ABSTRACT
Despite their serious disadvantages, which include higher upfront costs, the possibility of malfunctions due to corrosiveness, and a negative impact on the organoleptic properties of the food and possibly its nutritional importance, conventional antibacterial techniques such as pasteurization, pressure preparation, and radioactive substances are also valid as synthetic antiseptics, in fact, reduce bacterial growth in food to varying degrees. Most importantly, these cleaning techniques remove all contaminants, including various (often helpful) microorganisms found naturally in food. One potential solution to some of these issues is bacteriophage bio-control, a common and inexpensive method that uses lytic bacteriophages taken from the environment to selectively target harmful bacteria and eliminate significantly reduce their stages of feeding. It has been claimed that using bacteriophages on food is a novel way to prevent the growth of germs in vegetables. This review highlights the role of bacteriophages in food safety and their advantages in detail.
Introduction
Dieticians and health specialists globally promote the intake of fresh fruits and vegetables owing to their rich content of essential vitamins, minerals, and nutrients (Fan et al., Citation2009). Though, fresh produce remains a significant source of foodborne illnesses, with over 400 outbreaks related to produce reported since 1990, particularly associated with tomatoes, leafy greens, and sprouted seeds (Murray et al., Citation2017). Factors like open-field cultivation and handling add to the contamination of fruits and vegetables by microorganisms, leading to spoilage and food waste along the production process (Rawat, Citation2015). Human welfare and health are regularly impacted by foodborne infections, which can also occasionally result in hospitalization or even death. Between 2018 and 2019, outbreaks of Salmonella illness were detected in several US states, with the infection being linked to raw turkey products (Rashida et al., Citation2019). Due to the rise in the consumption of raw and undercooked chicken in recent years, particularly in China, the infection rate with Campylobacter has increased quickly (Zeng et al., Citation2016). A Hangzhou high school reported a severe case of Campylobacter jejuni infection in 2018, resulting in 84 pupils experiencing symptoms of foodborne disease, including vomiting and diarrhea (Yu et al., Citation2020).
Since the incidence of antibiotic generally drug-resistant microorganisms is increasing and the currently utilized procedures for the removal of foodborne pathogens are imprecise, new strategies against foodborne pathogens are badly needed (Moye et al., Citation2018). Since the beginning of the 20th century, bacteriophages have been known to be capable of eradicating specific bacteria. The initial focus of phage use was on medical cures, but it swiftly spread to several biotechnological and industrial fields. This relates to the food safety sector, where consumers are expressly put in danger by microbes (Sillankorva et al., Citation2012). Bacteriophages, which are bacterial viruses capable of infecting and killing specific bacterial hosts, have emerged as potential bio-regulator agents for enhancing food safety and reducing waste (O’Sullivan et al., Citation2019).
Bacteriophages have numerous advantages that make them valuable weapons in the fight against foodborne diseases. Because they are so specific, the product or the natural microbiota of the consumer organism (such as fermentation processes) are unaffected by the use of bacteria in the production process. Often, a single dose is adequate to achieve the desired therapeutic effect of the bacteriophage preparation. Moreover, phages can be readily isolated from their surroundings using simple, inexpensive techniques. Interestingly, and this is significant for customers, the phage addition had no effect on the product’s organoleptic, rheological, or nutritional qualities. By using bacteriophages, foodborne illnesses can be minimized, and food spoilage can be prevented, offering a promising solution to improving food safety and sustainability. Various studies have explored the application of bacteriophages as antibacterial agents to enhance microbiological food safety and reduce pathogenic and spoilage microorganisms in food products such as milk, poultry, cheeses, vegetables, and fresh fruits (Greer, Citation2005; Islam et al., Citation2022). This review highlights the prospective application of bacteriophages in regulating microbial contamination in fresh fruits and vegetables, dairy products, and convenience foods, presenting an innovative approach to ensure food safety and minimize food waste.
