assay. The ability of plant materials to inhibit the production of NO could be attributed to the synergistic impact of three different mechanisms; (1) suppressing the iNOS enzyme expression, (2) hindering the catalytic function of iNOS, and (3) actively removing NO radicals (Tsai et al., Citation2007). The diverse phytoconstituents found in plant samples can potentially employ a range of mechanisms to effectively suppress the production of excessive NO. These distinct mechanisms help clarify the variations observed in the inhibitory activity of NO (Tsai et al., Citation2007). Concerning the NO inhibitory activity of the studied pigmented rice, the result obtained was in full agreement with the previous study by Okonogi et al., highlighting the anti-inflammatory potential of the purple and black pigmented rice extracts in LPS-stimulated RAW264.7 macrophage cell model using enzyme-linked immunosorbent assay (Okonogi et al., Citation2018). In addition, the great anti-inflammatory activity of the crude extracts of glutinous BR was indicated by a study in Thailand (Ngamdee et al., Citation2016). It is worth noting that, in addition to the assessment of NO inhibitory activity discussed in this study, other bioactivities can also be employed to evaluate the anti-inflammatory potential of plant extracts or test substances. These include antinociceptive activity and the Zymosan-induced paw inflammation model (Mollica et al., Citation2014; Valle et al., Citation2020).
4.2. 1H NMR profiles of different pigmented rice extracts
The 1H NMR spectra of the pigmented rice extracts obtained from different solvent extractions and rice varieties displayed relative differences in peaks and peak intensities. Rice varieties and polarity solvents are associated with variations in composition, type, and content of metabolites in rice extracts (Jun et al., Citation2012). The metabolite profiles of pigmented rice grains were reported by a previous study in which sucrose was found to be a major sugar in rice samples including RR, BR, and white rice (Frank et al., Citation2012). In addition, the former study stated that BR revealed a higher level of free fatty acids, organic acids, amino acids, and biogenic amine GABA than RR and white rice. This finding agreed with the results of the present study in which sugars were found to be major metabolites found in the pigmented rice samples. Fatty acid derivatives, amino acids, phenolic compounds, and other organic acids were also detected in the current study as metabolites present in the pigmented rice samples.
4.3. Correlation between the metabolites and biological activities of the pigmented rice extracts
The PLS model suggested that chlorogenic acid (6), protocatechuic acid (8), and tyrosine (9) were the metabolites that contributed to the DPPH scavenging and α-glucosidase inhibitory activities of the RR methanol extract. A similar result was previously observed in which the great DPPH scavenging activity was found in the protocatechuic acid-rich PR and RR (Chen et al., Citation2012). The correlation of chlorogenic acid to DPPH scavenging activity was also perceived in sweet potato samples (Park et al., Citation2015).
On the other hand, nicotinic acid, GABA, and citric acid were found to correspond to the NO inhibitory activity of the RR aqueous extract. Nicotinic acid, a member of the vitamin B family, has been reported to have anti-inflammatory activity by inhibiting the production of reactive oxygen species and inflammatory mediators induced by different stimuli in cultured human endothelial cells (Ganji et al., Citation2009). GABA is a non-protein amino acid that is widely distributed in nature and is well known as the major inhibitory neurotransmitter in the mammalian central nervous system. Notably, GABA was reported to exhibit various biological activities such as anti-inflammation, anti-hypertension, anti-diabetes, anti-cancer, and antioxidant (Ngo & Vo, Citation2019). Citric acid has been reported to potentially inhibit brain and liver NO as well as lipid peroxidation in mice intoxicated with malathion (Abdel-Salam et al., Citation2016). In addition, the inflammation and lipid oxidation inhibitory activities of citric acid have been demonstrated in LPS-treated mice (Abdel-Salam et al., Citation2014). Moreover, the separation of the aqueous extracts of RR was due to higher carbohydrate contents (glucose, sucrose, and maltose), according to the loading plot. As carbohydrates are polar compounds, more sugars may be extracted by water that has higher dielectric constant than the other solvents.
