ABSTRACT
As obligate intracellular parasites, viruses rely completely on host metabolic machinery and hijack host nutrients for viral replication. Newcastle disease virus (NDV) causes acute, highly contagious avian disease and functions as an oncolytic agent. NDV efficiently replicates in both chicken and tumour cells. However, how NDV reprograms host cellular metabolism for its efficient replication is still ill-defined. We previously identified a significantly upregulated glutamate transporter gene, solute carrier family 1 member 3 (SLC1A3), during NDV infection via transcriptome analysis. To investigate the potential role of SLC1A3 during NDV infection, we first confirmed the marked upregulation of SLC1A3 in NDV-infected DF-1 or A549 cells through p53 and NF-κB pathways. Knockdown of SLC1A3 inhibited NDV infection. Western blot analysis further confirmed that glutamine, but not glutamate, asparagine, or aspartate, was required for NDV replication. Metabolic flux data showed that NDV promotes the decomposition of glutamine into the tricarboxylic acid cycle. Importantly, the level of glutamate and glutaminolysis were reduced by SLC1A3 knockdown, indicating that SLC1A3 propelled glutaminolysis for glutamate utilization and NDV replication in host cells. Taken together, our data identify that SLC1A3 serves as an important regulator for glutamine metabolism and is hijacked by NDV for its efficient replication during NDV infection. These results improve our understanding of the interaction between NDV and host cellular metabolism and lay the foundation for further investigation of efficient vaccines.
Introduction
Newcastle disease virus (NDV), an enveloped single stranded negative sense RNA virus, is a member of the Paramyxoviridae family [Citation1] and causes an acute, highly contagious disease. Animals susceptible to this disease include mainly chickens and turkeys. NDV infection leads to high morbidity in chickens and heavy losses to the poultry industry [Citation2,Citation3]. Glutamine is a nonessential amino acid, second only to glucose in terms of energy anaplerosis and biomass synthesis. The conversion of glutamine to α-ketoglutarate (α-KG) via glutamate serves as a route for entry of glutamine-derived carbons into the tricarboxylic acid (TCA) cycle [Citation4]. During the glutamine catabolism, glutamine can be converted into α-KG, which participates as a supplementary substrate in the TCA cycle. In addition, α-KG and aspartate/alanine can be converted into glutamate and oxaloacetic acid/pyruvate by glutamic-oxaloacetic transaminase 1, 2 (GOT1, GOT2) and alanine aminotransferase (ALT) [Citation5]. Glutamine can contribute nitrogen atoms; thus, it can also be used in the synthesis of glutathione, ammonia, and purines. In many cancer cells, glutamine is often utilized as a substrate to replenish the TCA cycle, given that the carbon source of glucose is used to produce lactic acid and fatty acids [Citation6]. Some studies have confirmed that glutamine is required for viral replication. The production of poliovirus virion in HeLa cells decreased in a medium free of glucose and glutamine, and reverted to normal levels after the medium was supplemented with glutamine [Citation7]. In 2008, Munger et al. have reported for the first time that glutamine uptake is markedly upregulated after human cytomegalovirus (HCMV) infection [Citation8]. Subsequently, in the absence of glutamine, ATP levels and viral production could be rescued after supplementation with TCA cycle [Citation9]. Some studies reported that vaccinia virus (VACV) relies on glutamine extracellular uptake or catabolism to ensure its replication in host cells [Citation10–12]. A global metabolomic screen of VACV-infected primary HFF cells showed that glutamate and glutamine levels increased. The lack of glutamine in growth media has significantly reduced the production of infectious virus particles [Citation10]. These data suggest that when glucose is diverted to produce lactic acid, the virus upregulates glutamine catabolism in infected cells, which supports the survival and replication of the virus. Amino acids are the basic units for the synthesis of proteins and peptides. They play vital roles during the processes of cell biology and contribute to the production of bioactive molecules, which participate in the regulation of cell signalling transduction and metabolism [Citation13–15]. Therefore, maintaining amino acid homoeostasis is essential for cell survival. There are three key factors which regulate homoeostasis in cells: (1) entry and exit through amino acid transporters (AATs); (2) biosynthesis of amino acids in cells; and (3) recycling of damaged organelles and protein degradation [Citation16,Citation17]. Solute carrier family 1 member 3 (SLC1A3), also known as EAAT1, a sodium-dependent glutamate/aspartate transporter 1, is a member of the high-affinity glutamate transporters [Citation18]. Glutamate transporters are critical to the termination of excitatory neurotransmission and the supply of glutamate to cells for metabolic processes [Citation18,Citation19]. In addition to astrocytes, SLC1A3 can be expressed in peripheral organs, indicating its metabolic significance beyond the central nervous system. SLC1A3 mediates the cellular transport and regulation of glutamate/aspartate and can also remove extracellular glutamate [Citation20]. Ralphe et al. reported that SLC1A3 is also localized in the mitochondrial membrane, as a glutamate carrier, to facilitate the malate/aspartate shuttle [Citation21,Citation22].
Few studies have been reported on the host metabolism of NDV. Lipid metabolism was significantly affected in NDV-infected black-bone chickens. In the early stage of NDV infection, lipogenesis was inhibited, whereas lipolysis was strengthened [Citation23]. Moreover, NDV replication can be inhibited by disturbing cholesterol homoeostasis [Citation24]. Our previous results showed that NDV had elevated glucose uptake and glycolysis in host cells [Citation25]. In addition, amino acid metabolism was significantly altered during NDV infection, but the detailed mechanism has not been elucidated [Citation26]. Regarding the important role of AATs in the homoeostasis of amino acid metabolism, we speculated that they might be involved with NDV replication. In this study, we found that SLC1A3 was required for effective infectious NDV production. SLC1A3 also contributed to the production of glutamate and to the maintenance of the TCA cycle. Moreover, glutamine was necessary for NDV replication. Our findings will help to understand the demand for amino acids and how to regulate the host cell amino acid metabolism during NDV replication, which may provide novel clues and directions for future research.
Materials and methods
Cells and viruses
DF-1, 293T, and A549 cell lines were purchased from ATCC (USA). These cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher Scientific, Waltham, MA, USA) or F12K with 10% foetal bovine serum (FBS, Thermo Fisher Scientific, Waltham, MA, USA) at 37°C with 5% CO2.
NDV strains Herts/33 (a standard NDV virulent strain) and LaSota (an attenuated strain) were obtained from the China Institute of Veterinary Drug Control (Beijing, China). NDV strain SH15 (a moderately virulent strain) was isolated and preserved by our laboratory in 2015 (GenBank accession number: KY247177). These viruses were proliferated in 10-day-old specific-pathogen free embryonic chicken eggs and viral titres were determined as 50% of the tissue culture infective dose (TCID50), as described previously [Citation27].
