ABSTRACT
The G-protein coupled receptor (GPCR) signaling was long believed to involve activation of receptor exclusively at the cell surface, followed by its binding to heterotrimeric G-proteins and arrestins to trigger various intracellular signaling cascades, and termination of signaling by internalization of the receptor. It is now accepted that many GPCRs continue to signal after internalization in the endosomes. Since the breakthrough discoveries of nuclear binding sites for their ligands in 1980s, several GPCRs have been detected at cell nuclei. But mechanisms of nuclear localization of GPCRs, many of whom contain putative nuclear localization signals, remain poorly understood to date. Nevertheless, it is known that subcellular trafficking of GPCRs is regulated by members of Ras superfamily of small GTPases, most notably by Rab and Arf GTPases. In this commentary, we highlight several recent studies which suggest novel roles of small GTPases, importins and sorting nexin proteins in the nuclear translocation of GPCRs via vesicular transport pathways. Taken together with increasing evidence for in vivo functionality of the nuclear GPCRs, better understanding of their trafficking will provide valuable clues in cell biology.
Introduction
Nuclear envelope (NE) is made up of 2 bilayered phospholipid membranes, named outer (ONM) and inner (INM) nuclear membranes. The NE is pierced by multiprotein assemblies, nuclear pore complexes (NPCs), which control trafficking of biomolecules (greater than ∼40 kDa) in and out of the nucleus.Citation1 The ONM is contiguous with outer membrane of endoplasmic reticulum (ER) and the space between 2 nuclear membranes is connected to ER lumen.Citation1 The basic structure of NPC comprises ∼30 nucleoporins forming a ring-shaped central channel with 8 identical subunits.Citation2 The ultrastructural analysis of NPC reveals that it is made up of distinct classes of nucleoporins; each with specific localization and function. Interestingly, several scaffold nucleoporins, which form the NPC framework, share structural similarities with constituents of COP-II vesicles which are part of endomembrane trafficking pathway.Citation3 ONM and INM are known to inhabit different transmembrane (TM) proteins, including receptors, ion channels, and linker proteins.Citation4 The recent evidence suggests that various protein and non-protein components of the NE play diverse roles in regulation of gene expression.Citation5 The proteomic analysis also shows that the composition of NE varies among tissues.Citation6
G-protein coupled receptors (GPCRs), which form the largest family of TM proteins with more than 800 members in the human genome,Citation7 were believed to be exclusively functional at the plasma membrane (PM). Typically, the heterotrimeric G-proteins act as links between the GPCR at PM and its intracellular second messengers.Citation8 GPCRs are also well-known to signal via G-protein-independent pathways.Citation9 Many components of both signaling pathways are found or are translocated at the NE or within the nucleus.Citation10,Citation11 To date, more than 30 different GPCRs have been localized at the cell nuclei.Citation11 In addition to GPCRs, TM proteins of the receptor tyrosine kinase (RTK) family have also been detected at the nucleus.Citation12 The phospholipids in nucleoplasmCitation13 and the intranuclear invaginations of NECitation14 could harbor lipophilic TM domains of these receptors. The interactions between various nuclear receptors, including cross-talk between their second messengers, might play a role in the regulation of nuclear signaling cascades.
The origin of nuclear GPCRs
The process of exit of GPCRs from ER requires passing quality control mechanisms and may involve specific motifs present within GPCRs as well as the action of Rab GTPases.Citation15,Citation16 Because ONM is contiguous with ER membrane, it has been proposed that some resident NE proteins could travel by lateral diffusion.Citation17,Citation18 However, GPCRs often undergo post-translational modifications in both ER and trans-golgi network (TGN), including glycosylation, which has been implicated in trafficking of various GPCRs to the PM via vesicular transport.Citation15 The anterograde transport of GPCRs from TGN is also regulated by Rab and Arf GTPases.Citation19 Based on the immunoblot evidence, PM and nuclear GPCRs have similar molecular weights which is an indirect proof for glycosylation of nuclear GPCRs. This, however, does not rule out presence of an alternative pathway of glycosylation at the nucleus. It has been proposed that synthesis of NE proteins could take place at the nucleus itself.Citation20 As discussed below, some GPCRs undergo ligand-induced nuclear translocation from PM via importin-regulated trafficking.Citation21,Citation22 More research is needed to delineate between transport pathways of nuclear GPCRs originating from TGN or PM from those translated locally.