Foodborne illnesses: the connection to contaminated food
Foodborne illness caused by food contamination is the chief contributor to disease and mortality around the world (Murray et al., Citation2017). Every year, contaminated food results in 600 million foodborne illness cases and 420,000 fatalities worldwide; children under the age of five account for 40% of these deaths (Sarno et al., Citation2021). The widely held of these cases are attributed to specific foodborne pathogens such as Shigella, Salmonella, Campylobacter, Listeria monocytogenes, and Escherichia coli pathotypes, along with other enteric microorganisms (Murray et al., Citation2017; Scallan et al., Citation2010). The claim for fresh produce is a significant factor contributing to these incidences, often related to inadequate thermal storage and microbiologic contamination of equipment (Żaczek et al., Citation2015).
In addressing the emergence of bacterial resistance, bacteriophages, specialized viruses that target bacteria by rupturing their cell walls, are being explored as an alternative to antibiotics. Bacteriophages possess RNA or DNA genomes and can yield endolysin enzymes that split peptidoglycan, leading to cell wall lysis (Woźnica et al., Citation2015). Furthermore, the bacteriophage genome comprises proteins known as amurins, which inhibit cell wall formation, causing cell wall rupture (Woźnica et al., Citation2015).
Bio-control capability of bacteriophages against dietary pathogens
Since the discovery of bacteriophages by Francis Type of circuit and Walter d’Herelle a century earlier (Wittebole et al., Citation2014), researchers have demonstrated their potential in curing microbial enterococcus illnesses like cholera, correctly selected, and giardiasis, as well as a variety of acute or prolonged pathogens in fields such as cardiology, gastroenterology, neonatology, and multiple surgeries. These infectious agents have been used for various agricultural, animal, and human applications, but their application in local food production remains unexplored (Sillankorva et al., Citation2012). Contaminated food episodes associated with fresh produce have emphasized the need for concrete methods to eradicate harmful bacteria from food. However, traditional commercial sanitizers have been shown to have limitations in removing pathogens from the surfaces of fruits and vegetables (Bhardwaj et al., Citation2015).
To find better alternatives for ensuring bacterial exclusion on fresh produce, researchers have explored techniques such as radioactivity, consumable covering, nitrogen oxides, ultraviolet, climate-controlled storage, potassium permanganate, water, and viral proteins (Islam et al., Citation2022; Mahajan et al., Citation2014). Phages, as operative and reasonable options for organic management, do not destroy the flavor of fresh food like conventional cleaning methods do. Investigating viral formulations for the overall bio-control capability of bacteriophages against dietary pathogens linked to bug of fruits and vegetables has been a focus. However, the unexpected outcomes have posed challenges in the application of phages for phytoremediation in the native food sector, which is attributed to inadequate treatment during viral concentration and limited knowledge of bacteriophage ecology (McCallin et al., Citation2013). Addressing these concerns can unlock the potential of phage-based bio-control strategies in ensuring food safety.
Bacteriophage treatment in antimicrobial resistance and infectious diseases
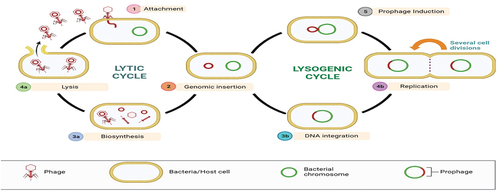
Bacteriophages, outnumbering bacterial cells by a factor of 10 in the environment as well as the intestines of both animal and human species, comprising a vast range of host organisms. Their genome sizes vary from 3.4 kilobases (kb) to around 500 kilobases (kb), containing numerous uncharacterized genes and proteins (Romero-Calle et al., Citation2019). Phages undergo two discrete life cycles: lysogenic and lytic. Lysogenic phages integrate their viral genome into the host’s genetic material, while lytic phages kill infected host cells (Garvey, Citation2020) (). They exhibit improved selectivity and a restricted host range, binding to host cells through various receptors, such as proteins, sugars, and lipopolysaccharides (Batinovic et al., Citation2019).
