ABSTRACT
Immunoglobulin G3 (IgG3) is the predominant IgG subclass elicited in response to polysaccharide antigens in mice. This specific subclass has been shown to crosslink its fragment crystallizable (Fc) regions following binding to multivalent polysaccharides. Crosslinking leads to increased affinity through avidity, which theoretically should lead to more effective protection against bacteria and yeast displaying capsular polysaccharides on their surface. To investigate this further we have analyzed the binding characteristics of 2 IgG monoclonal antibody (mAb) subclass families that bind to the capsular polysaccharide (CPS) of Burkholderia pseudomallei. The first subclass family originated from an IgG3 hybridoma cell line (3C5); the second family was generated from an IgG1 cell line (2A5). When the Fc region of the 3C5 IgG3 is removed by proteolytic cleavage, the resulting F(ab')2 fragments exhibit decreased affinity compared to the full-length mAb. Similarly, when the parent IgG3 mAb is subclass-switched to IgG1, IgG2b, and IgG2a, all of these subclasses exhibit decreased affinity. This decrease in affinity is not seen when the 2A5 IgG1 mAb is switched to an IgG2b or IgG2a, strongly suggesting the drop in affinity is related to the IgG3 Fc region.
Introduction
Burkholderia pseudomallei is a soil-dwelling bacillus that causes melioidosis, a severe disease common to Southeast Asia and northern Australia. Melioidosis cases in northeast Thailand are particularly severe; from 1997–2007 the mortality rate was nearly 43%.Citation1,2 In addition, Limmathurotsakul and colleagues estimated there to be 165,000 melioidosis cases globally per year resulting in 89,000 deaths.Citation3 The high mortality rate is in part due to the difficulty in diagnosing melioidosis and the inherent resistance of B. pseudomallei to commonly prescribed antibiotics.Citation3
B. pseudomallei produces a number of virulence factors that enhance pathogenesis, chief among them being the capsular polysaccharide (CPS).Citation2,4 Polysaccharide capsules are found on many pathogenic bacteria and fungi, and contribute to virulence by inhibiting complement activation and preventing phagocytosis.Citation4-8 They are high molecular weight antigens with repeating epitopes that are displayed on bacterial and fungal cell surfaces.Citation9-12 The B. pseudomallei CPS is comprised of an unbranched homopolymer of 1,3-linked 2-O-acetyl-6-deoxy-β-D-mannoheptopyranose residues.Citation13 CPS has been shown to inhibit phagocytosis by reducing the amount of complement factor C3b that is deposited on the bacterial cell surface.Citation4 Mutation of specific genes comprising the B. pseudomallei CPS operon results in the production of mutant strains attenuated for pathogenesis in animal models.Citation14-16
Anti-capsular antibodies are an important mechanism for host defense, thus capsules are appealing vaccine candidates; however, polysaccharide antigens do not illicit a robust humoral immune response by themselves. Normally, humoral immunity is induced in a T-cell dependent manner,Citation17 however, polysaccharides utilize a T-cell independent pathway and stimulate B-cells by cross-linking multiple cell surface antigen receptors. T-cell independent responses produce a short-lived and weak humoral immune response.Citation18 To circumvent this weak response polysaccharides can be conjugated to immunogenic proteins or toxoids.Citation19-21 For example, the Haemophilus influenzae capsule elicits a much stronger immune response when it is conjugated to tetanus toxoid.Citation19
The IgG subclass produced in response to T-cell independent polysaccharide antigens in mice is restricted to IgG3.Citation22-24 This begs the question of whether this subclass restriction is helpful when the immune system encounters an encapsulated pathogen. To address this question we produced subclass switch families of polysaccharide-specific mouse monoclonal antibodies and analyzed their immunochemical interactions. These subclass families possess identical variable regions (Fv), but different heavy chain constant regions. Subclass switching of hybridoma clones occurs infrequently in vitro,Citation25,26 although different clones secreting different subclasses can be isolated and expanded, allowing for efficient study of subclass-switch mAb families.
Two subclass-switch mAb families that bind to B. pseudomallei CPS were isolated and used in this study. The first family was derived from an IgG3 hybridoma cell line (3C5) that was isolated following immunization with heat-killed B. pseudomallei.Citation27 The additional IgG 3C5 subclass cell lines were isolated from the parental IgG3 cell line (IgG3→IgG1→IgG2b→IgG2a). Our group previously determined that mAb 3C5 is a relatively high affinity murine IgG3 that provides passive protection in a murine model of pulmonary melioidosis.Citation27 The second family was derived from an IgG1 cell line (2A5) and includes 3 IgG subclasses (IgG1→IgG2b→IgG2a). IgG1 mAb 2A5 has a similar affinity to mAb 3C5, but was generated with a CPS glycoconjugate.
Our study supports previous findings that murine IgG3 Fc regions have the ability to enhance affinity through Fc-Fc interactions when binding to antigens that contain repeating epitopes, such as polysaccharides.Citation28-30 Our experimental design includes binding and affinity studies of 2 subclass switch families of B. pseudomallei capsule-specific IgG mAbs via ELISA, Western blot and surface plasmon resonance (SPR). More specifically, we show that IgG3 Fc regions contribute to antibody binding to B. pseudomallei CPS, resulting in increased affinity.
