Staphylococcus aureus infections are associated with an enormous burden of morbidity, mortality and increase of patient hospital length of stay and costs. S. aureus is impressively fast in acquiring antibiotic resistance and multidrug resistant strains are a serious threat to human health. Due to resistance or insufficient effectiveness, antibiotics and bundle measures leave a tremendous unmet medical need worldwide. Vaccines and antibody-based biologic agents represent the most important alternatives to antibiotics. However, no licensed S. aureus vaccines or antibodies are available on the market yet despite the significant efforts done by public and private initiatives. Indeed, active vaccination and passive immunotherapy approaches tested in clinical trials in the last 2 decades have failed to show efficacy.Citation1-3 Reasons behind these failures have been proposedCitation4 and several research and development programs are still active.Citation5 The 2 options, vaccines and antibodies, may have different aims and applications against S. aureus infections. Vaccines, which need some time for inducing a protective immune response, may be more suitable for preventing infections associated with elective surgery (e.g., cardiothoracic-, orthopedic- and neuro-surgery) and prolonged exposure to the risk of the infection (e.g., catheter-associated infections, community-acquired infections). Antibodies, which exert their action immediately, could represent the best approach for infections associated with emergency interventions (e.g., trauma patients and emergency surgery as well as intensive care unit patients). The latter may also be the best option for immune-deficient patients, who may not properly respond to active vaccination. On top of the proper target population another difference stems from the mechanism of action of the 2 approaches. Vaccines targeting several antigens can be manufactured at relatively low costs and in addition they can contain adjuvants able to stimulate the cell-mediated immunity.Citation6 These 2 attributes are considered key protective elements against S. aureus by the scientific community. Indeed, the bacterium exploits several pathogenic pathways, and there is general consensus that a protective immunity needs to target several virulence factors with different roles. In addition, an important role of T-cell response involved in promoting bacterial clearance has been proposed.Citation3,7 Vaccines currently in more advanced stage of clinical development are listed in .
Table 1. Key characteristics of vaccines and antibody-based biologics currently in Phase II clinical testing (no active programs are currently in Phase III).
Antibody-based approaches currently in clinical development (for the ones information is available in clinicaltrials.gov) are described in . Medimmune and Aridis Pharmaceuticals independently developed a human monoclonal antibody (mAb) specific for the α-hemolysin (Hla), named respectively MEDI48938 and AR-301. Arsanis developed a combination of 2 mAbs (ASN100; see reference: Citation9,10). The latter product contains a Hla mAb (ASN-1) which has cross-reactive properties against 4 other toxins, the leukocidins HlgAB, HlgCB, LukED & LukSF and another mAb (ASN-2) against the leukocidin LukAB. Although ASN100 recognizes several antigens, they are all toxins. Therefore, this approach may not be able to induce bacterial clearance. Indeed, this mechanism is mediated by antibodies recognizing surface components. The human mAb developed by XBiotech (514G3) against the surface antigen Staphylococcal protein A (SpA) is expected to induce opsonophagocytosis. In addition, 514G3 is supposed to inhibit main immune evasion mechanisms of S. aureus associated to SpA functions (https://doi.org/10.1093/ofid/ofw172.1057 and Citation3).
A cocktail of antibodies would be needed for blocking toxins, immune evasion factors and induce phagocytosis of S. aureus. However, such a product may be too expensive for being cost-effective. Yet, it can be anticipated that technical improvements will allow a significant decrease of costs associated with mAb manufacturing in the near future.Citation11,12 In addition, some groups are attempting to develop engineered molecules which combine as fusion proteins mAbs against serine-aspartate repeat-containing surface antigens with novel protein-binding domains referred to as ‘centyrins’ which are able to bind to leukocidin toxins.Citation13
Additional target antigens for the development of mAbs are being considered at research level and one of those currently pursued by different groups is the staphylococcal enterotoxin B (SEB). Furthermore a mutated form of SEB has been included in a vaccine (STEBVax) tested in a Phase I trial. No safety concerns and a good immunogenicity profile (93% of the vaccine recipients seroconverted for SEB toxicity-neutralizing antibody titers) were observed following vaccination of healthy subjects.Citation14
SEB is a superantigen and as such is a protein able to induce a potent activation of T cells by binding to T-cell receptor and to class II major histocompatibility complex molecules.Citation15 Inflammatory cytokines released systemically by activated T-cells can cause toxic shock syndrome (TSS) with potential lethal outcomes for the patient. SEB is also considered a potential biologic weapon given its high toxicity and thermal stability.