Phages have a long history of being considered for the management of infectious diseases before the development of antibiotics. Recognized by microbiologist Felix d’Herelle in 1917, phages were primarily explored for their antibacterial activity against dangerous germ cells (Fruciano & Bourne, Citation2006). Phage therapy showed promising results in controlling diseases like bacillary dysentery and cholera outbreaks (Braga et al., Citation2020). However, the discovery of antibiotics in the 1930s and 1940s led to a decline in phage research. Challenges such as inconsistent findings, dosages, repeatability, and limited genetic information hindered further exploration (Garvey, Citation2020) ().
Recent resurgence in phage research comes as an unconventional route to combat the growing threat of antimicrobial resistance (AMR). In Eastern Europe, phage treatment has been effectively used for around 90 years without posing health risks to patients (Tang et al., Citation2019). In countries like Poland, bacteriophage treatment has proven effective against AMR diseases (Selle et al., Citation2020). As current therapeutic methods falter, bacteriophage therapy offers a promising option, particularly in managing infections like Clostridioides difficile infection (CDI) with high fatality rates. While challenges remain, bacteriophage treatment presents a solution to combat AMR and infectious diseases.
Life cycles of bacteriophages
Through certain receptors on the bacterial host cell, bacteriophages identify their host. The bacteriophage injects its genetic material into the host after attaching. Phage genome can either stay as unintegrated extragenomic genetic material or integrate into the host genetic material once it becomes intracytosolic. Whereas the latter life cycle is lytic, the first is lysogenic. Viral phages have a lytic life cycle and seize control of the host’s replication processes in order to multiply into mature virus particles and eventually cause the host cell to lyse. Reverting to lysogeny, temperate phages incorporate into the host genetic material until a mutagenic stimulus induces them into a lytic life cycle. Because they are restricted to the host in which they are incorporated, they are also highly host specific than virulent ones as shown in .
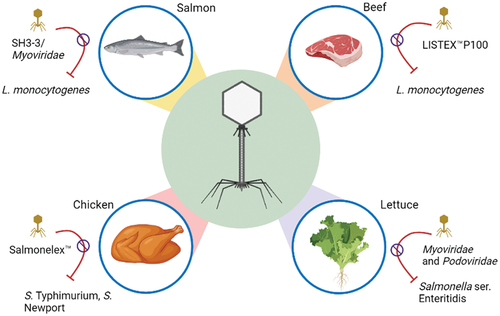
Phage-based biocontrol of food pathogens
Several experimental studies have investigated the use of phages to control pathogens in various food products and leafy green vegetables as described in . This indicates that bacteriophages are a promising regulator of food-borne pathogens. The table below describes the process of phage-regulation and their effects on pathogenic bacteria.
Table 1. Application of bacteriophages in regulating food-borne pathogens.
Benefits and drawbacks of using phages as antimicrobials
Phages, as antimicrobials, have both strengths and limitations as shown in . Their specificity in targeting diverse bacterial strains can be challenging, especially when dealing with illnesses caused by multiple strains. While some trials have shown the safety of oral phage administration, a key concern is ensuring proper translocation of phages through the intestinal epithelium. Studies have indicated that this translocation can be beneficial by directing the immune response to innate microbial antigens and averting the development of certain inflammatory factors (Ranveer et al., Citation2024). However, other research did not observe significant changes in cytokine levels after phage therapy. In spite of limited data on phage treatment, it appears to have fewer side effects than conventional antibiotics and can reduce pathogenic flora in the gut.
Table 2. Benefits and drawbacks of using bacteriophages as antimicrobials.
The regional specificity of phages allows for the selection of phages with the best infectivity contrary to target pathogens, making them potentially valuable in combating antibiotic-resistant bacteria, particularly in hospital settings (Ali et al., Citation2023). Additionally, phages carry enzymes that can break down bacterial biofilms and extracellular polymeric materials, providing an advantage over antibiotics, which are often ineffective against biofilm-forming bacteria (Liu et al., Citation2022). Nevertheless, further investigation is needed to completely understand and harness the potential of phages as antimicrobials.