Materials and methods
Immunization of mice and production of mAbs
Generation of mAb 3C5 IgG3 has been described.Citation27 Briefly, B. pseudomallei strain 1026b was incubated overnight under BSL-3 containment at 37°C in brain heart infusion (BHI) broth. Bacteria were heat-inactivated at 80°C for 2.5 h and confirmed killed by establishing no-growth in BHI broth and back-plating on BHI agar (each for 3 days). BALB/c mice were immunized via intraperitoneal (i.p.) injections with 2 × 108 heat-inactivated bacteria every 2 weeks for 8 weeks total. An ELISA was used to assess antibody titers to B. pseudomallei. A final boost was administered 3 d prior to splenectomy. Hybridoma cells were produced as previously described.Citation31 Western blot analysis was performed to identify hybridoma cell lines that were producing mAbs reactive with purified CPS.
Purified CPS was conjugated to cationized bovine serum albumin (cBSA;Pierce) as previously described.Citation21 In brief, B. pseudomallei LPS O-antigen mutant strain RR2683 was grown at 37°C in Luria Bertani-Lennox (LBL) broth and the CPS extracted via hot aqueous-phenol. CPS and rough LPS were separated on a Sephadex sG-50 column and the purified CPS activated with sodium meta-periodate (NaIO4; Pierce). cBSA was added, followed by sodium cyanoborohydride (NaBH3CN). Aliquots were incubated at room temperature for 4 d. Sodium borohydride (NaBH4) was added and the conjugate was lyophilized for later use.
This conjugate was used to immunize BALB/c mice and produce mAb 2A5 IgG1. Mice were immunized via i.p. injections with 5 µg of CPS-BSA every 2 weeks, for 6 weeks total. Antibody titers were measured by ELISA (see below) and hybridoma cell lines were generated as previously described.Citation31
Isolation of subclass-specific hybridoma cell lines
A modified protocol based on the method of Spira et al.Citation25 was used to isolate subclass-switch mAb families.Citation26 Switching from one subclass to another follows the germline order of heavy chain exons (IgG3, IgG1, IgG2b, IgG2a). The procedure was done in a sequential manner to obtain hybridoma clones that secrete each subclass. Briefly, the hybridoma cell line secreting the parent mAb (e.g. IgG3 mAb 3C5) was plated at 1000 cells/well in a 96-well tissue culture plate. The supernatant from these wells was added to an ELISA plate that contained goat anti-mouse IgG1 in the solid phase. A horseradish peroxidase (HRP)-labeled goat anti-mouse secondary antibody was used to identify the wells that contained IgG1 antibody. Selected high-positive hybridoma wells were then diluted to 100 cells/well and the ELISA was repeated. The dilutions were continued to 10 followed by 1 cell/well at which time multiple IgG1 clones were isolated. An IgG1 cell line was grown in culture and the cells were plated as previously described at 1000 cells/well to isolate an IgG2b secreting cell line. This protocol was repeated for isolation of each subclass. Cell lines were grown in Integra CL 1000 culture flasks (Integra Biosciences) with RPMI media containing 4.5 g glucose, 4 mM L-glutamine, 50 µM 2-mercaptoethanol, 20 mM HEPES, 1 mM sodium pyruvate, and 15% low IgG fetal bovine serum. Antibodies were purified by affinity chromatography over a protein-A column.
F(ab')2 fragments
Full-length mAb 3C5 IgG3 was digested with pepsin from porcine gastric mucosa (Sigma) to obtain F(ab')2 fragments. Briefly, mAbs (5 mg/mL) were incubated with shaking at 37°C for 30 min with pepsin at a final concentration of 0.2 µg/mL in 20 mM NaOAc, pH 4.4. Next, 10% (v/v) of Tris (2M) was used to stop the reaction. F(ab')2 fragments were purified over a Superose 12 (GE Healthcare) molecular sieve column. Eluted fractions were assessed by non-denaturing sodium dodecyl sulfate polyacrylamide (12%) gel electrophoresis (SDS-PAGE) with Coomassie blue staining. Fractions that contained F(ab')2 fragments (showing typical reduction in molecular weight vs. full length antibody) were combined.
Variable region sequencing of mAbs
Heavy and light chain variable regions were sequenced as previously described.Citation26 Briefly, total mRNA was isolated from each hybridoma cell line with an RNeasy Mini Kit (QIAGEN). cDNA was synthesized using a First Strand cDNA Synthesis Kit (Thermo Scientific) and amplified with a Mouse Ig-Primer Set (Novagen). PCR products were TA cloned into the pGem-T vector (Promega), and sequenced. Two independent clones were sequenced for each subclass switch cell line.
Western blot
Isolated subclass switch mAbs were confirmed to bind to purified CPS via Western blot as previously described.Citation32 Briefly, 1 µL of a 10x concentrated B. pseudomallei 1026b lysate, 1.1 × 105 inactivated whole cells of B. mallei China 7 (BEI Resources), 8 × 106 inactivated whole cells of B. thailandensis E264 (BEI Resources), or 0.5 µg purified CPS (see above) were incubated with 1 volume of proteinase K at 3.3 mg/mL for 1 hr at 60°C. Next, samples were separated by SDS PAGE (BioRad) at 160 V for 1 hr, followed by transfer to a nitrocellulose membrane (BioRad) via a TransBlot Turbo (BioRad). Membranes were blocked in Tris-buffered saline plus Tween 20 (TBST, 50 mM Tris-HCl, pH 7.6; 150 mM NaCl, 0.1% Tween 20) supplemented with, 5% milk overnight at 4°C (blocking solution). Membranes were then probed with mAbs at 0.1 µg/mL diluted in blocking solution for 1 hr while rocking at room temperature. Membranes were washed 3 times for 15 min with TBST followed by incubation for 30 min at room temperature with HRP-conjugated goat anti-mouse kappa chain antibody (Southern Biotech) diluted 1:10,000 in blocking solution. Membranes were washed 3 additional times and binding was detected with SuperSignal West Femto Chemiluminescent Substrate (Pierce). Binding was visualized with a Chemidoc imaging system (BioRad). Western blots were also performed to compare the binding activity of each mAb subclass. A B. pseudomallei whole cell lysate (87 μg/gel) was added to a 7.5% SDS PAGE gel that contained one regular sized well for the molecular weight marker and one large well (well sides cut out) for the B. pseudomallei whole cell lysate. Electrophoresis and blotting was performed as above. A miniblotter (with separate lane chambers for probing) was used so different concentrations of each subclass mAb could be used to probe the same blot. The nitrocellulose membranes were probed with either 1:100 or 1:1000 (stock solution of 1 mg/ml) dilutions of each subclass mAb as above. Membranes were washed, probed with a secondary antibody and imaged as above.