In this issue Karau and colleaguesCitation16 present a research study which aims at understanding the potential contribution of a passive therapy approach against S. aureus pneumonia using a pair of mAbs, targeting different epitopes of SEB. The antibodies are high-affinity human-mouse chimera and together they show enhanced neutralization of SEB superantigenicity both in vitro and in vivo as shown by the same group in previous publications.Citation17,18 To assess more reliably the protective potentials of the SEB mAbs, Karau et al. used humanized mice for the target bound by SEB. Indeed, as it happens for many virulence factors expressed by S. aureus, SEB shows high specificity for its human target (HLA-DR3) while the equivalent murine MHC class II molecule is bound with 100 fold less affinity.Citation19,20 The use of humanized mice is an important step forward for understanding the role of SEB and of antibodies against it. However, as S. aureus pathogenesis is mediated by several virulence factors able to recognize other human targets, a mouse model humanized only for one of those targets may still poorly resemble what happens in human infections.
HLA-DR3 transgenic mice infected intratracheally (for inducing pneumonia) with a S. aureus strain expressing SEB (not all strains express this toxin) were shown to be highly susceptible to the infection both in terms of T-cell activation and induction of pro-inflammatory cytokines induced by SEB as well as lethality. Using this infection model, the authors evaluated both prophylactic and therapeutic effects of the antibiotic linezolid, which is a new generation antibiotic approved for clinical therapy of S. aureus pneumonia. Administration of the antibiotic before infection prevented death and attenuated systemic inflammatory response. These data suggest that this antibiotic was able to inhibit production of SEB (supposedly due the ability of linezolid to inhibit protein synthesis). However, in the clinical setting antibiotics are often given at the appearance of symptoms when an infection is already established. Therefore, the authors decided to administer linezolid one hour after the infection and in this case the antibiotic was not able to significantly reduce neither the inflammatory response nor the lethality associated to the infection. On the other hand, both disease outcomes were significantly reduced in mice which received SEB antibodies after infection followed by linezolid.
Similarly, efficacy of antibiotics (vancomycin and linezolid) against S. aureus pneumonia in mice was shown to be increased by the previously mentioned mAbs against Hla developed by 2 independent groups.Citation9,21 Interestingly, Hla has been shown to facilitate penetration of a superantigen through an epithelium.Citation22 Therefore, it would be interesting to evaluate if strategies aimed at targeting both Hla and SEB have additive protective properties against S. aureus infection.
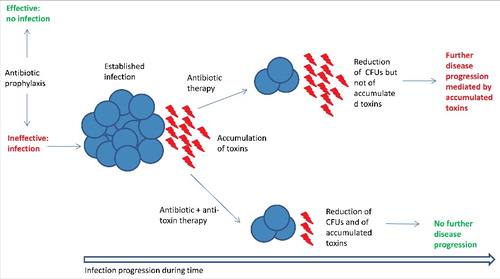
The data shown by Karau et al.Citation16 suggest that therapeutic effects of an antibiotic given once a S. aureus infection is established could be ineffective in blocking progression of disease mechanisms driven by secreted toxins such as SEB, Hla and leukocidins already accumulated in the lungs (). Therefore, anti-toxin immunotherapy co-administered with antibiotics may be a good option for decreasing mortality of patients affected by staphylococcal pneumonia. Indeed, mortality rate of 17% has been reported in patients treated with vancomycin, which is considered the primary antibiotic against pneumonia caused by methicillin-resistant S. aureus (MRSA).Citation23,24 Linezolid and telavancin are also approved antibiotics for the treatment of MRSA pneumonia and have been reported to have a comparable efficacy to vancomycin.Citation23-25 Such an approach is currently exploited in a Phase II trial for evaluating efficacy of AR-301 in patients who have severe pneumonia caused by S. aureus in addition to standard of care antibiotic treatment (). Given that not all S. aureus strains express the same toxins, this approach could be coupled with a diagnostic evaluation of the expression of the toxins for guiding the use of the most appropriate mAb.
Figure 1. Expected mechanism of action of anti-toxin antibody-based biologics in conjunction with antibiotic therapy. Antibiotic prophylaxis may prevent a S. aureus infection (blue spheres). Once an infection is established toxins accumulate in the tissues. Antibiotic therapy alone may not be able to effectively block disease progression mediated by accumulated toxins even if it is able to reduce bacterial counts. A combined anti-toxin/antibiotic therapy may be able to both reduce CFUs and neutralize toxin activity blocking disease progression. This mechanism may be particularly relevant in diseases such as S. aureus pneumonia in which toxins play a key role and disease reaches its acute phase quickly.
An alternative approach is represented by the prophylactic administration of mAb to patients at high risk of infection. Such strategy is being pursued with MEDI4893 and ASN100, which are currently being evaluated for the prevention of nosocomial pneumonia in high-risk ventilated patients (; Citation8,10,21). An innovative option of combining the antibody and antibiotic activity is the chemical conjugation of a mAb against a S. aureus surface antigen (wall teichoic acid) to an antibiotic.Citation26 This molecule promotes specific killing of bacteria that have been phagocytosed given that the antibiotic needs to be activated by proteolytic release in the phagolysosome.