Antibodies and reagents
An antibody against NDV nucleoprotein (NP) was produced and preserved in our laboratory. Antibodies against β-actin, p-p53, p53, p21, p-IκBα, IκBα, LAMP1, Na,K-ATPase, and COX IV were purchased from Cell Signalling Technology (Danvers, MA, USA). Anti-SLC1A3 antibody (NB100–1869) was purchased from NOVUS Biotechnology (Centennial, CO, USA).
Lipofectamine 2000, CellMask™ Green Plasma Membrane Stain (C37608), MitoTracker™ Deep Red FM (M22426), and Lysosome Enrichment Kit (89839) were purchased from Invitrogen Thermo Fisher Scientific (Waltham, MA, USA). DAPI (C1002), Enhanced Cell Counting Kit-8 (C0042), and Cell Mitochondria Isolation Kit (C3601) were purchased from Beyotime Biotechnology (Shanghai, China). The siRNA was synthesized by GenePharma Co., Ltd (Shanghai, China). SYBR Green qPCR Mix (p2092) was purchased from Dongsheng Biotech (Guangzhou, China). Enhanced chemiluminescence reagent kit was purchased from Share-Biotechnology (Shanghai, China). Plasma Membrane Protein Isolation and Fractionation Kit was purchased from Invent Biotechnology (Beijing, China). TFB-TBOA, an inhibitor of glutamate transporter, was purchased from Tocris Bioscience (Shanghai, China). The inhibitors, PFTα, BAY 11–7082, and chloroquine (CQ), were purchased from Selleck (Houston, TX, USA).
Quantitative real-time RT-PCR (qRT-PCR)
qRT-PCR was performed as described previously [Citation28]. Total RNAs from cell samples were extracted and reverse transcribed to cDNA. The primers used in this study are listed in Table S1. All experiments were performed in triplicate. The data were analysed using the comparative threshold cycle (2−ΔΔCT) method [Citation29].
Western blot and immunofluorescence
Western blot analysis was performed as described previously [Citation30]. Cells were treated with 200 μL of lysis buffer for 15 min and denatured in a 100°C water-bath for 5 min. Samples were then subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Finally, the bands were visualized using an enhanced chemiluminescence substrate kit.
Immunofluorescence assay was performed as described previously [Citation31]. A549 cells were inoculated on coverslips and infected with NDV at 1 MOI for 18 h. A549 cells were fixed in 4% paraformaldehyde for 10 min, and incubated with the primary antibody for 1 h at 37°C. After washing with PBS, the cells were incubated with the secondary antibody for 45 min. The cell nuclei were stained with DAPI (Beyotime, Shanghai, China) for 10 min. Lastly, the coverslips were observed using an LSM 880 Zeiss confocal microscope.
siRNA transfection and construction of SLC1A3 knockdown cells
The siRNA targeting human SLC1A3 (si-A3), and the nontargeting siRNA control (si-NC) were provided by GenePharma (Shanghai, China) and transfected as described previously [Citation30]. The si-SLC1A3 sense sequence was: 5′-GCUUGUGGGUGCUGUGAUATT-3′, and the si-SLC1A3 antisense sequence was: 5′-UAUCACAGCACCCACAAGCTT-3′. The si-NC sense sequence was: 5′-UUCUCCGAACGUGUCACGUTT-3′, and the si-NC antisense sequence was: 5′-ACGUGACACGUUCGGAGAATT. A short hairpin RNA targeting SLC1A3 (shSLC1A3, 5′-CGACAGTGAAACCAAGATGTA-3′) and a negative control (Control, 5′-TTCTCCGAACGTGTCACGT-3′) were synthesized by Sangon Biotech (Shanghai, China). To generate an SLC1A3 stable knockdown cell line, A549 cells were infected with lentiviral shRNA for SLC1A3 (shSLC1A3) and selected using puromycin as described previously [Citation32].
Amino acid quantitative analysis and targeted metabolomics analysis
NDV Herts/33 particles were obtained using a red blood cell absorption method as described previously [Citation33] for amino acid profiling. The cells (A549/DF-1) were washed using precooled phosphate-buffered saline (PBS), and the cell pellets were collected by centrifugation (4°C, 12,000 g for 10 min). After freeze-drying, the cell samples were dissolved in sterile water and then stored at −80°C. The resulting supernatant was extracted with 900 μL of methanol/acetonitrile/water (2:2:1, v/v/v), followed by incubation at −20°C for 1 h and centrifugation (4°C, 12,000 g for 15 min) for liquid chromatography – mass spectrometry detection. Pingli He Laboratory of China Agricultural University (Beijing, China) assisted with the amino acid profile analysis. For metabolomic analysis, the cell samples were obtained as described previously [Citation26]. Cells (A549/DF-1) were infected with NDV Herts/33 at 1 MOI. There were approximately 1 × 107 cells per sample. After freezing in liquid nitrogen, samples were stored at −80°C until metabolomic analysis.
Stable isotope tracing
A549 cells seeded in 100 mm dishes (5 × 106 cells/dish) were grown to 80% and were infected with NDV (Herts/33, MOI = 1). After washing with PBS, cells were incubated in DMEM with 10% dialysed FBS and [U-13C5]-glutamine. After 18 h of viral infection, cells were rinsed with 5% mannitol at room temperature. After adding ice-cold methanol (60%), the cells were frozen in liquid nitrogen for 30 s. The cell suspension was extracted using 900 μL of precooled methanol/acetonitrile/water (40:40:20, v/v/v), followed by incubation at −20°C for 1 h. Samples were then dried under vacuum and redissolved with 50 μL of acetonitrile/water (50:50, v/v) until LC-MS/MS analysis.
Nutrient deprivation studies
DMEM (Cat# D5030, Sigma-Aldrich, St. Louis, MO, USA) was used as the basic medium. The formula of 1 L complete medium was as follows: 8.3 g basic medium, 1.0 g glucose, 0.584 g L-glutamine, 3.7 g sodium bicarbonate, 2 mM glutamine, 0.2 mM glutamate, 0.2 mM aspartate and 0.2 mM asparagine. An incomplete medium was prepared according to the need without the addition of the corresponding nutrients.
Statistical analysis
GraphPad Prism 7.0 software was used for the statistical analyses. Values of p < 0.05 were considered statistically significant. Data are presented as the mean ± standard error of the mean. Significance in all figures is denoted as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Results
NDV infection induced reprogramming of host amino acid anabolism
NDV infection induced changes in host metabolism [Citation23,Citation25], and altered multiple metabolite contents and metabolic pathways in host cells, and most of which are involved in the amino acid metabolism [Citation26]. To confirm whether amino acid metabolism was affected in host cells after NDV infection, we quantitatively analysed amino acids in NDV-infected DF-1 and A549 cells. NDV infection altered the metabolism of 15 amino acids, among which 14 amino acids were upregulated and only L-Ala was downregulated, in DF-1 cells compared with mock-infection cells (). Under the same conditions, similar changes to the amino acid metabolism were observed in the NDV Herts/33-infected A549 cells (), demonstrating that mRNA levels of most amino acids, including aspartate and glutamate, were increased after NDV infection. Furthermore, the amino acid profile analysis indicated that proportions of glutamate and aspartate were higher than that of other amino acids in NDV-infected DF-1 and A549 cells ( and ()). Moreover, amino acid profile analysis of purified NDV Herts/33 particles indicated a similar composition and proportion of amino acids to NDV-infected DF-1/A549 cells, with the highest proportion of glutamic acid (). These results suggested that NDV infection induced reprogramming of host amino acid anabolism to benefit its own replication.