Surface plasmon resonance
Binding affinity was determined by surface plasmon resonance (SPR) with a BIAcore X100 (GE Healthcare). Purified CPS (see above) was benzoquinone-activated and conjugated to biotin as previously described,Citation33 and immobilized onto a streptavidin (SA) sensor chip at 30 response units (RU). A second flow cell was unmodified and used for reference subtractions. Affinity was evaluated with mAbs diluted in HBS-EP+ running buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.05% surfactant P20, pH 7.4) at a range of either 0.33–333 nM or 12–3333 nM. Two-fold serial dilutions of mAb were injected over the immobilized CPS at 30 µL/min for 60 s followed by 120 s of passive dissociation. The sensor chip surface was regenerated between each concentration with 10 mM HCl, pH 1.5. Each experiment was performed at least twice, except for 3C5 IgG3 F(ab')2 and 2A5 IgG2a. In both cases, the availability of purified reagent limited us to one assessment at several (6 or 8) different antibody concentrations. Dissociation constants (KD) were calculated using BIAevaluation software (GE Healthcare). The apparent kinetic constants were determined using the Bivalent Analyte model in BIAevaluation software. All evaluations passed the statistical internal quality controls of BIAevaluation software.
ELISA
Subclass-switch mAbs were evaluated via a direct antigen binding ELISA. Polystyrene plates (Thermo Scientific) were coated for 90 min at 37°C with 0.005% (w/v) Poly-L-Lysine (Sigma) diluted in PBS. Plates were washed with PBS and incubated overnight with 4 µg/mL of purified CPS.Citation21 Next, plates were washed with PBS+0.05% Tween 20 and blocked with PBS+0.05% Tween 20, 5% milk (blocking solution) at 37°C for 90 min. A 2-fold serial dilution of each mAb (starting from 2000 µg/mL) in blocking solution was added across the 96-well plate and the plates were incubated at 37°C for 90 min. Following three washes the wells were incubated for 90 min in HRP-conjugated goat anti-mouse kappa chain antibody (Southern Biotech) diluted in blocking solution at 1:10,000. Following a wash step, 100 µl of 3,3′,5,5′-tetramethylbenzidine substrate (KPL) was added to the wells for 30 min. The enzymatic reaction was stopped by adding 100 µL of 5% o-phosphoric acid to each well and the optical density was determined by measuring absorbance at 450 nm. Each experiment was completed in duplicate. To calculate the antibody concentration that induces a response halfway between baseline and maximum (EC50 value), a 4-parameter logistics curve fit was applied using Sigma Plot 11.0 (Systat Software Inc.).
Results
We previously generated IgG3 mAb 3C5, from BALB/c mice immunized with heat-inactivated whole B. pseudomallei.Citation27 IgG1 mAb 2A5 was generated from BALB/c mice immunized with purified CPS conjugated to BSA;Citation12 this glycoconjugate induces high titers of CPS-specific IgG antibodies in mice.Citation21 Subclass-switch families of both mAbs were generated with a modified sequential sib selection protocol where individual hybridoma clones were screened by ELISA to identify subclass-switch clones.Citation25 To verify that the heavy and light chain variable regions were identical for all subclasses, total mRNA was isolated from each hybridoma cell line and used for cDNA synthesis and PCR amplification followed by sequencing. Results indicate that the variable region sequences within each family are identical, and the sequences between each family (3C5 vs. 2A5) are different (data not shown).
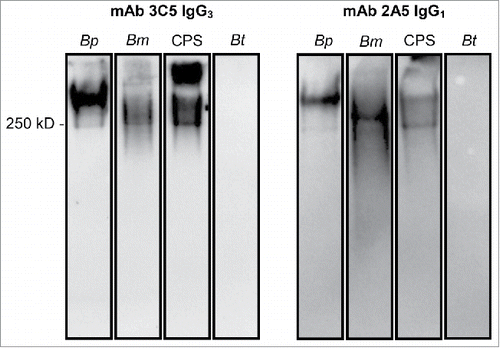
Western blot analysis was performed to verify that the parent mAb 2A5 IgG1 bound to CPS (). Our previous study confirmed that IgG3 mAb 3C5 is reactive with CPS from B. pseudomallei and B. mallei.Citation27 The CPS is a high molecular weight antigen comprised of an unbranched polymer of -3)-2-O-acetyl-6-deoxy-β-D-manno-heptopyranose-(1- residues that can be visualized by a characteristic high molecular weight smear via Western blot.Citation27 The IgG3 subclass of mAb 3C5 and the IgG1 subclass of mAb 2A5 both bound to the same proteinase K-resistant high molecular weight antigen found in B. pseudomallei, B. mallei and purified CPS preparations. Although a small percentage of B. thailandensis strains do produce CPS, E264 does not and both mAbs were not reactive to this strain ().Citation34,35
Figure 1. Western blot confirming binding of mAbs to CPS. B. pseudomallei (Bp) 1026b, B. mallei (Bm) China 7, CPS purified from Bp RR2683, or B. thailandensis (Bt) E264 were incubated with proteinase-K, separated by SDS PAGE, and subsequently transferred to a nitrocellulose membrane. Membranes were probed with 0.5 µg of mAb and binding was detected with an HRP-conjugated goat anti-mouse kappa chain antibody. IgG3 mAb 3C5 and IgG1 mAb 2A5 bind to a high molecular weight, proteinase-K resistant antigen found in B. pseudomallei, B. mallei, and purified CPS. No mAb binding was visualized against B. thailandensis, which lacks CPS.