Nevertheless the approaches mentioned above would not significantly decrease the use of antibiotics and the pressure toward the emergence of antibiotic resistance. Vaccines targeting multiple components including toxins and surface antigens may represent an important solution to that problem which could otherwise become overwhelming in the coming future. A recent study suggests that 10 million deaths attributable to antibiotic resistant infections every year, exceeding the ones caused by cancer, will occur by 2050 (https://amr-review.org/Publications). The ESKAPE pathogens (Enterococcus faecium, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) were recognized as the leading cause of antibiotic-resistant infections occurring worldwide in hospitals.Citation27 The Centers for Disease Control and Prevention (CDC) of the United States recently listed 18 drug-resistant human pathogens (which include the ESKAPE group and others such as Clostridium difficile, Neisseria gonorrhea, Candida albicans, Salmonella Typhi, etc…) as the most important infectious agents in the United States (https://www.cdc.gov/drugresistance/biggest_threats.html). Given the different features of these microorganisms and the wide range of diseases that they cause, there is no one single approach that can be used against all of them. Therefore, the only way to respond to such a tremendous threat is to improve the appropriate use of antibiotics and prioritize research and development efforts on new antibiotics and approaches that reduce the use of antibiotics such as, vaccines and antibody-based biologics.
Disclosure of potential conflicts of interest
Fabio Bagnoli is an employee of GSK Vaccines and owns patents on S. aureus vaccine candidates as well as GSK stocks. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
The author thanks Carine Goraj, David J. Payne and Anja K. Seubert at GSK for critical reading of the manuscript.
Funding
This work was funded by GSK Vaccines Srl.
References
- Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum Vaccin Immunother 2015; 11(3):632-41; PMID:25483694; http://dx.doi.org/10.4161/hv.34414
- Allen KB, Fowler VG Jr, Gammie JS, Hartzel JS, Onorato MT, DiNubile MJ, Sobanjo-Ter Meulen A. Staphylococcus aureus infections after elective cardiothoracic surgery: Observations from an international randomized Placebo-Controlled trial of an investigational S aureus vaccine. Open Forum Infect Dis 2014; 1(2):ofu071; PMID: 25734141; http://dx.doi.org/10.1093/ofid/ofu071
- Pozzi C., Lofano G, Mancini F, Soldaini E, Speziale P, De Gregorio E, Rappuoli R, Bertholet S, Grandi G, Bagnoli F et al. Phagocyte subsets and lymphocyte clonal deletion behind ineffective immune response to Staphylococcus aureus. FEMS Microbiol Rev 2015; 39(5):750-63; PMID: 25994610; http://dx.doi.org/10.1093/femsre/fuv024
- Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol 2012; 2:16; PMID: 22919608; http://dx.doi.org/10.3389/fcimb.2012.00016
- Yeaman MR, Filler SG, Schmidt CS, Ibrahim AS, Edwards JE Jr., Hennessey JP Jr Applying convergent immunity to innovative vaccines targeting Staphylococcus aureus. Front Immunol 2014; 5:463; PMID: 25309545; http://dx.doi.org/10.3389/fimmu.2014.00463
- Bagnoli F, Fontana MR, Soldaini E, Mishra RP, Fiaschi L, Cartocci E, Nardi-Dei V, Ruggiero P, Nosari S, De Falco MG et al. Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci U S A 2015; 112(12):3680-5; PMID: 25775551
- Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 2011; 11(8):505-18; PMID: 21720387; http://dx.doi.org/10.1038/nri3010
- Hua L, Cohen TS, Shi Y, Datta V, Hilliard JJ, Tkaczyk C, Suzich J, Stover CK, Sellman BR. MEDI4893* promotes survival and extends the antibiotic treatment window in a Staphylococcus aureus Immunocompromised Pneumonia Model. Antimicrob Agents Chemother 2015; 59(8):4526-32; PMID: 25987629; http://dx.doi.org/10.1128/AAC.00510-15
- Rouha H, Badarau A, Visram ZC, Battles MB, Prinz B, Magyarics Z, Nagy G, Mirkina I, Stulik L, Zerbs M et al. Five birds, one stone: neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs 2015; 7(1):243-54; PMID: 25523282; http://dx.doi.org/10.4161/19420862.2014.985132