5. Conclusions
The solvents and rice varieties influenced the TPC and biological activities of the pigmented rice extracts. The RR water extract exhibited the highest TPC and high NO inhibitory activity, whereas the RR methanol extract showed the highest DPPH scavenging and α-glucosidase inhibitory activities. The PCA results suggested the separation of each extract based on metabolite variation as the effects of extraction solvents on the varieties of rice. The water extracts of RR were separated from those of PR and BR, although no separation of acetone and methanol extracts was observed among three rice varieties. The PLS model described the correlation between metabolites and biological activities of the pigmented rice extracts, suggesting the relationship between the RR methanol extract and DPPH scavenging activity as well as α-glucosidase inhibitory activity. Chlorogenic acid, protocatechuic acid, and tyrosine were found to be the contributed metabolites to the DPPH scavenging and α-glucosidase inhibitory activities of the RR methanol extract. In addition, a strong relationship between the RR water extracts and NO inhibitory activity was implied by the model in which the activity of the extracts was associated with nicotinic acid, GABA, and citric acid. The potential of RR extract as a source of functional ingredients was suggested by NMR-based metabolomics. Efforts are performed in an anticipation to provide recommendations for yielding an optimal extract that has potential antioxidant and NO inhibitory properties.
Supplemental Material
Download MS Word (465 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19476337.2023.2284330.
Additional information
Funding
References
- Abas, F., Lajis, N. H., Israf, D. A., Khozirah, S., & Kalsom, Y. U. (2006). Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables. Food Chemistry, 95(4), 566–573. https://doi.org/10.1016/j.foodchem.2005.01.034
- Abdel-Salam, O. M. E., Youness, E. R., Mohammed, N. A., Morsy, S. M. Y., Omara, E. A., & Sleem, A. A. (2014). Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. Journal of Medicinal Food, 17(5), 588–598. https://doi.org/10.1089/jmf.2013.0065
- Abdel-Salam, O. M. E., Youness, E. R., Mohammed, N. A., Yassen, N. N., Khadrawy, Y. A., El-Toukhy, S. E., & Sleem, A. A. (2016). Novel neuroprotective and hepatoprotective effects of citric acid in acute malathion intoxication. Asian Pacific Journal of Tropical Medicine, 9(12), 1181–1194. https://doi.org/10.1016/j.apjtm.2016.11.005
- Abdul-Hamid, N. A., Abas, F., Ismail, I. S., Shaari, K., & Lajis, N. H. (2015). Influence of different drying treatments and extraction solvents on the metabolite profile and nitric oxide inhibitory activity of Ajwa dates. Journal of Food Science, 80(11), 2603–2611. https://doi.org/10.1111/1750-3841.13084
- Abdul-Hamid, N. A., Mediani, A., Maulidiani, M., Abas, F., Ismail, I. S., Shaari, K., & Lajis, N. H. (2016). Discrimination and nitric oxide inhibitory activity correlation of Ajwa dates from different grades and origin. Molecules, 21(1423), 1–14. https://doi.org/10.3390/molecules21111423
- Chen, M. H., Choi, S. H., Kozukue, N., Kim, H. J., & Friedman, M. (2012). Growth-inhibitory effects of pigmented rice bran extracts and three red bran fractions against human cancer cells: Relationships with composition and antioxidative activities. Journal of Agricultural and Food Chemistry, 60(36), 9151–9161. https://doi.org/10.1021/jf3025453
- Das, A. B., Goud, V. V., & Das, C. (2017). Extraction of phenolic compounds and anthocyanin from black and purple rice bran (Oryza sativa L.) using ultrasound: A comparative analysis and phytochemical profiling. Industrial Crops and Products, 95, 332–341. https://doi.org/10.1016/j.indcrop.2016.10.041
- Deng, G. F., Xu, X. R., Zhang, Y., Li, D., Gan, R. Y., & Li, H. B. (2013). Phenolic compounds and bioactivities of pigmented rice. Critical Reviews in Food Science and Nutrition, 53(3), 296–306. https://doi.org/10.1080/10408398.2010.529624