Current evidence for the involvement of small GTPases in localization of NE proteins is limited to Ran GTPase. The vesicular fusion has been a conserved mechanism necessary for reassembly of NE after mitosis in various eukaryotes and the activity of Ran GTPase is required for the process.Citation23,Citation24 Ran lacks CAAX membrane anchoring motif at its C-terminus, found in other members of Ras superfamily and is primarily involved in nucleocytoplasmic transport along with Karyopherins (discussed below).Citation25 The Ran GTPase also plays an important role in regulation of cell cycleCitation26 and in the trafficking of INM resident proteins. Many INM resident proteins contain nuclear localization signal (NLS).Citation27
GPCRs and Nuclear localization signal (NLS)
The classical monopartite NLS consists of small cluster of basic amino acids and was originally discovered in the simian virus 40 large-T antigen.Citation28 The bipartite NLS, on the other hand, consists of 2 clusters of basic amino acids which are separated by 10–12 residues.Citation29 Both NLSs are recognized by heterodimeric nuclear import receptor, composed of importin α and β. More recently, additional classes of NLSs, binding to different regions of importin α, have been identified.Citation30 Some of the earliest evidence for the presence of functional NLS in a GPCR was provided for agonist-induced internalization of rat angiotensin II type 1 (Agtr1b) receptor (307KKFKK311) in neurons.Citation31 In 2003, Lee and colleagues reported that several GPCRs, belonging to Rhodopsin-like receptor family, contain putative NLS which is located just after seventh transmembrane domain. An exception is the apelin receptor which contains a functional NLS in its third intracellular loop.Citation32 Human formyl peptide receptor 2 (FPR2) has been recently reported to contain a NLS in its 3rd intracellular loop (227KIHKK231) and has been localized at the nuclei in lung and gastric cancer cell lines.Citation33 Human cysteinyl leukotriene receptor 1 (CYSLTR1) but not CYSLTR2 contains a functional bipartite NLS at its C-terminus.Citation34 Our group identified presence of 2 monopartite NLS motifs (in first and third intracellular loops, respectively) in human F2R like trypsin receptor 1 (F2RL1), which is a member of protease activated receptor sub-family of class-A GPCRs.Citation21 The mutational disruption of these motifs revealed that both NLSs are necessary for agonist-induced nuclear translocation of the receptor from PM.Citation21 We first reported presence of putative monopartite NLS (298KKFRKH302) in the C-terminus of human platelet-activating factor receptor (PTAFR)Citation35 and recently showed that the NLS is not functional by its mutational disruption.Citation36 However, internalization motif between 311 and 330 amino acids, present at the C-terminus, is essential for nuclear translocation.Citation36 It is interesting to note that some GPCR ligands also contain NLS [e.g., parathyroid hormone-related protein].Citation37 Functional NLS has also been identified as being responsible for nuclear localization of non-GPCR receptors such as erb-b2 receptor tyrosine kinase 2 (also known as HER-2).Citation38
NLS and nuclear importins
As described earlier, classical NLSs (monopartite and bipartite) are recognized by the importin-α.β heterodimer. Both importins are members of Karyopherin (Kap) family of proteins. Out of 19 human Kapβs, 11 are involved in nuclear import.Citation39 To date, consensus NLSs have been identified only for importin (Imp) α.β (classical NLS) and transportin (PY-NLS) pathways.Citation39 The PY-NLS consists of weak consensus motifs and physical rules such as structural disorder, overall positive charge, which together identify transportin-mediated nuclear import cargos.Citation40 The role of importins in nuclear translocation of full-length F2RL1 is further evidenced by RNAi (small interfering RNA) mediated silencing of Impβ1 (along with Impα3 and Impα5) affecting nuclear localization of the receptor.Citation21 On the other hand, nuclear import of parathyroid hormone receptor 1 is regulated by its interaction with Impα1 and Impβ.Citation41 In case of PTAFR, a novel interaction between Rab11a GTPase and importin-5 (Imp5) is essential for nuclear translocation of the receptor.Citation36 To date, 3 GPCRs, 2 of which belong to chemokine-receptor family (CCR2 and CXCR4 receptors)Citation42,Citation43 and oxytocin receptor (Oxtr)Citation22 have been reported to undergo transportin 1 mediated nuclear translocation.