Conclusion and future perspectives
Despite improvements in safety procedures for food, adulteration of fresh fruits and vegetables stays a significant issue. Pathogenic and deteriorative bacteria can undermine product quality and contribute to food waste. Current research in food microbiology has highlighted the effectiveness of bacteriophages in averting the growth of harmful bacteria on fresh produce. Bacteriophages are advantageous at various points of the food manufacture chain. They offer a promising auxiliary tool to combat foodborne infections, particularly concerning vulnerable populations like children, the elderly, and expectant mothers. Phages are strong, definite, and self-replicating pillagers of bacterial species, making them valuable for disease mitigation, farm-level disinfection, and food preservation. They are increasingly recognized as GRAS-Generally Recognized as Safe for use in food products and are considered organic and legitimate. Phage cocktails, in particular, have shown remarkable activity to counter Multi-Drug Resistant (MDR) species and can be combined with other safe antimicrobials, like bacteriocins, to augment effectiveness and selectivity. However, issues that require further investigation include phage resistance mechanisms, potential transmission of pathogenic genes, phage traceability in the environment, and formulation and stability challenges for therapeutic applications. Overall, Bacteriophages are essential components of ecosystems, playing a crucial character in bacterial evolution. Their use as biocontrol agents during before-harvest, harvest, and after-harvest stages provides several benefits for refining food safety and sustainability, aligning with the Sustainable Development Goals (SDGs).
Consent for publication
All authors are willing for publication of this manuscript.
Consent to participate
All the co-authors are willing to participate in this manuscript.
Acknowledgements
Authors are thankful to Government College University for providing literature collection facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated used and/or analyzed during the current study available from the corresponding author on reasonable request.
Additional information
Funding
References
- Ali, Y., Inusa, I., Sanghvi, G., Mandaliya, V. B., & Bishoyi, A. K. (2023). The current status of phage therapy and its advancement towards establishing standard antimicrobials for combating multi drug-resistant bacterial pathogens. Microbial Pathogenesis, 181, 106199. https://doi.org/10.1016/j.micpath.2023.106199
- Batinovic, S., Wassef, F., Knowler, S. A., Rice, D. T., Stanton, C. R., Rose, J., Tucci, J., Nittami, T., Vinh, A., Drummond, G. R., Sobey, C. G., Chan, H. T., Seviour, R. J., Petrovski, S., & Franks, A. E. (2019). Bacteriophages in natural and artificial environments. Pathogens, 8(3), 100. https://doi.org/10.3390/pathogens8030100
- Bhardwaj, N., Bhardwaj, S. K., Deep, A., Dahiya, S., & Kapoor, S. (2015). Lytic bacteriophages as biocontrol agents of foodborne pathogens. Asian Journal of Animal and Veterinary Advances, 10(11), 708–7. https://doi.org/10.3923/ajava.2015.708.723
- Bigwood, T., Hudson, J. A., Billington, C., Carey-Smith, G. V., & Heinemann, J. A. (2008). Phage inactivation of foodborne pathogens on cooked and raw meat. Food Microbiology, 25(2), 400–406. https://doi.org/10.1016/j.fm.2007.11.003
- Braga, L. P., Spor, A., Kot, W., Breuil, M.-C., Hansen, L. H., Setubal, J. C., & Philippot, L. (2020). Impact of phages on soil bacterial communities and nitrogen availability under different assembly scenarios. Microbiome, 8(1), 1–14. https://doi.org/10.1186/s40168-020-00822-z