Figure 1. NDV infection induced amino acid metabolic reprogramming in host cells to match composition of NDV particles. (a-b) Changes to amino acid metabolism in NDV-infected DF-1 cells (a) and A549 cells (b). DF-1/A549 cells were infected with NDV Herts/33 at 1 MOI, then harvested at 12 h.p.i. For metabolomic analyses. (c-e) Amino acid profile analysis of DF-1 cells (c), A549 cells (d), and NDV Herts/33 particles (e). DF-1/A549 cells were washed with ice-cold PBS, and the supernatant was centrifugated. After freeze-drying, DF-1/A549 cell samples were dissolved in sterile water. NDV Herts/33 particles were prepared using a red blood cell absorption method as described previously [Citation33]. These samples were then subjected to amino acid profile analysis.
![Figure 1. NDV infection induced amino acid metabolic reprogramming in host cells to match composition of NDV particles. (a-b) Changes to amino acid metabolism in NDV-infected DF-1 cells (a) and A549 cells (b). DF-1/A549 cells were infected with NDV Herts/33 at 1 MOI, then harvested at 12 h.p.i. For metabolomic analyses. (c-e) Amino acid profile analysis of DF-1 cells (c), A549 cells (d), and NDV Herts/33 particles (e). DF-1/A549 cells were washed with ice-cold PBS, and the supernatant was centrifugated. After freeze-drying, DF-1/A549 cell samples were dissolved in sterile water. NDV Herts/33 particles were prepared using a red blood cell absorption method as described previously [Citation33]. These samples were then subjected to amino acid profile analysis.](/cms/asset/e6a6eb5b-5bf0-4f25-ae70-bbfc3d950a2f/kvir_a_2112821_f0001_b.gif)
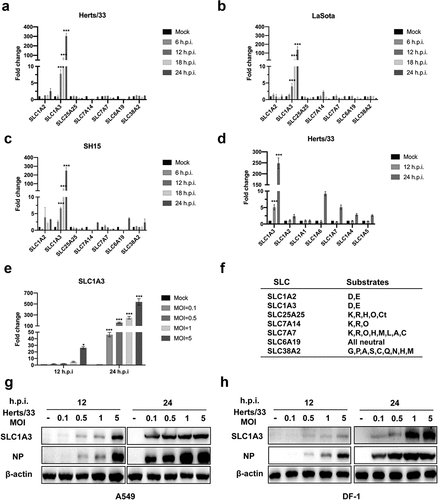
NDV infection induced significant upregulation of SLC1A3
AATs are membrane-bound transport proteins that participate in amino acid transport between cells and cellular organelles. AATs are divided into cationic, anionic, and neutral AATs according to different transport substrates and mechanisms [Citation16,Citation34]. Our previous results showed that NDV significantly upregulated of SLC1A3 during infection by transcriptome (data not shown). To further investigate the potential role of AATs in amino acid homoeostasis of NDV-infected cells, we harvested cells at 6, 12, 18, and 24 h after NDV infection (Herts/33, LaSota and SH15 strains). The mRNA levels of various AATs (i.e. SLC1A2, SLC1A3, SLC25A25, SLC7A14, SLC7A7, SLC6A19, and SLC38A2) were analysed using qRT-PCR. SLC1A3 was distinctly increased in Herts/33-infected A549 cells (). Furthermore, both LaSota and SH15 strains upregulated SLC1A3 ( and ()). Solute carrier family 1 (SLC1) consists of five high-affinity glutamate transporters, namely SLC1A1, SLC1A2, SLC1A3, SLC1A6, and SLC1A7, as well as two neutral amino acid transporters, SLC1A4 and SLC1A5. We further analysed whether these seven transporters were affected during NDV infection (). The results showed that the mRNA levels of SLC1A3 were significantly enhanced after NDV infection. SLC1A3 expression in A549 cells infected with NDV at 0.1, 0.5, 1 and 5 MOI was upregulated in a dose-dependent pattern (). The enhanced expression of SLC1A3, a glutamate and aspartate transporter, is essential in maintaining cell viability [Citation4]. We considered the possibility that the transport of glutamate and aspartate by SLC1A3 adapted to the stimulation induced by viral infection, which is beneficial to viral replication. With further verification, this result was confirmed in other cell lines, similar to H1299, HeLa, LoVo, HuH-7, HN13, CAL27, DF-1, and CEF samples harvested at different time points and analysed using qRT-PCR (Fig. S1). We further examined the protein levels of SLC1A3 and NDV NP in A549 () and DF-1 () cells after Herts/33 infection. Clearly, the protein levels of SLC1A3, induced by NDV infection at various doses, displayed a similar pattern to the mRNA levels of SLC1A3. The expression levels of SLC1A3 in mock-infected A549/DF-1 cells were considerably low or undetectable. These results demonstrated that NDV infection upregulated SLC1A3 expression to enhance the transport of glutamate and aspartate, which may promote viral replication in host cells.
Figure 2. NDV infection induced significant SLC1A3 upregulation. (a-c) A549 cells were harvested at 6, 12, 18 and 24 h.p.i. after NDV infection (Herts/33 or LaSota or SH15), and non-infected cells were used as the control. The mRNA levels of different amino acid transporters (SLC1A2, SLC1A3, SLC25A25, SLC7A14, SLC7A7, SLC6A19, SLC38A2) were determined using qRT-PCR. β-actin was used as an internal reference gene. (d) A549 cells were harvested at 12 and 24 h.p.i. after NDV infection (Herts/33, MOI=1). The mRNA levels of the SLC1 family were detected after NDV infection in A549 cells. (e) A549 cells were harvested after NDV infection (Herts/33, MOI=0.1, 0.5, 1, 5) and were used to detect the mRNA levels of SLC1A3. (f) Amino acid transporters and their substrates. (g-h) the expression levels of SLC1A3 were detected in A549/DF-1 cells post-NDV infection.