Protein interactions involving Rab GTPases and Importins
The trafficking of GPCRs between various cellular membranes is regulated by small GTPases of the Ras superfamily; especially members of the Rab and Arf families. These small GTPases control various steps of vesicular trafficking including cargo selection, vesicular budding from donor membrane, interaction with cellular motors, and docking of vesicle to the acceptor membrane.Citation44 Recently, evidence for presence of vesicles in the NE (in the space between ONM and INM) was provided for the nuclear export of herpes viral nucleocaspidCitation45 and it has been suggested that such a pathway might exist for endogenous TM nuclear proteins as well.Citation46 Some Rab GTPases link specific intracellular compartments to nuclear signaling via their effector proteins.Citation47 Others show direct nuclear localization. The latter category includes Rab24 which has been proposed to play a role in NE assembly and/or transport.Citation48 We found that Rab11a (along with Imp5) plays a role in agonist-independent nuclear translocation of PTAFR.Citation36 Rab11 effectors (known as family of Rab11 interacting proteins or Rab11 FIPs) are divided into 2 classes based on their sequence homology.Citation49 class-I FIPs are known to regulate endosomal recycling of TM proteins back to PM; while class-II FIPs participate in the regulation of cell division. class-II FIPs also interact with Arf6 GTPase.Citation50 Recent evidence suggests that membrane phosphoinositides are involved in recruiting Rab11 effectors to the intracellular membranes.Citation51 Whether Rab11a is involved directly or indirectly (via one of its effectors) in the nuclear translocation of PTAFR is unknown. Along similar lines, Rab23 has been recently reported to exist in a complex with transportin 1.Citation52 Finally, several members of Ras and Rho families of small GTPases contain putative NLS.Citation53 Small GTPases of RGK family are involved in cell shape remodeling by nuclear transport but underlying molecular mechanisms are unknown.Citation54
Role of endosomal sorting proteins in nuclear localization of GPCRs
The sortin nexins (SNXs) form another class of evolutionarily conserved eukaryotic proteins which contain Bin/Amphiphysin/Rvs (BAR) and phox homology (PX) domains. These domains are essential for interaction of SNX proteins with various biologic membranes.Citation55 Recent in vitro studies suggest that BAR domain proteins play a role in the regulation of cellular membrane curvature.Citation56 Snx1 is known to associate with C-terminal tails of many GPCRs.Citation57 The SNXs were first identified regulators of retromer-dependent endosomal trafficking but current evidence suggests for more diverse roles.Citation58 Snx6 enhances localization of Lamin-A at the NE.Citation59 Using confocal microscopy and subcellular fractionation, Zhu and colleagues reported that Snx10 shows nuclear localization in osteoblasts derived from human peripheral blood mononuclear cells as well as from RAW 264.7 mouse cell-line.Citation60 We found that, Snx11, which lacks BAR domain but contains an extended PX domain and shares the highest sequence homology with Snx10 among the sorting nexin family members, regulates endosomal sorting of F2rl1 (along with aforementioned importins) to the nucleus via trafficking through microtubule network.Citation21 The extended PX domain of Snx11 is essential for its function in vivo.Citation61 Moreover, it has been proposed that Snx10 contains the extended PX domain, based on its sequence alignment with Snx11.Citation61
Arrestins and GPCR trafficking