- Carter, C. D., Parks, A., Abuladze, T., Li, M., Woolston, J., Magnone, J., Senecal, A., Kropinski, A. M., & Sulakvelidze, A. (2012). Bacteriophage cocktail significantly reduces Escherichia coli O157: H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage, 2(3), 178–185. https://doi.org/10.4161/bact.22825
- Chibeu, A., Agius, L., Gao, A., Sabour, P. M., Kropinski, A. M., & Balamurugan, S. (2013). Efficacy of bacteriophage LISTEX™ P100 combined with chemical antimicrobials in reducing listeria monocytogenes in cooked turkey and roast beef. International Journal of Food Microbiology, 167(2), 208–214. https://doi.org/10.1016/j.ijfoodmicro.2013.08.018
- Culot, A., Grosset, N., & Gautier, M. (2019). Overcoming the challenges of phage therapy for industrial aquaculture: A review. Aquaculture, 513, 734423. https://doi.org/10.1016/j.aquaculture.2019.734423
- Ding, Y., Nan, Y., Qiu, Y., Niu, D., Stanford, K., Holley, R., Narváez‐Bravo, C., & McAllister, T. (2023). Use of a phage cocktail to reduce the numbers of seven Escherichia coli strains belonging to different STEC serogroups applied to fresh produce and seeds. Journal of Food Safety, 43(4), e13044. https://doi.org/10.1111/jfs.13044
- Duc, H. M., Son, H. M., Yi, H. P. S., Sato, J., Ngan, P. H., Masuda, Y., Honjoh, K.-I., & Miyamoto, T. (2020). Isolation, characterization and application of a polyvalent phage capable of controlling Salmonella and Escherichia coli O157: H7 in different food matrices. Food Research International, 131, 108977. https://doi.org/10.1016/j.foodres.2020.108977
- Fan, X., Niemira, B. A., Doona, C. J., Feeherry, F. E., & Gravani, R. B. (2009). Microbial safety of fresh produce (Vol. 41). John Wiley & Sons.
- Firlieyanti, A. S., Connerton, P. L., & Connerton, I. F. (2016). Campylobacters and their bacteriophages from chicken liver: The prospect for phage biocontrol. International Journal of Food Microbiology, 237, 121–127. https://doi.org/10.1016/j.ijfoodmicro.2016.08.026
- Fruciano, D. E., & Bourne, S. (2006). Phage as an antimicrobial agent: D’Herelle’s heretical theories and their role in the decline of phage prophylaxis in the west. The Canadian Journal of Infectious Diseases & Medical Microbiology, 18(1), 19–26. https://doi.org/10.1155/2007/976850
- Garvey, M. (2020). Bacteriophages and the one health approach to combat multidrug resistance: Is this the way? Antibiotics, 9(7), 414. https://doi.org/10.3390/antibiotics9070414
- Garvey, M. (2022). Bacteriophages and food production: Biocontrol and bio-preservation options for food safety. Antibiotics, 11(10), 1324. https://doi.org/10.3390/antibiotics11101324
- Gencay, Y. E., Ayaz, N. D., Copuroglu, G., & Erol, I. (2016). Biocontrol of Shiga toxigenic Escherichia coli O157: H7 in Turkish raw meatball by bacteriophage. Journal of Food Safety, 36(1), 120–131. https://doi.org/10.1111/jfs.12219
- Giau, V. V., Lee, H., An, S. S. A., & Hulme, J. (2019). Recent advances in the treatment of C. Difficile using biotherapeutic agents. 12, 1597–1615. https://doi.org/10.2147/IDR.S207572
- Greer, G. G. (2005). Bacteriophage control of foodborne bacteria. Journal of Food Protection, 68(5), 1102–1111. https://doi.org/10.4315/0362-028X-68.5.1102
- Imran, A., Shehzadi, U., Islam, F., Afzaal, M., Ali, R., Ali, Y. A., Chauhan, A., Biswas, S., Khurshid, S., Usman, I., Hussain, G., Zahra, S. M., Shah, M. A., & Rasool, A. (2023). Bacteriophages and food safety: An updated overview. Food Science & Nutrition, 11(7), 3621–3630. https://doi.org/10.1002/fsn3.3360
- Ishaq, A., Ebner, P. D., Syed, Q. A., & Ur Rahman, H. U. (2020). Employing list-shield bacteriophage as a bio-control intervention for Listeria monocytogenes from raw beef surface and maintain meat quality during refrigeration storage. LWT- Food Science Technology, 132, 109784. https://doi.org/10.1016/j.lwt.2020.109784