SLC1A3 was involved in NDV replication
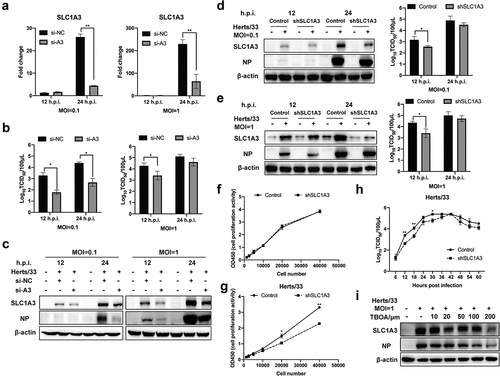
To further study the role of SLC1A3 during NDV replication in host cells, we effectively knocked down SLC1A3 expression using siRNA. SLC1A3 levels and NDV replication were detected by qRT-PCR and western blot, respectively. As expected, the mRNA and protein expression levels of SLC1A3 in si-A3 cells were markedly inhibited compared with the si-NC control cells ( and ()). By silencing SLC1A3, expression of NDV NP in the cells, and the titres of NDV in the cell supernatant were both decreased ( and ()). SLC1A3 knockdown A549 cells (shSLC1A3) were successfully constructed by using the shRNA and lentivirus packaging system. Western blot analysis and qRT-PCR confirmed the reduction in shSLC1A3 cells. We then evaluated whether SLC1A3 knockdown affected NDV replication. In shSLC1A3 cells, the NP protein level and the viral titres in the cell supernatant were significantly decreased at 12 hours post infection (h.p.i.) () and ()). In addition, SLC1A3 could transport glutamate and aspartate, which may be closely related to cell metabolism. We also investigated the cell proliferation activity of control and shSLC1A3 cells. Interestingly, we observed that SLC1A3 knockdown significantly decreased the proliferative activity of the host cell after NDV infection. However, no significant difference was observed between knockdown and uninfected cells ( and ()). Subsequently, to further validate the significance of SLC1A3 in NDV replication, we distinguished the replication curve of Herts/33 in the control and shSLC1A3 cells (). The results showed that the knockdown of SLC1A3 notably impaired viral infection, and the difference was significant at 12, 18, and 30 h.p.i., indicating the complex role of SLC1A3 during NDV infection. Moreover, after treatment with TFB-TBOA, a glutamate transporter inhibitor, the expression levels of SLC1A3 and viral NP, were decreased (). Taken together, these results suggested that NDV-mediated SLC1A3 upregulation benefited NDV replication in A549 cells.
Figure 3. NDV replication level was decreased after knocking down SLC1A3. (a) A549 cells were transfected with siRNA (si-A3 and si-NC) followed by NDV (Herts/33, MOI= 0.1/1) infection, and cells were harvested to determine the effect of SLC1A3 siRNA using qRT-PCR. (b, c) After treatment with siRNA, NDV-infected A549 cell samples and supernatant were harvested for TCID50 measurement of NDV titers, and the cells were subjected to western blot analysis of the SLC1A3 and NP expression. (d, e) A549 cells (Control and shSLC1A3) were infected with NDV (Herts/33, MOI=0.1/1), and cell samples and supernatant were harvested for western blot and TCID50 detection. (f, g) Cell proliferation activity of A549 cells (Control and shSLC1A3) (F) and cells infected with NDV Herts/33 (MOI=0.1) (g) were measured using the Enhanced Cell Counting Kit-8. (H) the growth curve of NDV in A549 cells (Control and shSLC1A3). Cells were infected with NDV Herts/33 (MOI=0.1). Supernatants were harvested at the indicated times and were subjected to the TCID50 assay. (I) After treatment with TFB-TBOA, a glutamate transporter inhibitor, cells infected with NDV (Herts/33, MOI=1) were harvested at 12 h for western blot detection.
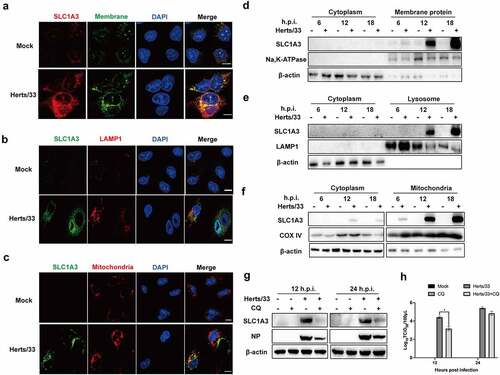
SLC1A3 was located on cell membranes, mitochondria, and lysosomes
Previous studies have reported that AATs can be found in intracellular compartments (vesicular, lysosome, mitochondria, etc.) or the plasma membrane [Citation16,Citation21]. Therefore, they can promote the absorption, excretion, and exchange of amino acids. Considering the low or undetectable level of SLC1A3 in uninfected A549 cells, localization of SLC1A3 was identified following NDV infection. Colocalization of SLC1A3 on the cell membrane was confirmed using immunofluorescence in infected cells (). Furthermore, SLC1A3 was also specifically located on the mitochondria and lysosomes ( and ()). The fluorescence of SLC1A3 in infected A549 cells was greater than that in uninfected cells. To further verify the location of SLC1A3, A549 cells underwent plasma membrane protein isolation, lysosome enrichment, and cell mitochondria isolation followed by NDV infection. The proteins of Na, K-ATPase, COX IV, and LAMP1 proteins were selected as markers for western blot analysis, respectively. As shown in , the signals of these three marker proteins were strong, indicating that the extraction effect was good, and the target and cytoplasmic components were clearly distinguished. As expected, SLC1A3 was detected in the components of membrane proteins, mitochondria, and lysosomes, but not in the cytoplasmic components () –()). In NDV-infected samples, the expression levels of SLC1A3 were noticeably higher, and SLC1A3 also gradually increased over time. Using immunofluorescence and western blot analysis, these results indicated that SLC1A3 was located on the cell membrane, mitochondria, and lysosomes, which contribute to glutamate uptake from media and cytoplasm. In addition, CQ treatment inhibited the expression levels of SLC1A3 and NP in NDV-infected A549 cells (). Viral titres in the cell supernatant were significantly decreased at 12 h.p.i after CQ treatment (). These findings suggest that SLC1A3 located on lysosomes was favourable for glutamate circulation and viral replication.
Figure 4. SLC1A3 was located on the cell membranes, mitochondria, and lysosomes. (a-c) A549 cells were infected with NDV (Herts/33, MOI=1) for 18 h, followed by western blot analysis. SLC1A3 was detected using anti-SLC1A3 antibody. Cell plasma membranes were stained green. Lysosomes were detected using anti-LAMP1 antibody and stained red. Mitochondria were stained red. Cell nuclei were stained blue. Scale bars: 10 μm. (d-f) NDV-infected A549 cell samples were harvested at 6, 12, and 18 h.p.i., then underwent plasma membrane protein isolation, lysosome enrichment, and cell mitochondria isolation. Expressions of Na, K-ATPase (d), LAMP1(e), and COX IV (f) in isolated samples were analyzed using western blot and antibodies against Na, K-ATPase, LAMP1 and COX IV. Expression levels of SLC1A3 and β-actin were also detected. (g, h) Treatment with CQ, a lysosome inhibitor, inhibited SLC1A3 and NDV NP expression (g) and decreased virus titers (H), as detected using western blot and TCID50 assay at 12 and 24 h.p.i.