Roles of β-arrestins in the regulation of endocytic trafficking of agonist-induced phosphorylated GPCRs are reviewed elsewhere.Citation62 In addition, they can act as effector molecules in non-canonical GPCR signaling.Citation63 β-arrestins are also known to activate Arf6 GTPaseCitation64 and this process has been shown to control recycling of β2 adrenergic receptor.Citation65 Out of the 2 non-visual β-arrestins, only β-arrestin1 (arrestin2) shows nuclear localization, where it is involved in histone acetylation and control of gene transcription.Citation66 β-arrestin2 (arrestin3), on the other hand, is constitutively exported out of the nucleus as it contains leucine-rich nuclear export signal.Citation67 We found that C-terminal truncated F2rl1 fails to go to the nucleus. However, the mutational disruption of its C-terminal amino acid residues 363 and 366, which are required for arrestin interaction,Citation68 does not affect agonist-induced nuclear localization of F2rl1.Citation21 We speculate that agonist-induced binding of some other protein(s) to the C-terminus of F2rl1 might result in the receptor's conformational change to expose its aforementioned NLS motifs. On the other hand, RNAi mediated silencing of β-arrestin1/2 in osteoblasts has shown that they are required for expression of differentiation-inducing genes in the cells such as osterix (Sp7) and bone sialoprotein (Ibsp), a function reported to be mediated by nuclear Oxtr.Citation22 Conversely, mutational disruption of serine-rich clusters in the C-terminus of Oxtr (a common site of GPCR phosphorylation and required for arrestin recruitment) partially impairs its nuclear localization, without affecting the Erk phosphorylation.Citation22 How arrestin2 regulates Oxtr-induced gene expression in osteoblasts remains to be elucidated. In summary, the role of arrestin2 in governing localization of GPCRs to the nucleus varies according to the GPCR and thus entails additional interacting partners and/or their effect on conformational changes of the complex.
Agonist-dependent vs. -independent nuclear localization of GPCRs
Agonist-dependency for nuclear localization seems to differ according to GPCRs. Following agonist stimulation at the PM, the C-terminus of Frizzled 2 receptor gets cleaved and is translocated to the nucleus by importins (Impβ11 and Impα2).Citation69 Multiple full-length GPCRs with peptide ligands, such as F2rl1 and Oxtr, also need agonist-induced internalization via one of the endocytic pathways for their nuclear translocation.Citation31,Citation21 Valdehita and colleagues have shown that exogenous vasoactive intestinal polypeptide increases nuclear localization of vasoactive intestinal peptide receptor 1 (VIPR1, previously known as VPAC1) but not VIPR2 in human breast cancer cell-lines.Citation70 Others such as apelin receptor and bradykinin receptor B2 have been reported to show agonist-independent nuclear localization.Citation32,Citation71 However, there have been reports of autocrine apelin signaling in other cellular systems.Citation72 How endogenous apelin-APJ pathway might affect nuclear translocation of the receptor is unknown. The GPCRs with bioactive lipids as their ligands, like PTAFR and Lysophosphatidic acid receptor 1 seem to show their nuclear localization is not dependent of agonist stimulation.Citation35,Citation73 Along these lines, our latest work indicates that exogenous or endogenous ligand (platelet-activating factor) stimulation is not required for nuclear translocation of PTAFR in primary human retinal microvascular endothelial cells.Citation36 In PC12 cell line, endogenous nuclear localization of lysophosphatidic acid receptor 1 (Lpar1) is regulated by integrin signaling and this has been attributed to the action of Rho family of small GTPases.Citation74 Whereas sphingosine-1-phosphate receptor 1 (S1PR1) is reported to undergo agonist-induced nuclear translocation in human umbilical vein endothelial cells,Citation75 issue is further complicated by the fact that most phospholipid ligands can be synthesized locally at the nuclear membranes and these ligands are capable of transversing biomembranes. In case of the PTAFR, its ligand is mainly retained intracellularly in several cell types after synthesis.Citation76 Current understanding of possible mechanisms of nuclear translocation of GPCRs is summarized in and .