- Frank, T., Reichardt, B., Shu, Q., & Engel, K. H. (2012). Metabolite profiling of colored rice (Oryza sativa L.) grains. Journal of Cereal Science, 55(2), 112–119. https://doi.org/10.1016/j.jcs.2011.09.009
- Ganji, S. H., Qin, S., Zhang, L., Kamanna, V. S., & Kashyap, M. L. (2009). Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis, 202(1), 68–75. https://doi.org/10.1016/j.atherosclerosis.2008.04.044
- Gunaratne, A., Wu, K., Li, D., Bentota, A., Corke, H., & Cai, Y. Z. (2013). Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chemistry, 138(2–3), 1153–1161. https://doi.org/10.1016/j.foodchem.2012.11.129
- Jun, H. I., Song, G. S., Yang, E. I., Youn, Y., & Kim, Y. S. (2012). Antioxidant activities and phenolic compounds of pigmented rice bran extracts. Journal of Food Science, 77(7), 1–6. https://doi.org/10.1111/j.1750-3841.2012.02763.x
- Kowsalya, P., Sharanyakanth, P. S., & Mahendran, R. (2022). Traditional rice varieties: A comprehensive review on its nutritional, medicinal, therapeutic and health benefit potential. Journal of Food Composition and Analysis, 114, 1–11. https://doi.org/10.1016/j.jfca.2022.104742
- Kusano, M., Yang, Z., Okazaki, Y., Nakabayashi, R., Fukushima, A., & Saito, K. (2015). Using metabolomic approaches to explore chemical diversity in rice. Molecular Plant, 8(1), 58–67. https://doi.org/10.1016/j.molp.2014.11.010
- Lee, S. Y., Abas, F., Khatib, A., Ismail, I. S., Shaari, K., & Zawawi, N. (2016). Metabolite profiling of Neptunia oleracea and correlation with antioxidant and α-glucosidase inhibitory activities using 1H NMR-based metabolomics. Phytochemistry Letters, 16, 23–33. https://doi.org/10.1016/j.phytol.2016.02.014
- Lee, S. Y., Mediani, A., Ismail, I. S., Maulidiani, & Abas, F. (2019). Antioxidants and α-glucosidase inhibitors from Neptunia oleracea fractions using 1H NMR-based metabolomics approach and UHPLC-MS/MS analysis. BMC Complementary and Alternative Medicine, 19(1), 1–15. https://doi.org/10.1186/s12906-018-2413-4
- Lee, S. Y., Mediani, A., Nur Ashikin, A. H., Azliana, A. B. S., & Abas, F. (2014). Antioxidant and α-glucosidase inhibitory activities of the leaf and stem of selected traditional medicinal plants. International Food Research Journal, 21(1), 165–172.
- Maulidiani, M., Abas, F., Shaari, M., Shitan, K., & Lajis, N. H. (2013). Comparison of partial least squares and artificial neural network for the prediction of antioxidant activity in extract of Pegaga (Centella) varieties from 1H nuclear magnetic resonance spectroscopy. Food Research International, 54(1), 852–860. https://doi.org/10.1016/j.foodres.2013.08.029
- Mediani, A., Abas, F., Maulidiani, M., Choi, K., Shaari, Y. H., & Lajis, N. H. (2012). 1H-NMR-based metabolomics approach to understanding the drying effects on the phytochemicals in cosmos caudatus. Food Research International, 49(2), 763–770. https://doi.org/10.1016/j.foodres.2012.09.022
- Min, B., McClung, A., & Chen, M. H. (2014). Effects of hydrothermal processes on antioxidants in brown, purple and red bran whole grain rice (Oryza sativa L.). Food Chemistry, 159, 106–115. https://doi.org/10.1016/j.foodchem.2014.02.164
- Mollica, A., Carotenuto, A., Novellino, E., Limatola, A., Costante, R., Pinnen, F., Stefanucci, A., Pieretti, S., Borsodi, A., Samavati, R., Zador, F., Benyhe, S., Davis, P., Porreca, F., & Hruby, V. J. (2014). Novel cyclic biphalin analogue with improved antinociceptive properties. ACS Medicinal Chemistry Letters, 5(9), 1032–1036. https://doi.org/10.1021/ml500241n
- Muntana, N., & Prasong, S. (2010). Study on total phenolic contents and their antioxidant activities of Thai white, red and black rice bran extracts. Pakistan Journal of Biological Sciences, 13(4), 170–174. https://doi.org/10.3923/pjbs.2010.170.174
- Ngamdee, P., Jiamyangyuen, S., & Parkin, K. L. (2016). Phase II enzyme induction and anti-inflammatory effects of crude extracts and secondary fractions obtained from bran from five black glutinous rice cultivars. International Journal of Food Science and Technology, 51(2), 333–341. https://doi.org/10.1111/ijfs.12967