Glutamine is essential for NDV replication
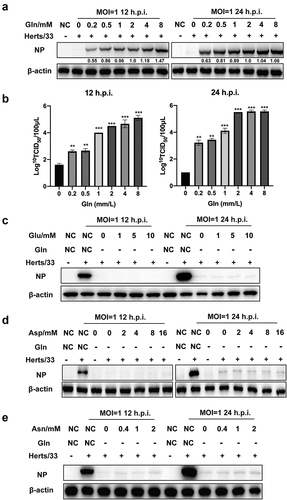
Given the important role of AATs in amino acid homoeostasis, some evidence showed that glutamine transporter SLC1A5 was increased in latent KSHV-infected endothelial cells and included increased glutamine uptake for glutaminolysis [Citation35]. We previously showed that SLC1A3 was involved in NDV replication, and aimed to determine if SLC1A3 affects the replication of NDV by regulating the transport of glutamate and aspartate. To determine if exogenous glutamate, aspartate, glutamine, or other nutritional components were required for A549 cells during NDV infection, we prepared alternative culture media for the experiment as follows: a complete culture medium (NC); media that only lacked glutamine, glutamate, asparagine, aspartate, or glucose; and media that only contained glutamine, glutamate, asparagine, or aspartate. We observed that cells in media containing no glucose could not grow or had died, thereby hindering NDV replication. However, cells grew well in other incomplete culture media (). Therefore, glucose was added to all culture media for all subsequent experiments. We then used western blotting to analyse the replication levels of NDV in cells grown in media lacking a certain nutrient component. The results showed that NDV replication was inhibited only when the culture medium lacked glutamine, whereas no significant effect was observed when other nutrients were absent compared with that in the NC (). To verify this result, we used the incomplete culture medium containing only a single nutrient component for cells infected with NDV. We found that NDV successfully replicated only in the culture medium with glutamine (). These results indicated that glutamine contributed to NDV replication. Besides, the culture media with different concentrations of glutamine were prepared for the next experiment. Following NDV infection, the supernatant and cell samples were harvested for TCID50 and western blot analysis. The results showed that there was in a dose-dependent manner between NDV replication and glutamine concentration within the limits, as shown in and (). Under glutamine starvation, the NDV replication level could not be rescued after supplementation with high concentrations of glutamate, aspartate, and asparagine ( –()). These results showed that glutamine was essential and irreplaceable for successful NDV replication.
Figure 5. Detection of NDV replication levels after treatment with different culture media. (a) Microscopic observation of NDV-infected A549 cells in different culture media at 18 h.p.i. Cells were infected with NDV (Herts/33, MOI=1) and incubated at 37°C with 5% CO2 for 1 h. Thereafter, the virus inoculum was removed by washing with PBS and were exposed to various culture media. Culture media were prepared as follows: complete culture medium (NC), and media that only lacked glutamine (−gln), glutamate (−glu), asparagine (−asn), aspartate (−asp), or glucose (−glc). (b) the replication level of NDV in different culture media. A549 cells were infected with NDV Herts/33 at 1 MOI in the absence of a certain nutrient component and cell samples were harvested at 12 and 24 h.p.i. For western blot analysis. (c) the NDV replication level in culture media containing only one nutrient (glutamine, glutamate, asparagine, or aspartate). A549 cells were infected with NDV Herts/33 at 1 MOI and cell samples were harvested at 12 and 24 h.p.i. For western blot analysis.
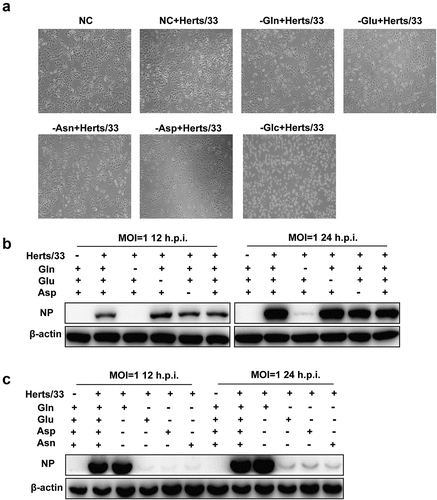
Figure 6. NDV replication relied on glutamine in a dose-dependent manner. (a, b) A549 cells were infected with NDV (Herts/33, MOI=1) for 1 h, and the supernatant was removed by washing with PBS. Thereafter, cells were exposed to culture media containing various glutamine concentrations at 1 h post infection, followed by western blot analysis and TCID50 assay. (c-e) A549 cells were infected with NDV (Herts/33, MOI=1) for 1 h and then were exposed to complete culture medium (NC), glutamine-free medium (−gln), or glutamine-free medium supplemented with various concentrations of glutamate (Glu), aspartate (Asp), and asparagine (Asn). Cells were then harvested for western blot analysis.
SLC1A3 was involved in the regulation of glutamine catabolism during NDV infection
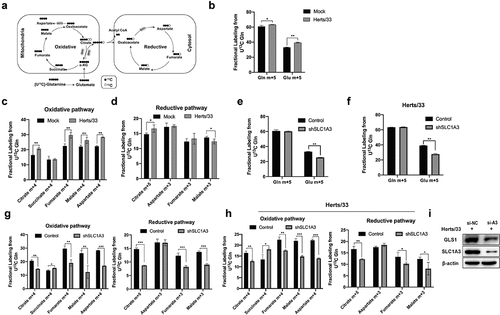
Our results showed that both SLC1A3 and glutamine played important roles in NDV replication. To investigate a possible synergistic effect between SLC1A3 and glutamine in NDV replication, we investigated NDV metabolism of glutamine by NDV and performed a metabolic flow analysis on U-13C5-labelled-glutamine cultured cells (). The labelled cells promoted the uptake of glutamine and catabolism to produce glutamate isotopologue [M + 5] under NDV infection (). Glutamine is known to serve as a crucial carbon source to replenish the TCA cycle and as a source of reduced nitrogen for some biological reactions [Citation36–38]. Metabolic flux data showed that NDV promotes the decomposition of glutamine into the TCA cycle, and the intermediate metabolites, including the isotopologues of citrate[M+4], fumarate [M+4], malate [M+4], and aspartate [M+4], were markedly higher than those in mock cells (). Glutamine is converted to glutamate after catalysis by glutaminase (GLS), and the derived α-KG can then be converted and catalysed by GLS. Thereafter, the derived α-KG can be converted to isocitrate and provide acetyl-CoA for lipid synthesis by NADP+/NADPH-dependent isocitrate dehydrogenase 1 in the cytoplasm. In the reductive pathway, the intermediate metabolites including citrate [M + 5] were noticeably higher than those in the mock group, which indicated that NDV infection also activated the reductive pathway (). These results showed that NDV infection disrupted glutamine metabolism and increased glutamine catabolism by activating both pathways. To further investigate the effect of SLC1A3 on glutamine metabolism regarding viral infection, we selected control and shSLC1A3 cells and evaluated the metabolic flow of U-13C5-labelled glutamine. Compared with control cells, shSLC1A3 cells displayed lower intracellular glutamate levels, suggesting that the SLC1A3 knockdown markedly reduced the ability of cells to generate glutamate (). A similar pattern was consistently observed after NDV infection () in both control and knockdown cells. We also examined changes to TCA cycle intermediates and glutamine metabolism pathways. shSLC1A3 cells contained lower levels of citrate, fumarate, malate, and aspartate compared to control cells, suggesting that the oxidative reaction and reductive reactions were weakened (). The results in the NDV-infected groups were consistent with those in the mock groups (). By silencing SLC1A3, GLS was decreased (). SLC1A3 was involved in the regulation of GLS, which may be used to promote glutaminolysis. Taken together, our data suggested that SLC1A3 contributed to glutamate production and maintenance of the TCA cycle, in agreement with the previous work indicating that SLC1A3 provides reducing equivalents to benefit the electron transport chain and TCA cycle [Citation39].