Figure 1. Subcellular GPCR trafficking occurs via vesicular transport mechanisms which are regulated by members of Ras superfamily of small GTPases. Upon binding to its ligand at PM, 1) full-length GPCRs (e.g., F2rl1 and oxytocin receptor) can undergo agonist-induced nuclear translocation by importins and sorting nexinsCitation21,Citation22 or 2) can be recycled back by recycling Rabs (e.g., Rab 4, 11) or 3) targeted for degradation by proteasomes/lysosomes, the process regulated by Rab 7 and sorting nexin 1., 4) In case of the frizzed 2 receptor, its intracellular C-terminus is cleaved off by the action of cellular proteases and only C-terminus is then translocated to nucleus by importins and Ran GTPase.Citation69 Lastly, agonist-independent translocation of GPCRs directly from TGN 5) and 6) is controlled by rab/arf GTPases and (in case of nuclear translocation 6)) importins as well (Rab11a and importin-5 in case of the platelet-activating factor receptor).Citation36 More research is needed to understand trafficking of GPCRs between nuclear membranes and their orientation of at the NE.
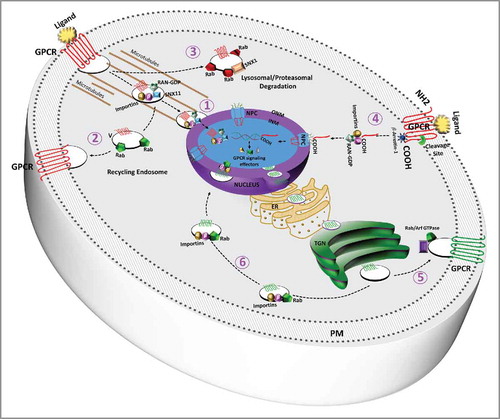
Table 1. Known mechanisms and receptor motifs required for nuclear translocation of GPCRs.
Future perspectives
GPCRs are prominent drug targets. It is of critical importance to know the origin and trafficking of nuclear receptors to be able to develop successful pharmacologic strategies to target them. It is improtant to note that in recent years, functional GPCRs have been localized at other intracellular compartments, such as endosomes and mitochondria.Citation85,Citation86 The charateristic subcellular distribution of small GTPases makes them attractive tools to modify intracellular GPCR trafficking and signaling. Moreover, studying spatio-temporal disctribution and interactions of proteins involved in the GPCR translocation will help to unravel their physiologic roles. These mechanisms appear to be depedent on the type of receptor as well as the cell-type.Citation11,Citation36 The trafficking of nuclear GPCRs is only part of the puzzle. The recent studies also indicate that nuclear GPCRs perform functions which differ from their plasma membrane counterparts, both in vitro and in vivo.Citation36,Citation21 New subcellular delivery systems such as nanoparticles might help to better understand the physiologic significance of nuclear GPCRs.Citation11
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from Canadian Institutes of Health Research (CIHR). V.K.B. was a recipient of CIHR Systems Biology studentship award at McGill University (Montréal, QC, Canada). S.C. is supported by grants from the CIHR, March of Dimes Birth Defects Foundation, Fonds de la Recherche du Québec—Santé (FRQS) — Vision Health Network. S.C. also holds a Canada Research Chair (Vision Science) and the Leopoldine Wolfe Chair in translational research in age-related macular degeneration.
References
- Harris JR. The biochemistry and ultrastructure of the nuclear envelope. Biochim Biophys Acta 1978; 515:55-104; PMID:346065; http://dx.doi.org/10.1016/0304-4157(78)90008-4
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. The molecular architecture of the nuclear pore complex. Nature 2007; 450:695-701; PMID:18046406; http://dx.doi.org/10.1038/nature06405
- Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol 2004; 2:e380; PMID:15523559; http://dx.doi.org/10.1371/journal.pbio.0020380
- Schirmer EC, Gerace L. The nuclear membrane proteome: extending the envelope. Trends Biochem Sci 2005; 30:551-8; PMID:16125387; http://dx.doi.org/10.1016/j.tibs.2005.08.003