- Badarau A, Rouha H, Malafa S, Battles MB, Walker L, Nielson N, Dolezilkova I, Teubenbacher A, Banerjee S, Maierhofer B et al. Context matters: The importance of dimerization-induced conformation of the LukGH leukocidin of Staphylococcus aureus for the generation of neutralizing antibodies. MAbs 2016; 8(7):1347-1360; PMID: 27467113; http://dx.doi.org/10.1080/19420862.2016.1215791
- Chon JH, Zarbis-Papastoitsis G. Advances in the production and downstream processing of antibodies. N Biotechnol 2011; 28(5):458-63; PMID: 21515428; http://dx.doi.org/10.1016/j.nbt.2011.03.015
- Shukla AA, Thommes J. Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol 2010; 28(5):253-61; PMID: 20304511; http://dx.doi.org/10.1016/j.tibtech.2010.02.001
- Diem MD, Hyun L, Yi F, Hippensteel R, Kuhar E, Lowenstein C, Swift EJ, O'Neil KT, Jacobs SA. Selection of high-affinity Centyrin FN3 domains from a simple library diversified at a combination of strand and loop positions. Protein Eng Des Sel 2014; 27(10):419-29; PMID: 24786107; http://dx.doi.org/10.1093/protein/gzu016
- Chen WH, Pasetti MF, Adhikari RP, Baughman H, Douglas R, El-Khorazaty J, Greenberg N, Holtsberg FW, Liao GC, Reymann MK et al. Safety and immunogenicity of a parenterally administered, Structure-Based rationally modified recombinant Staphylococcal Enterotoxin B Protein Vaccine, STEBVax. Clin Vaccine Immunol 2016; 23(12):918-925; PMID: 27707765; http://dx.doi.org/10.1128/CVI.00399-16
- Krakauer T, Stiles BG. The staphylococcal enterotoxin (SE) family: SEB and siblings. Virulence 2013; 4(8):759-73; PMID: 23959032; http://dx.doi.org/10.4161/viru.23905
- Karau MJ, Tilahun ME, Krogman A, Osborne BA, Goldsby RA, David CS, Mandrekar JN, Patel R, Rajagopalan G. Passive therapy with humanized anti-staphylococcal enterotoxin B antibodies attenuates systemic inflammatory response and protects from lethal pneumonia caused by staphylococcal enterotoxin B-producing Staphylococcus aureus. Virulence 2016; 1-12; PMID: 27925510; http://dx.doi.org/10.1080/21505594.2016.1267894
- Tilahun ME, Rajagopalan G, Shah-Mahoney N, Lawlor RG, Tilahun AY, Xie C, Natarajan K, Margulies DH, Ratner DI, Osborne BA et al. Potent neutralization of staphylococcal enterotoxin B by synergistic action of chimeric antibodies. Infect Immun 2010; 78(6):2801-11; PMID: 20308304; http://dx.doi.org/10.1128/IAI.01121-09
- Tilahun ME, Kwan A, Natarajan K, Quinn M, Tilahun AY, Xie C, Margulies DH, Osborne BA, Goldsby RA, Rajagopalan G et al. Chimeric anti-staphylococcal enterotoxin B antibodies and lovastatin act synergistically to provide in vivo protection against lethal doses of SEB. PLoS One 2011; 6(11):e27203; PMID: 22102880; http://dx.doi.org/10.1371/journal.pone.0027203
- DaSilva L, Welcher BC, Ulrich RG, Aman MJ, David CS, Bavari S. Humanlike immune response of human leukocyte antigen-DR3 transgenic mice to staphylococcal enterotoxins: a novel model for superantigen vaccines. J Infect Dis 2002; 185(12):1754-60; PMID: 12085321; http://dx.doi.org/10.1086/340828
- Fraser JD. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature 1989; 339(6221):221-3; PMID: 2785644; http://dx.doi.org/10.1038/339221a0
- Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R et al. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 2014; 58(2):1108-17; PMID: 24295977; http://dx.doi.org/10.1128/AAC.02190-13
- Brosnahan AJ, Mantz MJ, Squier CA, Peterson ML, Schlievert PM. Cytolysins augment superantigen penetration of stratified mucosa. J Immunol 2009; 182(4):2364-73; PMID: 19201891; http://dx.doi.org/10.4049/jimmunol.0803283
- Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012; 54(5):621-9; PMID: 22247123; http://dx.doi.org/10.1093/cid/cir895
- Holmes NE, Tong SY, Davis JS, van Hal SJ. Treatment of methicillin-resistant Staphylococcus aureus: vancomycin and beyond. Semin Respir Crit Care Med 2015; 36(1):17-30; PMID: 25643268; http://dx.doi.org/10.1055/s-0034-1397040
- Rubinstein E, Lalani T, Corey GR, Kanafani ZA, Nannini EC, Rocha MG, Rahav G, Niederman MS, Kollef MH, Shorr AF et al. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis 2011; 52(1):31-40; PMID: 21148517; http://dx.doi.org/10.1093/cid/ciq031
- Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, DePalatis L, Raab H, Hazenbos WL, Morisaki JH et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 2015; 527(7578):323-8; PMID: 26536114; http://dx.doi.org/10.1038/nature16057
- Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 2008; 197(8):1079-81; PMID: 18419525; http://dx.doi.org/10.1086/533452