- Islam, F., Saeed, F., Afzaal, M., Ahmad, A., Hussain, M., Khalid, M. A., Saewan, S. A., & Khashroum, A. O. (2022). Applications of green technologies-based approaches for food safety enhancement: A comprehensive review. Food Science & Nutrition, 10(9), 2855–2867. https://doi.org/10.1002/fsn3.2915
- Lawpidet, P., Tengjaroenkul, B., Saksangawong, C., & Sukon, P. (2021). Global prevalence of vancomycin-resistant enterococci in food of animal origin: A meta-analysis. Foodborne Pathogens and Disease, 18(6), 405–412. https://doi.org/10.1089/fpd.2020.2892
- Liu, S., Lu, H., Zhang, S., Shi, Y., & Chen, Q. (2022). Phages against pathogenic bacterial biofilms and biofilm-based infections: A review. Pharmaceutics, 14(2), 427. https://doi.org/10.3390/pharmaceutics14020427
- Lu, Y. T., Ma, Y., Wong, C. W., & Wang, S. (2022). Characterization and application of bacteriophages for the biocontrol of Shiga-toxin producing Escherichia coli in romaine lettuce. Food Control, 140, 109109. https://doi.org/10.1016/j.foodcont.2022.109109
- Mahajan, P. V., Caleb, O. J., Singh, Z., Watkins, C. B., & Geyer, M. (2014). Postharvest treatments of fresh produce. Philosophical Transactions Series A, Mathematical, Physical, and Engineering Sciences, 372(2017), 20130309. https://doi.org/10.1098/rsta.2013.0309
- McCallin, S., Sarker, S. A., Barretto, C., Sultana, S., Berger, B., Huq, S., Krause, L., Bibiloni, R., Schmitt, B., Reuteler, G., & Reuteler, G. (2013). Safety analysis of a Russian phage cocktail: From MetaGenomic analysis to oral application in healthy human subjects. Virology, 443(2), 187–196. https://doi.org/10.1016/j.virol.2013.05.022
- Moye, Z. D., Woolston, J., & Sulakvelidze, A. (2018). Bacteriophage applications for food production and processing. Viruses, 10(4), 205. https://doi.org/10.3390/v10040205
- Murray, K., Wu, F., Shi, J., Jun Xue, S., & Warriner, K. (2017). Challenges in the microbiological food safety of fresh produce: Limitations of post-harvest washing and the need for alternative interventions. Food Quality and Safety, 1(4), 289–301. https://doi.org/10.1093/fqsafe/fyx027
- Orquera, S., Gölz, G., Hertwig, S., Hammerl, J., Sparborth, D., Joldic, A., & Alter, T. (2012). Control of Campylobacter spp. And Yersinia enterocolitica by virulent bacteriophages. Journal of Molecular and Genetic Medicine: An International Journal of Biomedical Research, 6(1), 273. https://doi.org/10.4172/1747-0862.1000049
- O’Sullivan, L., Bolton, D., McAuliffe, O., & Coffey, A. (2019). Bacteriophages in food applications: From foe to friend. Annual Review of Food Science and Technology, 10(1), 151–172. https://doi.org/10.1146/annurev-food-032818-121747
- Ranveer, S. A., Dasriya, V., Ahmad, M. F., Dhillon, H. S., Samtiya, M., Shama, E., Anand, T., Dhewa, T., Chaudhary, V., Chaudhary, P., Behare, P., Ram, C., Puniya, D. V., Khedkar, G. D., Raposo, A., Han, H., & Puniya, A. K. (2024). Positive and negative aspects of bacteriophages and their immense role in the food chain. Npj Science of Food, 8(1), 1–13. https://doi.org/10.1038/s41538-023-00245-8
- Rashida, H., Sean, B., Douglas, N., Carlota, M., Alida, S., Jessica, L., Rotstein, D., Schlater, L., Freiman, J., Douris, A., Simmons, M., Donovan, D., Henderson, J., Tewell, M., Snyder, K., Oni, O., Von Stein, D., Dassie, K. … Gieraltowski, L. (2019). Multistate outbreak of salmonella infections linked to raw turkey products — United States, 2017–2019. MMWR Morbidity & Mortality Weekly, 68(46), 1045–1049. https://doi.org/10.15585/mmwr.mm6846a1
- Rawat, S. (2015). Food spoilage: Microorganisms and their prevention. Asian Journal of Plant Science and Research, 5(4), 47–56.