- Arib G, Akhtar A. Multiple facets of nuclear periphery in gene expression control. Curr Opin Cell Biol 2011; 23:346-53; PMID:21242077; http://dx.doi.org/10.1016/j.ceb.2010.12.005
- Korfali N, Wilkie GS, Swanson SK, Srsen V, de Las Heras J, Batrakou DG, Malik P, Zuleger N, Kerr AR, Florens L, et al. The nuclear envelope proteome differs notably between tissues. Nucleus 2012; 3:552-64; PMID:22990521; http://dx.doi.org/10.4161/nucl.22257
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 2003; 63:1256-72; PMID:12761335; http://dx.doi.org/10.1124/mol.63.6.1256
- Hamm HE. The many faces of G protein signaling. J Biol Chem 1998; 273:669-72; PMID:9422713; http://dx.doi.org/10.1074/jbc.273.2.669
- Hur EM, Kim KT. G protein-coupled receptor signalling and cross-talk: achieving rapidity and specificity. Cell Signal 2002; 14:397-405; PMID:11882384; http://dx.doi.org/10.1016/S0898-6568(01)00258-3
- Zhu T, Gobeil F, Vazquez-Tello A, Leduc M, Rihakova L, Bossolasco M, Bkaily G, Peri K, Varma DR, Orvoine R, et al. Intracrine signaling through lipid mediators and their cognate nuclear G-protein-coupled receptors: a paradigm based on PGE2, PAF, and LPA1 receptors. Can J Physiol Pharmacol 2006; 84:377-91; PMID:16902584; http://dx.doi.org/10.1139/y05-147
- Joyal JS, Bhosle VK, Chemtob S. Subcellular G-protein coupled receptor signaling hints at greater therapeutic selectivity. Expert Opin Ther Targets 2015; 19:717-21; PMID:25976229; http://dx.doi.org/10.1517/14728222.2015.1042365
- Song S, Rosen KM, Corfas G. Biological function of nuclear receptor tyrosine kinase action. Cold Spring Harb Perspect Biol 2013; 5(7) pii: a009001; PMID:23818495; http://dx.doi.org/10.1101/cshperspect.a009001
- Maraldi NM, Mazzotti G, Capitani S, Rizzoli R, Zini N, Squarzoni S, Manzoli FA. Morphological evidence of function-related localization of phospholipids in the cell nucleus. Adv Enzyme Regul 1992; 32:73-90; PMID:1496925; http://dx.doi.org/10.1016/0065-2571(92)90009-O
- Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J Cell Biol 1997; 136:531-44; PMID:9024685; http://dx.doi.org/10.1083/jcb.136.3.531
- Duvernay MT, Filipeanu CM, Wu G. The regulatory mechanisms of export trafficking of G protein-coupled receptors. Cell Signal 2005; 17:1457-65; PMID:16014327; http://dx.doi.org/10.1016/j.cellsig.2005.05.020
- Saraste J. Spatial and functional aspects of ER-Golgi Rabs and tethers. Front Cell Dev Biol 2016; 4:28; PMID:27148530; http://dx.doi.org/10.3389/fcell.2016.00028
- Schindler M, Holland JF, Hogan M. Lateral diffusion in nuclear membranes. J Cell Biol 1985; 100:1408-14; PMID:3988794; http://dx.doi.org/10.1083/jcb.100.5.1408
- Ungricht R, Klann M, Horvath P, Kutay U. Diffusion and retention are major determinants of protein targeting to the inner nuclear membrane. J Cell Biol 2015; 209:687-703; PMID:26056139; http://dx.doi.org/10.1083/jcb.201409127
- Wang G, Wu G. Small GTPase regulation of GPCR anterograde trafficking. Trends Pharmacol Sci 2012; 33:28-34; PMID:22015208; http://dx.doi.org/10.1016/j.tips.2011.09.002
- Iborra FJ, Jackson DA, Cook PR. Coupled transcription and translation within nuclei of mammalian cells. Science 2001; 293:1139-42; PMID:11423616; http://dx.doi.org/10.1126/science.1061216
- Joyal JS, Nim S, Zhu T, Sitaras N, Rivera JC, Shao Z, Sapieha P, Hamel D, Sanchez M, Zaniolo K, et al. Subcellular localization of coagulation factor II receptor-like 1 in neurons governs angiogenesis. Nat Med 2014; 20:1165-73; PMID:25216639; http://dx.doi.org/10.1038/nm.3669