Figure 7. SLC1A3 was involved in glutamine catabolism regulation during NDV infection. (a) Schematic diagram of the metabolic flow of labeled carbon atoms in the TCA cycle. Black dots denote 13C atoms originating from uniformly U-13C5-labeled glutamine. (b) A549 cells infected with NDV (Herts/33, MOI=1) received complete culture medium labeled with U-13C5-labeled glutamine. At 18 h.p.i., glutamine and glutamate were analyzed via metabolic analysis. (c, d) TCA cycle intermediates were analyzed using LC-MS. (E-H) A549 cells (Control and shSLC1A3) were or were not infected with NDV (Herts/33, MOI=1) and received complete culture medium labeled with U-13C5-labeled glutamine. At 18 h.p.i., intracellular glutamine and glutamate were quantified by metabolic analysis (e, f). Stable isotopomer tracing analysis of U-13C5-labeled glutamine incorporation into reduction reactions and TCA cycle intermediates was conducted (G, H). (I) After treatment with siRNA, A549 cells infected with NDV (Herts/33, MOI=1) were harvested at 24 h.p.i. For western blot analysis. GLS1, SLC1A3, and β-actin were detected using corresponding antibodies.
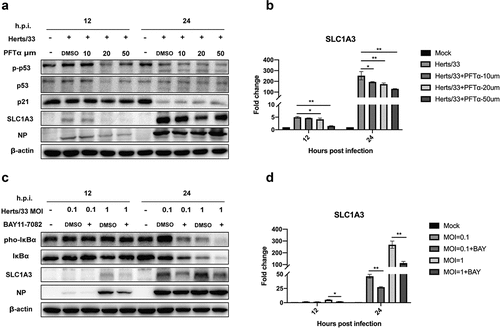
The NF-κB pathway plays a positive role in regulating SLC1A3 at the transcriptional level [Citation40,Citation41]. In addition, Tajan et al. demonstrated that SLC1A3 expression was induced by p53 [Citation38]. p53 increased the expression of SLC1A3 to promote cancer cell survival under glutamine starvation. We validated the two pathways using inhibitor treatments (). As shown in and (), levels of SLC1A3 were decreased slightly after treatment with inhibitors (PFTα and BAY11–7082) for 24 h. Additionally, the transcription levels of SLC1A3 decreased significantly ( and ()). Therefore, activation of the p53 or NF-κB pathway was related to the increase of SLC1A3 induced by NDV infection.
Figure 8. The expression levels of SLC1A3 decreased after treatment with p53 and NF-κB pathway inhibitors. (a, b) in the presence of the p53 inhibitor PFTα (10, 20, or 50 μM), A549 cells infected with NDV (Herts/33, MOI=1) were harvested for western blot analysis and qRT-PCR. Antibodies against phopho-p53, total p53, p21, SLC1A3, NDV-NP, and β-actin were used. The mRNA levels of SLC1A3 were analyzed using qRT-PCR. (c, d) Under BAY 11–7082 (10 μM) treatment, A549 cells infected with NDV (Herts/33, MOI=0.1, 1) were harvested for western blot and qRT-PCR analysis. Antibodies against phopho-IκBα, total IκBα, SLC1A3, NDV-NP, and β-actin were used. DMSO was used as a control. The mRNA levels of SLC1A3 were analyzed using qRT-PCR.
Discussion
Amino acid homoeostasis plays an important role during cell metabolism. Our results demonstrated that amino acid metabolism of DF-1 and A549 cells was reprogrammed after NDV infection. In this study, we determined that NDV-induced SLC1A3 upregulation benefited viral replication. We also showed that glutamine was essential for viral replication in cells. Glutamine is a vital nutrient that enables cancer cells to survive and grow [Citation42,Citation43]. Glutamine provides carbon and nitrogen for biosynthesis, energy, and redox homoeostasis [Citation44]. Some evidence has recently proved that viruses modify specific metabolic pathways to meet the requirements for viral replication [Citation8–10,Citation45,Citation46]. We found that NDV infection induced glutamine catabolism in A549 cells. Furthermore, increased glutamine catabolism and glutamate production were involved with SLC1A3 upregulation. This work provided some new information about the demand for amino acids during NDV replication and the interaction between NDV and glutamine metabolism in the host cells.
Our data showed that NDV infection caused reprogramming of amino acid metabolism in DF-1/A549 cells ( and ()), indicating that NDV could regulate the overall amino acid metabolism of infected cells to facilitate its own replication. The amino acids required for viral survival and replication are provided by infected cells; thus, the infected cells require reprogramming of amino acid metabolism to meet the needs of viral protein synthesis during viral replication. This concept was confirmed by the results of amino acid metabolism changes in NDV-infected cells. The amino acid profile in NDV-infected DF-1 and A549 cells showed high similarity to that of purified NDV particles, with glutamate and aspartate accounting for a larger proportion (–()).This suggests that NDV prefers glutamate and aspartate, and NDV infection reprogrammed cellular amino acid metabolism of the cells to benefit its own replication.