- Romero-Calle, D., Guimarães Benevides, R., Góes-Neto, A., & Billington, C. J. A. (2019). Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics (Basel), 8(3), 138. https://doi.org/10.3390/antibiotics8030138
- Sadekuzzaman, M., Mizan, M. F. R., Yang, S., Kim, H. S., & Ha, S. D. (2018). Application of bacteriophages for the inactivation of Salmonella spp. In biofilms. Food Science and Technology International, 24(5), 424–433. https://doi.org/10.1177/1082013218763424
- Salmond, G. P., & Fineran, P. C. (2015). A century of the phage: Past, present and future. Nature Reviews Microbiology, 13(12), 777–786. https://doi.org/10.1038/nrmicro3564
- Sarno, E., Pezzutto, D., Rossi, M., Liebana, E., & Rizzi, V. (2021). A review of significant European foodborne outbreaks in the last decade. Journal of Food Protection, 84(12), 2059–2070. https://doi.org/10.4315/JFP-21-096
- Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, A., Roy, S. L., Jones, J. L., & Griffin, P. M. (2010). Foodborne illness acquired in the United States—major pathogens. Emerging Infectious Diseases, 17(1), 7–15. https://doi.org/10.3201/eid1701.P11101
- Selle, K., Fletcher, J. R., Tuson, H., Schmitt, D. S., McMillan, L., Vridhambal, G. S., Rivera, A. J., Montgomery, S. A., Fortier, C., Barrangou, R., Theriot, C. M., Ousterout, D. G., & Ballard, J. D. (2020). In vivo targeting of clostridioides difficile using phage-delivered CRISPR-Cas3 antimicrobials. MBio, 11(2). https://doi.org/10.1128/mBio.00019-20
- Shebs-Maurine, E. L., Giotto, F. M., Laidler, S. T., & de Mello, A. S. (2021). Effects of bacteriophages and peroxyacetic acid applications on beef contaminated with Salmonella during different grinding stages. Meat Science, 173, 108407. https://doi.org/10.1016/j.meatsci.2020.108407
- Sillankorva, S. M., Oliveira, H., & Azeredo, J. (2012). Bacteriophages and their role in food safety. International Journal of Microbiology, 2012, 1–13. https://doi.org/10.1155/2012/863945
- Sisakhtpour, B., Mirzaei, A., Karbasizadeh, V., Hosseini, N., Shabani, M., & Moghim, S. (2022). The characteristic and potential therapeutic effect of isolated multidrug-resistant Acinetobacter baumannii lytic phage. Annals of Clinical Microbiology and Antimicrobials, 21(1), 1–11. https://doi.org/10.1186/s12941-022-00492-9
- Sukumaran, A. T., Nannapaneni, R., Kiess, A., & Sharma, C. S. (2015). Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. International Journal of Food Microbiology, 207, 8–15. https://doi.org/10.1016/j.ijfoodmicro.2015.04.025
- Tang, S., Biswas, S. K., Tan, W. S., Saha, A. K., & Leo, F. (2019). Efficacy and potential of phage therapy against multidrug resistant shigella spp. PeerJ, 7, 7. https://doi.org/10.7717/peerj.6225
- Thanki, A. M., Hooton, S., Gigante, A. M., Atterbury, R. J., & Clokie, M. R. (2021). Potential roles for bacteriophages in reducing Salmonella from poultry and swine. Salmonella spp-a global challenge, IntechOpen.