Binding kinetics of each mAb were measured by SPR to determine if Fc regions from each IgG subclass contribute to mAb affinity. Purified CPS was conjugated to biotin Citation33 and immobilized on a streptavidin-coated SPR sensor chip. Total immobilization of CPS was 30 response units (RU). Injection of mAbs occurred over a 60 second pulse and a titratable increase in RU was observed (). A steady state model was applied to each graph to determine the dissociation constant for each mAb (). The calculated binding affinity of each mAb is in . Notably, the subclass-switch variants of mAb 3C5 have a substantially lower affinity for CPS than the parent IgG3 (8 to 20-fold). To determine if this affinity change was in part due to Fc region variation, F(ab')2 fragments were produced from the 3C5 IgG3. The F(ab')2 fragment affinity is comparable to the 3C5 non-IgG3 mAbs (29-fold reduction compared to IgG3 mAb 3C5). However, the 2A5 mAb subclasses show minor affinity variation, meaning the overall affinity is similar to that of IgG3 mAb 3C5.
Figure 2. Binding specificity of subclass-switch family mAbs and 3C5 IgG3 F(ab')2 fragments given as a function of response units (RU) generated over time. Data are shown from a representative experiment. A BIAcore X100 instrument was used to determine the affinity of each mAb for CPS. CPS purified from Bp RR2683 was conjugated to biotin and immobilized on a SA sensor chip. Binding was analyzed by injecting 8 samples diluted 2-fold (dilutions; 0.33–333 or 12–3,333 nM) for 60 s, followed by 120 s of passive dissociation.
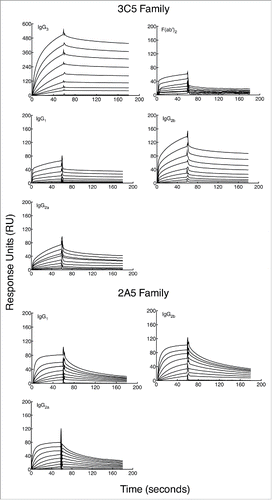
Figure 3. Binding affinity of subclass-switch family mAbs and 3C5 IgG3 F(ab')2 fragments given as a function of RUs generated by concentration. Data are shown from a representative experiment. The steady-state model from BIAevaluation software was applied to each graph in to determine the dissociation constant (KD) of each mAb. A smaller KD corresponds to a higher affinity. Note the higher mAb concentrations needed for calculation of affinity for the 3C5 non-IgG3 mAbs.
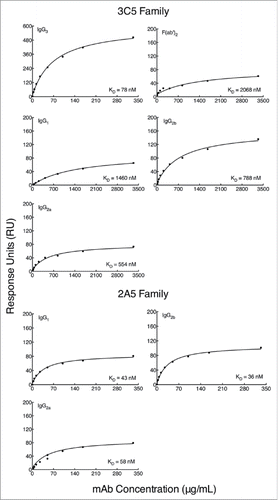
Table 1. Summary of antibodies generated for this study.
The SPR data indicated that the non-IgG3 3C5 antibodies had lower affinity, while the 2A5 subclass mAbs had comparable affinity. As further support we performed a Western blot experiment to compare binding of the 3C5 and 2A5 subclass switch mAbs. A miniblotter (with separate lane chambers for probing) was used so different concentrations of each subclass mAb could be used to probe the same blot containing a B. pseudomallei lysate. As shown in at equal mAb probing concentrations the most CPS reactivity was seen with mAb 3C5 IgG3 with far less intense reactivity for the non-IgG3 subclasses. Subclass-switching mAb 2A5 generated mAbs that bound to CPS at similar signal levels as the parent IgG1 (). The exposure time for Western blots in panel A and B were different to prove this point.
Figure 4. Comparison of binding activity within each mAb subclass by Western blot. B. pseudomallei Bp82 total cell lysate (87 μg) was separated by SDS-PAGE and transferred onto nitrocellulose membrane. The membranes were probed with mAb 3C5 family (panel A) and 2A5 family (panel B) using a miniblotter. 3C5 IgG3 shows substantially higher reactivity to CPS compared to the other subclasses. The reactivity between different subclasses of mAb 2A5 and CPS was comparable. 2A5 and 3C5 antibody concentration was prepared from 1mg/ml stock. Exposure time for Western blots in panel A and B were different.
An ELISA was performed to compare the relative antigen binding characteristics of the 3C5 and 2A5 subclass families (). Purified CPS was incubated in the solid phase at 4 µg/mL and 2-fold serial dilutions of mAbs were added in the fluid phase starting at 2,000 µg/mL. Each line on the plot represents a different mAb subclass. These plots were used to calculate the half-maximal effective concentration value (EC50), which is the antibody concentration that generates a response halfway between the baseline and maximum (). The calculated EC50 value for the parent 3C5 IgG3 mAb is 0.9 µg/mL, whereas the calculated EC50 values for the 3C5 subclass-switch mAbs are higher, by roughly 10 to 20-fold. In addition, F(ab')2 fragments were generated from 3C5 IgG3 and the EC50 values of these fragments were substantially higher than IgG3 mAb 3C5. Finally, the EC50 values of the 2A5 subclass-switch mAbs were comparable. The calculated EC50 values of the 2A5 subclasses ranged from 0.36–0.76 μg/mL, which is similar to 3C5 IgG3.