Starvation is one of several stress types occurring in all organisms. When the free amino acid pool is insufficient to support viral replication, it causes amino acid starvation in infected cells. All organisms have evolved various mechanisms to respond and adapt to starvation [Citation47]. Cells respond to amino acid starvation by inducing autophagy, which captures and degrades proteins and damaged organelles [Citation48,Citation49]. Our previous study showed that autophagy is triggered by NDV infection in U251 glioma cells and is beneficial for efficient viral replication [Citation50]. Recently, increasing evidence has shown that tumour cells need to upregulate certain AATs to meet their high demand for amino acids [Citation51,Citation52]. Therefore, we investigated the relationship between NDV infection and AATs. Our results revealed that AAT levels were upregulated in A549 cells infected with different strains of NDV, and SLC1A3 was significantly increased (p < 0.001; undefined –()). Further experiments on five members of the SLC1 family showed that only SLC1A3 was significantly upregulated after NDV infection, which occurred in a dose-dependent manner ( and ()). The upregulation of SLC1A3 caused by NDV infection was confirmed in other cell lines, such as H1299 and HeLa cells, etc. (Fig. S1). However, it is unknown whether other viral infections may also lead to SLC1A3 upregulation. After downregulating SLC1A3, the NDV replication level decreased (). Consistently, SLC1A3 knockdown significantly decreased the proliferative activity of host cells after NDV infection, whereas there was no significant difference between them in uninfected cells ( and ()). The results suggested that SLC1A3 knockdown may meet the basic growth needs of A549 cells. However, additional cellular metabolic resources are required after NDV infection and replication; thus, SLC1A3 may play a complex and important role in the preferential use of cellular metabolic resource for viral replication, resulting in the limited proliferation level of infected cells. These results suggested that SLC1A3 may contribute to the survival and proliferation of NDV-infected cells.
SLC1A3 was detected in the components of membrane proteins, mitochondria, and lysosomes, and it was upregulated in NDV-infected cells. (). However, we noticed that the LAMP1 levels were low at 12 and 18 h.p.i. in NDV-infected cells. We suggested that disrupted lysosome integrity was one of the reasons. When reactive oxygen species (ROS) levels are high, they can induce lysosomal membrane permeabilization, resulting in the translocation of cathepsins to the cytosol, inducing lysosomal-dependent cell death [Citation53]. A previous study showed that NDV infection induces elevated mitochondrial ROS and mitochondrial damage [Citation25], which may compromise lysosomal integrity.
Glutamine is a critical nutrient for cell metabolism, which viruses increase in hosts to support replication [Citation8,Citation45,Citation54]. Viruses may upregulate glutamine metabolism for efficient replication. Our recent results confirmed that glutamine is essential for NDV replication and thus could not be replaced (). Glutamine may provide carbon or nitrogen sources for the synthesis of viral proteins and nucleotides, which is necessary for viral replication. NDV infection may alter the metabolic pathway of glutamine, as well as increase of glutamine catabolism. Importantly, NDV replication supported by glutamine catabolism was regulated by SLC1A3, which contributed to the production of glutamate and maintenance of the TCA cycle (). Previous study showed that NDV infection induced energy metabolism dysfunction and electron transport chain (ETC) dysfunction [Citation25]. Therefore, increasing glutamine catabolism may promote ATP production and supply energy for viral replication. The mRNA levels of GLS, glutamine synthetase (GLUL), glutamate dehydrogenase (GLUD2), GOT1, GOT2 and ALT, which are related to glutamine metabolism, were also detected (Fig. S2). The results showed that the mRNA level of GLUL initially decreased, then increased, whereas GLS increased after NDV infection, suggesting that glutamine anabolism decreased, and catabolism increased. Furthermore, the mRNA levels of GLUD2, GOT1, GOT2 and ALT were also upregulated. These results confirmed that NDV infection significantly altered the glutamine metabolism in host cells.
Our results also indicated that the activation of p53 and NF-κB pathway activation was involved in increased SLC1A3 induced by NDV (). Kan et al. reported that NDV infection activated p53 in NDV-infected U251 cells and regulated glutathione metabolism [Citation55]. Glutathione is composed of glutamate, cysteine, and glycine, and plays critical roles in protecting cells from oxidative damage and maintaining redox homoeostasis. These results collectively suggest that p53 activation plays an important role in NDV-infected cells and the relationship between different metabolic pathways is complex and interrelated.
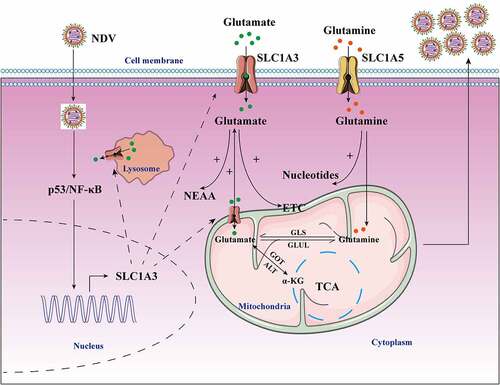
In summary, our results revealed that NDV infection distinctly altered the amino acid metabolism of infected cells, and NDV showed a preference for glutamate and aspartate. For the first time, our findings revealed that NDV infection enhanced the expression and function of SLC1A3, a glutamate/aspartate transporter, to facilitate viral replication. SLC1A3 upregulation increased glutamate uptake and transportation from media and cytoplasm to satisfy the needs of NDV replication, which may promote glutamine catabolism and glutamate production (). These results will improve our understanding of the demand for amino acids, how to regulate the host cell amino acid metabolism during NDV replication, and the effect of amino acid metabolism on NDV replication.
Figure 9. Schematic diagram of the molecular mechanism of NDV-mediated glutamine metabolism. NDV infection induced metabolic reprogramming in host cells to benefit viral protein synthesis and replication. NDV infection facilitated glutamate transport by increasing SLC1A3 expression on cell membranes, lysosomes, and mitochondria in infected cells by activating p53 and NF-κB pathways. The transportation of glutamate contributes to maintaining functionality of the TCA cycle and the electron transport chain, as well as providing energy for viral replication. Glutamate can also produce some nonessential amino acids. In addition, glutamine can also be used for viral nucleotide synthesis. All these factors provide favorable conditions for viral replication during NDV infection.
Supplemental Material
Download Zip (2.8 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request ([email protected]).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2022.2112821.
Additional information
Funding
References
- Ganar K, Das M, Sinha S, et al. Newcastle disease virus: Current status and our understanding. Virus Res. 2014;184:71–81.
- Alexander DJ, Aldous EW, Fuller CM The long view: A selective review of 40 years of Newcastle disease research. Avian Pathol. 2012;41:329–335.
- Brown VR, Bevins SN A review of virulent Newcastle disease viruses in the United States and the role of wild birds in viral persistence and spread. Vet Res. 2017;48:68.
- Kodama M, Oshikawa K, Shimizu H, et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nat Commun. 2020;11:1320.
- Sookoian S, Pirola CJ Liver enzymes, metabolomics and genome-wide association studies: From systems biology to the personalized medicine. World J Gastroenterol. 2015;21:711–725.
- Cluntun AA, Lukey MJ, Cerione RA, et al. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. 2017;3:169–180.
- Eagle H, Habel K The nutritional requirements for the propagation of poliomyelitis virus by the HeLa cell. J Exp Med. 1956;104:271–287.
- Munger J, Bennett BD, Parikh A, et al. Systems-Level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol. 2008;26:1179–1186.
- Chambers JW, Maguire TG, Alwine JC Glutamine metabolism is essential for human cytomegalovirus infection. J Virol. 2010;84:1867–1873.
- Fontaine KA, Camarda R, Lagunoff M Vaccinia virus requires glutamine but not glucose for efficient replication. J Virol. 2014;88:4366–4374.