- Vikram, A., Woolston, J., & Sulakvelidze, A. (2021). Phage biocontrol applications in food production and processing. Current Issues in Molecular Biology, 40(1), 267–302. https://doi.org/10.21775/cimb.040.267
- Villa, T. G., Feijoo-Siota, L., Rama, J. R., Sánchez-Pérez, A., & Viñas, M. (2019). Horizontal gene transfer between bacteriophages and bacteria: Antibiotic resistances and toxin production. Horizontal Gene Transfer: Breaking Borders Between Living Kingdoms, 97–142 https://link.springer.com/chapter/10.1007/978-3-030-21862-1_3.
- Wittebole, X., De Roock, S., & Opal, S. M. (2014). A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence, 5(1), 226–235. https://doi.org/10.4161/viru.25991
- Witte, S., Huijboom, L., Klamert, S., van de Straat, L., Hagens, S., Fieseler, L., de Vegt, B. T., & van Mierlo, J. T. (2022). Application of bacteriophages EP75 and EP335 efficiently reduces viable cell counts of Escherichia coli O157 on beef and vegetables. Food Microbiology, 104, 103978. https://doi.org/10.1016/j.fm.2022.103978
- Woźnica, W. M., Bigos, J., & Łobocka, M. B. (2015). Liza komórek bakteryjnych w procesie uwalniania bakteriofagów–kanoniczne i nowo poznane mechanizmy. Postępy Higieny i Medycyny Doświadczalnej, 69, 114–126.
- Yang, S., Sadekuzzaman, M., & Ha, S. D. (2017). Reduction of Listeria monocytogenes on chicken breasts by combined treatment with UV-C light and bacteriophage ListShield. Lwt, 86, 193–200. https://doi.org/10.1016/j.lwt.2017.07.060
- Yeh, Y., Purushothaman, P., Gupta, N., Ragnone, M., Verma, S. C., & De Mello, A. S. (2017). Bacteriophage application on red meats and poultry: Effects on Salmonella population in final ground products. Meat Science, 127, 30–34. https://doi.org/10.1016/j.meatsci.2017.01.001
- Yu, H., Elbediwi, M., Zhou, X., Shuai, H., Lou, X., Wang, H., Li, Y., & Yue, M. (2020). Epidemiological and genomic characterization of campylobacter jejuni isolates from a foodborne outbreak at Hangzhou, China. International Journal of Molecular Sciences, 21(8), 3001. https://doi.org/10.3390/ijms21083001
- Żaczek, M., Weber-Dąbrowska, B., & Górski, A. (2015). Phages in the global fruit and vegetable industry. Journal of Applied Microbiology, 118(3), 537–556. https://doi.org/10.1111/jam.12700
- Zampara, A., Sørensen, M. C. H., Elsser-Gravesen, A., & Brøndsted, L. (2017). Significance of phage-host interactions for biocontrol of campylobacter jejuni in food. Food Control, 73, 1169–1175. https://doi.org/10.1016/j.foodcont.2016.10.033
- Zeng, D., Zhang, X., Xue, F., Wang, Y., Jiang, L., & Jiang, Y. (2016). Phenotypic characters and molecular epidemiology of Campylobacter jejuni in East China. Journal of Food Science, 81(1), M106–M113. https://doi.org/10.1111/1750-3841.13146
- Zhang, X., Niu, Y. D., Nan, Y., Stanford, K., Holley, R., McAllister, T., & Narváez-Bravo, C. (2019). SalmoFresh™ effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. International Journal of Food Microbiology, 305, 108250. https://doi.org/10.1016/j.ijfoodmicro.2019.108250
- Zhou, C., Zhu, M., Wang, Y., Yang, Z., Ye, M., Wu, L., Bao, H., Pang, M., Zhou, Y., Wang, R., Sun, L., Wang, H., Zheng, C., & Zhang, H. (2020). Broad host range phage vB-LmoM-SH3-3 reduces the risk of Listeria contamination in two types of ready-to-eat food. Food Control, 108, 106830. https://doi.org/10.1016/j.foodcont.2019.106830