Figure 5. Direct antigen binding ELISA comparing 3C5 IgG3 and 2A5 IgG1 binding with subclass with switch family mAbs. CPS purified from Bp RR2683 was immobilized in the solid phase at 4 µg/mL. Two-fold serial dilutions of mAb or F(ab')2 were added in the fluid phase starting at 2,000 µg/mL and mAb binding was detected with an HRP-conjugated goat anti-mouse kappa chain antibody. All trials were completed in duplicate.
Discussion
Many pathogenic microorganisms produce capsular structures, including Haemophilus influenzae,Citation36 Neisseria meningitidis,Citation37 Bacillus anthracis, and Cryptococcus neoformans.Citation38 These capsules are comprised of polysaccharides or, in the case of B. anthracis, a polypeptide.Citation39 Capsules are antiphagocytic and are generally required for virulence.Citation40 Antibodies that target capsules are opsonic and protective in many cases.Citation40 As such, capsules are ideal vaccine targets, however, most capsular polysaccharides do not elicit a strong immune response, especially in infants and young children.Citation41 Capsular polysaccharides are high molecular weight antigens comprised of identical repeating units, making them T-cell independent type 2 antigens (TI-2).Citation42 They can activate B-cells by multivalent cross-linking of B-cell receptors, which produces a very specific antibody response.Citation18 Mice generally produce IgG3 in response to capsular polysaccharides;Citation22-24 humans generally produce IgG2.Citation43 As such, it is important to study the immunochemistry between antibodies and microbial capsules to develop effective vaccines and immunotherapeutics that elicit the appropriate humoral response against encapsulated pathogenic microbes.
Subclass-switch families are antibodies with identical Fv regions but different heavy chain constant regions, thereby making them invaluable for determining how the Fc region contributes to affinity and protection.Citation26,44,45 Previous research has shown that subclass-switch antibodies bind to their target with variable specificity and affinity, despite having identical Fv regions. Seminal research by Greenspan and colleagues established the enhanced binding of murine IgG3 to polysaccharides over that of non-IgG3 subclass-switch mAbs containing identical variable regions.Citation28-30 Specifically, it was shown that a partial murine IgG subclass family (IgG3→IgG1→IgG2b) interacts with the same Group A streptococcal antigen in very different ways. Murine IgG3 bound cooperatively and thus possessed a higher affinity than IgG1 or IgG2b. In addition, IgG3-derived F(ab')2 fragments did not show cooperative binding and bound with a much lower affinity, similar to that of the IgG1 and IgG2b.Citation28 Our recent studies in Bacillus anthracis have shown that an IgG3 mAb possessed greater binding affinity to the capsular polypeptideCitation26 when compared to all 3 other switched subclasses. The reduced binding affinity of the non-IgG3 subclass mAbs results from alterations in the CH2 and CH3 domains, which comprise the Fc region in IgG molecules. When altering this same murine IgG3 Fc region by engineering human chimeric antibodies (chAbs), the resulting chAbs have significantly reduced binding affinities for the capsular polypeptide.Citation46 Together, these studies strongly support that changes in the Fc region can alter antibody-antigen interactions.
There have been multiple studies showing passive transfer of antibodies can provide protection in animal models of melioidosis,Citation27,47-52 however, there have been no studies to date comparing different antibody subclasses. In the current study, we generated 2 unique subclass-switch families that bind to the CPS of B. pseudomallei. One family originated from a high affinity IgG3 mAb (3C5), while the other originated from a high affinity IgG1 mAb (2A5). Removal of the Fc region of mAb 3C5 by proteolytic cleavage generates IgG3 F(ab')2 fragments with binding ability as shown by SPR and ELISA. Similarly, subclass-switching mAb 3C5 from an IgG3 to an IgG1 results in a substantial loss in affinity and a large increase in EC50 values. Subclass-switching initiated from a high affinity IgG1 mAb (2A5) to an IgG2b or IgG2a subclass yields similar binding results. However, the IgG1 and IgG2b variants showed slightly improved binding characteristics over the IgG2a. These small affinity changes between variable region identical mAbs are not surprising. For example, it is well established that variable region structure can be altered by switching constant regions, resulting in subtle changes in affinity and fine specificity.Citation53,54 Taken together, the 3C5 IgG3 Fc region clearly enhances binding affinity to the B. pseudomallei CPS compared to non-IgG3 Fc regions.
These findings are supported by recent results published by our group.Citation26. In this study, mice were immunized with 2 subclass-switch families of mAbs targeting the capsule of B. anthracis. In these studies, the IgG3 subclasses were protective in a murine model of pulmonary anthrax and the lower affinity non-IgG3 mAbs were not protective (26). In addition to enhanced protection and affinity the IgG3 mAbs were deposited at the capsular edge and resembled a rim formation under DIC microscopy. The non-IgG3 mAbs were deposited throughout the capsule and were described as a puffy pattern. The rim formation by IgG3 appears to be due to Fc-Fc crosslinking since the other non-IgG3 subclasses were not able to form the rim. The rim formation may have the protective benefit of depositing more antibody on the capsular edge where it can interact efficiently with immune molecules and effector cells. It is not know whether the B. pseudomallei CPS mAbs bind in a rim or puffy pattern. Unfortunately, the capsular binding pattern is very difficult to visualize with a small bacterium such as B. pseudomallei under DIC microscopy.