- Greseth MD, Traktman P, Alwine JC De Novo fatty acid biosynthesis contributes significantly to establishment of a bioenergetically favorable environment for vaccinia virus infection. PLoS Pathog. 2014;10:e1004021.
- Mazzon M, Castro C, Roberts LD, et al. A role for vaccinia virus protein C16 in reprogramming cellular energy metabolism. J Gen Virol. 2015;96:395–407.
- Dai Z, Wu Z, Hang S, et al. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol Hum Reprod. 2015;21:389–409.
- Wu G Functional amino acids in nutrition and health. Amino Acids. 2013;45:407–411.
- Wu G, Bazer FW, Dai Z, et al. Amino acid nutrition in animals: Protein synthesis and beyond. Annu Rev Anim Biosci. 2014;2:387–417.
- Bröer S, Bröer A Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J. 2017;474:1935–1963.
- Galluzzi L, Kroemer G Amino acid deprivation promotes intestinal homeostasis through autophagy. Oncotarget. 2016;7:29877–29878.
- Kanai Y, Clémençon B, Simonin A, et al. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol Aspects Med. 2013;34:108–120.
- Kanai Y, Hediger MA The glutamate/neutral amino acid transporter family SLC1: Molecular, physiological and pharmacological aspects. Pflugers Arch. 2004;447:469–479.
- Arriza JL, Fairman WA, Wadiche JI, et al. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569.
- Ralphe JC, Segar JL, Schutte BC, et al. Localization and function of the brain excitatory amino acid transporter type 1 in cardiac mitochondria. J Mol Cell Cardiol. 2004;37:33–41.
- Ralphe JC, Bedell K, Segar JL, et al. Correlation between myocardial malate/aspartate shuttle activity and EAAT1 protein expression in hyper- and hypothyroidism. Am J Physiol Heart Circ Physiol. 2005;288:H2521–6.
- Renli Q, Chao S, Jun Y, et al. Changes in fat metabolism of black-bone chickens during early stages of infection with Newcastle disease virus. Animal. 2012;6:1246–1252.
- Sheng XX, Sun YJ, Zhan Y, et al. The LXR ligand GW3965 inhibits Newcastle disease virus infection by affecting cholesterol homeostasis. Arch Virol. 2016;161:2491–2501.
- Gong Y, Tang N, Liu P, et al. Newcastle disease virus degrades SIRT3 via PINK1-PRKN-dependent mitophagy to reprogram energy metabolism in infected cells. Autophagy. 2022;18(7):1503–1521.
- Liu P, Yin Y, Gong Y, et al. In vitro and in vivo metabolomic profiling after infection with virulent Newcastle disease virus. Viruses. 2019;12:11. DOI:10.3390/v12010011
- Römer-Oberdörfer A, Werner O, Veits J, et al. Contribution of the length of the HN protein and the sequence of the F protein cleavage site to Newcastle disease virus pathogenicity. J Gen Virol. 2003;84:3121–3129.
- Sun Y, Ding N, Ding SS, et al. Goose RIG-I functions in innate immunity against Newcastle disease virus infections. Mol Immunol. 2013;53:321–327.
- Schmittgen TD, Livak KJ Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108.
- Sun Y, Zheng H, Yu S, et al. Newcastle disease virus V protein degrades mitochondrial antiviral signaling protein to inhibit host type I interferon production via E3 Ubiquitin Ligase RNF5. J Virol. 2019;93. DOI:10.1128/JVI.00322-19.
- Ren S, Ur Rehman Z, Gao B, et al. ATM-Mediated DNA double-strand break response facilitated oncolytic Newcastle disease virus replication and promoted syncytium formation in tumor cells. PLoS Pathog. 2020;16:e1008514.
- Wu W, Qu Y, Yu S, et al. Caspase-Dependent cleavage of DDX21 suppresses host innate immunity. Mbio. 2021;12:e0100521.
- Yi J, Liu C Detecting Newcastle disease virus in combination of RT-PCR with red blood cell absorption. Virol J. 2011;8:202.
- Hyde R, Taylor PM, Hundal HS Amino acid transporters: Roles in amino acid sensing and signalling in animal cells. Biochem J. 2003;373:1–18.
- Sanchez EL, Carroll PA, Thalhofer AB, et al. Latent KSHV infected endothelial cells are glutamine addicted and require glutaminolysis for survival. PLoS Pathog. 2015;11:e1005052.
- Pavlova NN, Thompson CB The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47.
- DeBerardinis RJ, Chandel NS Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200.
- Altman BJ, Stine ZE, Dang CV From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–634.
- Tajan M, Hock AK, Blagih J, et al. A role for p53 in the adaptation to Glutamine starvation through the expression of SLC1A3. Cell Metab. 2018;28:721–36.e6.
- Karki P, Kim C, Smith K, et al. Transcriptional regulation of the Astrocytic Excitatory Amino Acid Transporter 1 (EAAT1) via NF-κB and Yin Yang 1 (YY1). J Biol Chem. 2015;290:23725–23737.
- Karki P, Hong P, Johnson J Jr., et al. Arundic acid increases expression and function of Astrocytic Glutamate Transporter EAAT1 via the ERK, Akt, and NF-κB Pathways. Mol Neurobiol. 2018;55:5031–5046.
- Vander Heiden MG, Cantley LC, Thompson CB Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033.
- Yuneva M, Zamboni N, Oefner P, et al. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105.
- Hensley CT, Wasti AT, DeBerardinis RJ Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678–3684.
- Vastag L, Koyuncu E, Grady SL, et al. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 2011;7:e1002124.
- Fontaine KA, Sanchez EL, Camarda R, et al. Dengue virus induces and requires glycolysis for optimal replication. J Virol. 2015;89:2358–2366.
- Mejlvang J, Olsvik H, Svenning S, et al. Starvation induces rapid degradation of selective autophagy receptors by endosomal microautophagy. J Cell Biol. 2018;217:3640–3655.
- Mizushima N, Komatsu M Autophagy: Renovation of cells and tissues. Cell. 2011;147:728–741.
- Kim KH, Lee MS Autophagy–a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10:322–337.
- Meng C, Zhou Z, Jiang K, et al. Newcastle disease virus triggers autophagy in U251 glioma cells to enhance virus replication. Arch Virol. 2012;157:1011–1018.
- Kaira K, Oriuchi N, Imai H, et al. L-Type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci. 2008;99:2380–2386.
- Lewerenz J, Hewett SJ, Huang Y, et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18:522–555.
- Wang F, Gómez-Sintes R, Boya P Lysosomal membrane permeabilization and cell death. Traffic. 2018;19:918–931.
- Thai M, Graham NA, Braas D, et al. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19:694–701.
- Kan X, Yin Y, Song C, et al. Newcastle-Disease-Virus-Induced ferroptosis through nutrient deprivation and ferritinophagy in tumor cells. iScience. 2021;24:102837.