- Di Benedetto A, Sun L, Zambonin CG, Tamma R, Nico B, Calvano CD, et al. Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proc Natl Acad Sci U S A 2014; 111:16502-7; PMID:25378700; http://dx.doi.org/10.1073/pnas.1419349111
- Zhang C, Clarke PR. Chromatin-independent nuclear envelope assembly induced by Ran GTPase in Xenopus egg extracts. Science 2000; 288:1429-32; PMID:10827954; http://dx.doi.org/10.1126/science.288.5470.1429
- Askjaer P, Galy V, Hannak E, Mattaj IW. Ran GTPase cycle and importins alpha and beta are essential for spindle formation and nuclear envelope assembly in living Caenorhabditis elegans embryos. Mol Biol Cell 2002; 13:4355-70; PMID:12475958; http://dx.doi.org/10.1091/mbc.E02-06-0346
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol 1999; 15:607-60; PMID:10611974; http://dx.doi.org/10.1146/annurev.cellbio.15.1.607
- Kalab P, Weis K, Heald R. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science 2002; 295:2452-6; PMID:11923538; http://dx.doi.org/10.1126/science.1068798
- King MC, Lusk CP, Blobel G. Karyopherin-mediated import of integral inner nuclear membrane proteins. Nature 2006; 442:1003-7; PMID:16929305; http://dx.doi.org/10.1038/nature05075
- Kalderon D, Richardson WD, Markham AF, Smith AE. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature 1984; 311:33-8; PMID:6088992; http://dx.doi.org/10.1038/311033a0
- Robbins J, Dilworth SM, Laskey RA, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 1991; 64:615-23; PMID:1991323; http://dx.doi.org/10.1016/0092-8674(91)90245-T
- Kosugi S, Hasebe M, Matsumura N, Takashima H, Miyamoto-Sato E, Tomita M, Yanagawa H. Six classes of nuclear localization signals specific to different binding grooves of importin alpha. J Biol Chem 2009; 284:478-85; PMID:19001369; http://dx.doi.org/10.1074/jbc.M807017200
- Lu D, Yang H, Shaw G, Raizada MK. Angiotensin II-induced nuclear targeting of the angiotensin type 1 (AT1) receptor in brain neurons. Endocrinology 1998; 139:365-75; PMID:9421435
- Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, Chemtob S, George SR, O'Dowd BF. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem 2004; 279:7901-8; PMID:14645236; http://dx.doi.org/10.1074/jbc.M306377200
- Cattaneo F, Parisi M, Fioretti T, Sarnataro D, Esposito G, Ammendola R. Nuclear localization of Formyl-Peptide Receptor 2 in human cancer cells. Arch Biochem Biophys 2016; 603:10-9; PMID:27177968; http://dx.doi.org/10.1016/j.abb.2016.05.006
- Nielsen CK, Campbell JI, Ohd JF, Morgelin M, Riesbeck K, Landberg G, Sjölander A. A novel localization of the G-protein-coupled CysLT1 receptor in the nucleus of colorectal adenocarcinoma cells. Cancer Res 2005; 65:732-42; PMID:15705869
- Marrache AM, Gobeil F, Jr, Bernier SG, Stankova J, Rola-Pleszczynski M, Choufani S, Bkaily G, Bourdeau A, Sirois MG, Vazquez-Tello A, et al. Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J Immunol 2002; 169:6474-81; PMID:12444157; http://dx.doi.org/10.4049/jimmunol.169.11.6474
- Bhosle VK, Rivera JC, Zhou TE, Omri S, Sanchez M, Hamel D, Zhu T, Rouget R, Rabea AA, Hou X, et al. Nuclear localization of platelet-activating factor receptor controls retinal neovascularization. Cell Discov 2016; 2:16017; PMID:27462464; http://dx.doi.org/10.1038/celldisc.2016.17
- Lam MH, Briggs LJ, Hu W, Martin TJ, Gillespie MT, Jans DA. Importin beta recognizes parathyroid hormone-related protein with high affinity and mediates its nuclear import in the absence of importin alpha. J Biol Chem 1999; 274:7391-8; PMID:10066803; http://dx.doi.org/10.1074/jbc.274.11.7391