In summary, this study suggests that IgG3 Fc regions contribute to affinity of IgG3 antibodies binding to the CPS of B. pseudomallei. Switching the IgG3 Fc subclass to a non-IgG3 Fc region or removing the IgG3 Fc region reduces antibody affinity. Therefore, as our current and previous studies suggest, it may be sensible not to start with a murine IgG3 variable region when planning to produce a chimeric or humanized antibody for use as an immunotherapeutic targeting a microbial capsular structure. To further support this thought, future studies will include testing the current subclass mAbs for protection in a murine model of melioidosis. This should provide additional insights into the contributions of subclass and affinity to protection.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The project described was supported by grant U54AI065359 and grant 1R4AI1202481 from the National Institute of Allergy and Infectious Diseases.
References
- Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg 2010; 82:1113-7; PMID:20519609; http://dx.doi.org/10.4269/ajtmh.2010.10-0038
- Reckseidler SL, DeShazer D, Sokol PA, Woods DE. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect Immun 2001; 69:34-44; PMID:11119486; http://dx.doi.org/10.1128/IAI.69.1.34-44.2001
- Limmathurotsakul D, Golding N, Dance D, Messina JP, Pigott DM, Moyes CL, Rolim DB, Bertherat E, Day NP, Peacock SJ, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nature Microbiology 2016; 1.
- Reckseidler-Zenteno SL, DeVinney R, Woods DE. The capsular polysaccharide of Burkholderia pseudomallei contributes to survival in serum by reducing complement factor C3b deposition. Infect Immun 2005; 73:1106-15; PMID:15664954; http://dx.doi.org/10.1128/IAI.73.2.1106-1115.2005
- Shaw BM, Daubenspeck JM, Simmons WL, Dybvig K. EPS-I polysaccharide protects Mycoplasma pulmonis from phagocytosis. FEMS Microbiol Lett 2013; 338:155-60; PMID:23190331; http://dx.doi.org/10.1111/1574-6968.12048
- Lemire P, Houde M, Lecours MP, Fittipaldi N, Segura M. Role of capsular polysaccharide in Group B Streptococccus interactions with dendritic cells. Microbes Infect 2012; 14:1064-76; PMID:22683668; http://dx.doi.org/10.1016/j.micinf.2012.05.015
- Melin M, Jarva H, Siira L, Meri S, Kayhty H, Vakevainen M. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect Immun 2009; 77:676-84; PMID:19047408; http://dx.doi.org/10.1128/IAI.01186-08
- Agarwal S, Vasudhev S, DeOliveira RB, Ram S. Inhibition of the classical pathway of complement by meningococcal capsular polysaccharides. J Immunol 2014; 193:1855-63; PMID:25015832; http://dx.doi.org/10.4049/jimmunol.1303177
- Berti F, Campisi E, Toniolo C, Morelli L, Crotti S, Rosini R, Romano MR, Pinto V, Brogioni B, Torricelli G, et al. Structure of the type IX group B Streptococcus capsular polysaccharide and its evolutionary relationship with types V and VII. J Biol Chem 2014; 289:23437-48; PMID:24990951; http://dx.doi.org/10.1074/jbc.M114.567974
- Xie O, Bolgiano B, Gao F, Lockyer K, Swann C, Jones C, Delrieu I, Njanpop-Lafourcade BM, Tamekloe TA, Pollard AJ, et al. Characterization of size, structure and purity of serogroup X Neisseria meningitidis polysaccharide, and development of an assay for quantification of human antibodies. Vaccine 2012; 30:5812-23; PMID:22835740; http://dx.doi.org/10.1016/j.vaccine.2012.07.032
- Bruckner V, Kovacs J, Denes G. Structure of poly-D-glutamic acid isolated from capsulated strains of B. anthracis. Nature 1953; 172:508; PMID:13099252; http://dx.doi.org/10.1038/172508a0
- Heiss C, Burtnick MN, Wang Z, Azadi P, Brett PJ. Structural analysis of capsular polysaccharides expressed by Burkholderia mallei and Burkholderia pseudomallei. Carbohydrate research 2012; 349:90-4; PMID:22221792; http://dx.doi.org/10.1016/j.carres.2011.12.011
- Perry MB, MacLean LL, Schollaardt T, Bryan LE, Ho M. Structural characterization of the lipopolysaccharide O antigens of Burkholderia pseudomallei. Infect Immun 1995; 63:3348-52; PMID:7543882
- Warawa JM, Long D, Rosenke R, Gardner D, Gherardini FC. Role for the Burkholderia pseudomallei capsular polysaccharide encoded by the wcb operon in acute disseminated melioidosis. Infect Immun 2009; 77:5252-5261; PMID:19752033; http://dx.doi.org/10.1128/IAI.00824-09
- Atkins T, Prior R, Mack K, Russell P, Nelson M, Prior J, Ellis J, Oyston PC, Dougan G, Titball RW. Characterisation of an acapsular mutant of Burkholderia pseudomallei identified by signature tagged mutagenesis. J Med Microbiol 2002; 51:539-47; PMID:12132769; http://dx.doi.org/10.1099/0022-1317-51-7-539