- Ngo, D. H., & Vo, T. S. (2019). An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules, 24(15), 1–23. https://doi.org/10.3390/molecules24152678
- Oki, T., Masuda, M., Kobayashi, M., Nishiba, Y., Furuta, S., Suda, I., & Sato, T. (2002). Polymeric procyanidins as radical-scavenging components in red-hulled rice. Journal of Agricultural and Food Chemistry, 50(26), 7524–7529. https://doi.org/10.1021/jf025841z
- Okonogi, S., Kaewpinta, A., Junmahasathien, T., & Yotsawimonwat, S. (2018). Effect of rice variety and modification on antioxidant and anti-inflammatory activities. Drug Discoveries & Therapeutics, 12(4), 206–213. https://doi.org/10.5582/ddt.2018.01041
- Paiva, F. F., Vanier, N. L., Berrios, J. D. J., Pan, J., Villanova, F. D. A., Takeoka, G., Elias, M. C., & Vanier, N. L. (2014). Physicochemical and nutritional properties of pigmented rice subjected to different degrees of milling. Journal of Food Composition and Analysis, 35(1), 10–17. https://doi.org/10.1016/j.jfca.2014.05.003
- Park, J. S., Woo, J. W., Choi, G. H., Choi, D. S., & Jung, M. Y. (2015). Chlorogenic acid profiles and antioxidant potentials of 17 sweet potato varieties cultivated in Korea: Impact of extraction condition and classification by hierarchical clustering analysis. Journal of Food Chemistry and Nanotechnology, 1(1), 3–12. https://doi.org/10.17756/jfcn.2015-001
- Pingret, D., Fabiano-Tixier, A. S., Bourvellec, C. L., Renard, C. M. G. C., & Chemat, F. (2012). Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. Journal of Food Engineering, 111(1), 73–81. https://doi.org/10.1016/j.jfoodeng.2012.01.026
- Pramai, P., Abdul-Hamid, N. A., Mediani, A., Maulidiani, M., Abas, F. & Jiamyangyuen, S. (2017). Metabolite profiling, antioxidant, and α-glucosidase inhibitory activities of germinated rice: Nuclear-magnetic-resonance-based metabolomics study. Journal of Food & Drug Analysis, 26, 47–57. https://doi.org/10.1016/j.jfda.2016.11.023
- Priya, T. S. R., Nelson, A. R. L. E., Ravichandran, K., & Antony, U. (2019). Nutritional and functional properties of coloured rice varieties of South India: A review. Journal of Ethnic Foods, 6(1), 1–11. https://doi.org/10.1186/s42779-019-0017-3
- Sati, R., & Singh, S. (2019). Pigmented rice: A potential ingredient for extruded products review paper. Journal of Pharmacognosy & Phytochemistry, 8(3), 700–702.
- Sompong, R., Siebenhandl-Ehn, S., Linsberger-Martin, G., & Berghofer, E. (2011). Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chemistry, 124(1), 132–140. https://doi.org/10.1016/j.foodchem.2010.05.115
- Tripathi, P., Tripathi, P., Kashyap, L., & Singh, V. (2007). The role of nitric oxide in inflammatory reactions. FEMS Immunology and Medical Microbiology, 51(3), 443–452. https://doi.org/10.1111/j.1574-695X.2007.00329.x
- Tsai, P. J., Tsai, T. H., Yu, C. H., & Ho, S. C. (2007). Comparison of NO-scavenging and NO-suppressing activities of different herbal teas with those of green tea. Food Chemistry, 103(1), 181–187. https://doi.org/10.1016/j.foodchem.2006.08.013
- Valle, A. D., Dimmito, M. P., Zengin, G., Pieretti, S., Mollica, A., Locatelli, M., Cichelli, A., Novellino, E., Ak, G., Yerlikaya, S., Baloglu, M. C., Altunoglu, Y. C., & Stefanucci, A. (2020). Exploring the nutraceutical potential of dried pepper Capsicum annuum L. on market from Altino in Abruzzo region. Antioxidants, 9(5), 1–19. https://doi.org/10.3390/antiox9050400
- Wei, L., Audrey, K., Foong, S., Maulidiani, K., May, M., Ang, Y., Yin, W., Ming, C., Lee, T., Ping, C., Khozirah, T., Chau, S., & Tham, L. (2019). 1 H-NMR metabolomics for evaluating the protective effect of Clinacanthus nutans (Burm. f) Lindau water extract against nitric oxide production in LPS-IFN- γ activated RAW 264.7 macrophages. Phytochemical Analysis, 30(1), 46–61. https://doi.org/10.1002/pca.2789
- Yao, Y., Sang, W., Zhou, M., & Ren, G. (2010). Antioxidant and α-glucosidase inhibitory activity of colored grains in China. Journal of Agricultural and Food Chemistry, 58(2), 770–774. https://doi.org/10.1021/jf903234c