- Wikraiphat C, Charoensap J, Utaisincharoen P, Wongratanacheewin S, Taweechaisupapong S, Woods DE, Bolscher JG, Sirisinha S. Comparative in vivo and in vitro analyses of putative virulence factors of Burkholderia pseudomallei using lipopolysaccharide, capsule and flagellin mutants. FEMS Immunol Med Microbiol 2009; 56:253-9; PMID:19549172; http://dx.doi.org/10.1111/j.1574-695X.2009.00574.x
- Parker DC. T cell-dependent B cell activation. Annu Rev Immunol 1993; 11:331-60; PMID:8476565; http://dx.doi.org/10.1146/annurev.iy.11.040193.001555
- Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev 2000; 176:154-70; PMID:11043775; http://dx.doi.org/10.1034/j.1600-065X.2000.00607.x
- Claesson BA, Trollfors B, Lagergard T, Taranger J, Bryla D, Otterman G, Cramton T, Yang Y, Reimer CB, Robbins JB, et al. Clinical and immunologic responses to the capsular polysaccharide of Haemophilus influenzae type b alone or conjugated to tetanus toxoid in 18- to 23-month-old children. J Pediatr 1988; 112:695-702; PMID:3361379; http://dx.doi.org/10.1016/S0022-3476(88)80684-X
- Beuvery EC, Miedema F, van Delft R, Haverkamp J. Preparation and immunochemical characterization of meningococcal group C polysaccharide-tetanus toxoid conjugates as a new generation of vaccines. Infect Immun 1983; 40:39-45; PMID:6187693
- Burtnick MN, Heiss C, Roberts RA, Schweizer HP, Azadi P, Brett PJ. Development of capsular polysaccharide-based glycoconjugates for immunization against melioidosis and glanders. Front Cell Infect Microbiol 2012; 2:108; PMID:22912938
- Perlmutter RM, Hansburg D, Briles DE, Nicolotti RA, Davie JM. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol 1978; 121:566-72; PMID:79606
- Slack J, Der-Balian GP, Nahm M, Davie JM. Subclass restriction of murine antibodies. II. The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med 1980; 151:853-62; PMID:6966310; http://dx.doi.org/10.1084/jem.151.4.853
- Hansburg D, Perlmutter RM, Briles DE, Davie JM. Analysis of the diversity of murine antibodies to dextran B1355. III. Idiotypic and spectrotypic correlations. Eur J Immunol 1978; 8:352-9; PMID:689075; http://dx.doi.org/10.1002/eji.1830080512
- Spira G, Bargellesi A, Teillaud JL, Scharff MD. The identification of monoclonal class switch variants by sib selection and an ELISA assay. J Immunol Methods 1984; 74:307-15; PMID:6438240; http://dx.doi.org/10.1016/0022-1759(84)90298-9
- Hovenden M, Hubbard MA, AuCoin DP, Thorkildson P, Reed DE, Welch WH, Lyons CR, Lovchik JA, Kozel TR. IgG subclass and heavy chain domains contribute to binding and protection by mAbs to the poly gamma-D-glutamic acid capsular antigen of Bacillus anthracis. PLoS Pathog 2013; 9:e1003306; PMID:23637599; http://dx.doi.org/10.1371/journal.ppat.1003306
- AuCoin DP, Reed DE, Marlenee NL, Bowen RA, Thorkildson P, Judy BM, Torres AG, Kozel TR. Polysaccharide specific monoclonal antibodies provide passive protection against intranasal challenge with Burkholderia pseudomallei. PLoS One 2012; 7:e35386; PMID:22530013; http://dx.doi.org/10.1371/journal.pone.0035386
- Cooper LJ, Schimenti JC, Glass DD, Greenspan NS. H chain C domains influence the strength of binding of IgG for streptococcal group A carbohydrate. J Immunol 1991; 146:2659-63; PMID:1901882
- Cooper LJ, Robertson D, Granzow R, Greenspan NS. Variable domain-identical antibodies exhibit IgG subclass-related differences in affinity and kinetic constants as determined by surface plasmon resonance. Mol Immunol 1994; 31:577-84; PMID:7515151; http://dx.doi.org/10.1016/0161-5890(94)90165-1
- Schreiber JR, Cooper LJ, Diehn S, Dahlhauser PA, Tosi MF, Glass DD, Patawaran M, Greenspan NS. Variable region-identical monoclonal antibodies of different IgG subclass directed to Pseudomonas aeruginosa lipopolysaccharide O-specific side chain function differently. J Infect Dis 1993; 167:221-6; PMID:8418172; http://dx.doi.org/10.1093/infdis/167.1.221
- Kozel TR, Murphy WJ, Brandt S, Blazar BR, Lovchik JA, Thorkildson P, Percival A, Lyons CR. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc Natl Acad Sci U S A 2004; 101:5042-7; PMID:15051894; http://dx.doi.org/10.1073/pnas.0401351101
- Nuti DE, Crump RB, Dwi Handayani F, Chantratita N, Peacock SJ, Bowen R, Felgner PL, Davies DH, Wu T, Lyons CR, et al. Identification of circulating bacterial antigens by in vivo microbial antigen discovery. mBio 2011; 2.
- Kozel TR, Hermerath CA. Benzoquinone activation of Cryptococcus neoformans capsular polysaccharide for construction of an immunoaffinity column. J Immunol Methods 1988; 107:53-8; PMID:3125258; http://dx.doi.org/10.1016/0022-1759(88)90008-7
- Houghton RL, Reed DE, Hubbard MA, Dillon MJ, Chen H, Currie, BJ, Mayo M, Sarovich D, Theobald V, Limmathurotsakul D, et al. Development of a prototype lateral flow immunoassay (LFI) for the rapid diagnosis of melioidosis. PLoS Negl Trop Dis 2014; 8:e2727; PMID:24651568; In Press.
- DeShazer D, Waag DM, Fritz DL, Woods DE. Identification of a Burkholderia mallei polysaccharide gene cluster by subtractive hybridization and demonstration that the encoded capsule is an essential virulence determinant. Microb Pathog 2001; 30:253-69; PMID:11373120; http://dx.doi.org/10.1006/mpat.2000.0430
- Crisel RM, Baker RS, Dorman DE. Capsular polymer of Haemophilus influenzae, type b. I. Structural characterization of the capsular polymer of strain Eagan. J Biol Chem 1975; 250:4926-30; PMID:1080151
- DeVoe IW. The meningococcus and mechanisms of pathogenicity. Microbiol Rev 1982; 46:162-90; PMID:6126800
- Cherniak R, Valafar H, Morris LC, Valafar F. Cryptococcus neoformans chemotyping by quantitative analysis of 1H nuclear magnetic resonance spectra of glucuronoxylomannans with a computer-simulated artificial neural network. Clin Diagn Lab Immunol 1998; 5:146-59; PMID:9521136
- Zwartouw HT, Smith H. Polyglutamic acid from Bacillus anthracis grown in vivo; structure and aggressin activity. Biochem J 1956; 63:437-42; PMID:13341899; http://dx.doi.org/10.1042/bj0630437
- Wilkinson JF. The extracellualr polysaccharides of bacteria. Bacteriol Rev 1958; 22:46-73; PMID:13522509
- Kanaphun P, Thirawattanasuk N, Suputtamongkol Y, Naigowit P, Dance DA, Smith MD, White NJ. Serology and carriage of Pseudomonas pseudomallei: a prospective study in 1000 hospitalized children in northeast Thailand. J Infect Dis 1993; 167:230-33; PMID:7678106; http://dx.doi.org/10.1093/infdis/167.1.230
- Feldmann M, Easten A. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response. J Exp Med 1971; 134:103-19; PMID:4104294; http://dx.doi.org/10.1084/jem.134.1.103
- Scott MG, Shackelford PG, Briles DE, Nahm MH. Human IgG subclasses and their relation to carbohydrate antigen immunocompetence. Diagn Clin Immunol 1988; 5:241-8; PMID:3282712
- Beenhouwer DO, Yoo EM, Lai CW, Rocha MA, Morrison SL. Human immunoglobulin G2 (IgG2) and IgG4, but not IgG1 or IgG3, protect mice against Cryptococcus neoformans infection. Infect Immun 2007; 75:1424-35; PMID:17220317; http://dx.doi.org/10.1128/IAI.01161-06
- Yuan RR, Spira G, Oh J, Paizi M, Casadevall A, Scharff MD. 1998. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect Immun 2007; 66:1057-62; PMID:9488395
- Hubbard MA, Thorkildson P, Kozel TR, AuCoin DP. Constant domains influence binding of mouse-human chimeric antibodies to the capsular polypeptide of Bacillus anthracis. Virulence 2013; 4:483-8; PMID:23863605; http://dx.doi.org/10.4161/viru.25711
- Jones SM, Ellis JF, Russell P, Griffin KF, Oyston PC. Passive protection against Burkholderia pseudomallei infection in mice by monoclonal antibodies against capsular polysaccharide, lipopolysaccharide or proteins. J Med Microbiol 2002; 51:1055-62; PMID:12466403; http://dx.doi.org/10.1099/0022-1317-51-12-1055
- Zhang S, Feng SH, Li B, Kim HY, Rodriguez J, Tsai S, Lo SC. In vitro and in vivo studies of monoclonal antibodies with prominent bactericidal activity against burkholderia pseudomallei and burkholderia mallei. Clin Vaccine Immunol 2011; 18:825-34; PMID:21450976; http://dx.doi.org/10.1128/CVI.00533-10
- Bottex C, Gauthier YP, Hagen RM, Finke EJ, Splettstosser WD, Thibault FM, Neubauer H, Vidal DR. Attempted passive prophylaxis with a monoclonal anti-Burkholderia pseudomallei exopolysaccharide antibody in a murine model of melioidosis. Immunopharmacol Immunotoxicol 2005; 27:565-83; PMID:16435577; http://dx.doi.org/10.1080/08923970500493995
- Nelson M, Prior JL, Lever MS, Jones HE, Atkins TP, Titball RW. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J Med Microbiol 2004; 53:1177-82; PMID:15585494; http://dx.doi.org/10.1099/jmm.0.45766-0
- Brett PJ, Woods DE. Structural and immunological characterization of Burkholderia pseudomallei O-polysaccharide-flagellin protein conjugates. Infect Immun 1996; 64:2824-8; PMID:8698517
- Bryan LE, Wong S, Woods DE, Dance DA, Chaowagul W. Passive protection of diabetic rats with antisera specific for the polysaccharide portion of the lipopolysaccharide isolated from Pseudomonas pseudomallei. Can J Infect Dis 1994; 5:170-8; PMID:22346496; http://dx.doi.org/10.1155/1994/856850
- Janda A, Eryilmaz E, Nakouzi A, Cowburn D, Casadevall A. Variable region identical immunoglobulins differing in isotype express different paratopes. J Biol Chem 2012; 287:35409-17; PMID:22930758; http://dx.doi.org/10.1074/jbc.M112.404483
- Pritsch O, Hudry-Clergeon G, Buckle M, Petillot Y, Bouvet JP, Gagnon J, Dighiero G. Can immunoglobulin C(H)1 constant region domain modulate antigen binding affinity of antibodies? J Clin Invest 1996; 98:2235-43; PMID:8941639