- Beguelin W, Diaz Flaque MC, Proietti CJ, Cayrol F, Rivas MA, Tkach M, Rosemblit C, Tocci JM, Charreau EH, Schillaci R, et al. Progesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 function as a coactivator of Stat3. Mol Cell Biol 2010; 30:5456-72; PMID:20876300; http://dx.doi.org/10.1128/MCB.00012-10
- Chook YM, Suel KE. Nuclear import by karyopherin-betas: recognition and inhibition. Biochim Biophys Acta 2011; 1813:1593-606; PMID:21029754; http://dx.doi.org/10.1016/j.bbamcr.2010.10.014
- Lee BJ, Cansizoglu AE, Suel KE, Louis TH, Zhang Z, Chook YM. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell 2006; 126:543-58; PMID:16901787; http://dx.doi.org/10.1016/j.cell.2006.05.049
- Pickard BW, Hodsman AB, Fraher LJ, Watson PH. Type 1 parathyroid hormone receptor (PTH1R) nuclear trafficking: association of PTH1R with importin alpha1 and beta. Endocrinology 2006; 147:3326-32; PMID:16574786; http://dx.doi.org/10.1210/en.2005-1408
- Favre N, Camps M, Arod C, Chabert C, Rommel C, Pasquali C. Chemokine receptor CCR2 undergoes transportin1-dependent nuclear translocation. Proteomics 2008; 8:4560-76; PMID:18846510; http://dx.doi.org/10.1002/pmic.200800211
- Don-Salu-Hewage AS, Chan SY, McAndrews KM, Chetram MA, Dawson MR, Bethea DA, Hinton CV. Cysteine (C)-x-C receptor 4 undergoes transportin 1-dependent nuclear localization and remains functional at the nucleus of metastatic prostate cancer cells. PLoS One 2013; 8:e57194; PMID:23468933; http://dx.doi.org/10.1371/journal.pone.0057194
- Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem 2012; 81:637-59; PMID:22463690; http://dx.doi.org/10.1146/annurev-biochem-052810-093700
- Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol 2011; 9:382-94; PMID:21494278; http://dx.doi.org/10.1038/nrmicro2559
- Burns LT, Wente SR. Trafficking to uncharted territory of the nuclear envelope. Curr Opin Cell Biol 2012; 24:341-9; PMID:22326668; http://dx.doi.org/10.1016/j.ceb.2012.01.009
- Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, et al. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 2004; 116:445-56; PMID:15016378; http://dx.doi.org/10.1016/S0092-8674(04)00117-5
- Maltese WA, Soule G, Gunning W, Calomeni E, Alexander B. Mutant Rab24 GTPase is targeted to nuclear inclusions. BMC Cell Biol 2002; 3:25; PMID:12323076; http://dx.doi.org/10.1186/1471-2121-3-25
- Horgan CP, McCaffrey MW. The dynamic Rab11-FIPs. Biochem Soc Trans 2009; 37:1032-6; PMID:19754446; http://dx.doi.org/10.1042/BST0371032
- Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J 2005; 24:3389-99; PMID:16148947; http://dx.doi.org/10.1038/sj.emboj.7600803
- Campa CC, Hirsch E. Rab11 and phosphoinositides: A synergy of signal transducers in the control of vesicular trafficking. Adv Biol Regul 2016; pii: S2212–4926(16)30043-4; PMID:27658318; http://dx.doi.org/10.1016/j.jbior.2016.09.002
- Lim YS, Tang BL. A role for Rab23 in the trafficking of Kif17 to the primary cilium. J Cell Sci 2015; 128:2996-3008; PMID:26136363; http://dx.doi.org/10.1242/jcs.163964
- Williams CL. The polybasic region of Ras and Rho family small GTPases: a regulator of protein interactions and membrane association and a site of nuclear localization signal sequences. Cell Signal 2003; 15:1071-80; PMID:14575862; http://dx.doi.org/10.1016/S0898-6568(03)00098-6
- Mahalakshmi RN, Ng MY, Guo K, Qi Z, Hunziker W, Beguin P. Nuclear localization of endogenous RGK proteins and modulation of cell shape remodeling by regulated nuclear transport. Traffic 2007; 8:1164-78; PMID:17605760; http://dx.doi.org/10.1111/j.1600-0854.2007.00599.x
- Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol 2008; 9:574-82; PMID:18523436; http://dx.doi.org/10.1038/nrm2427
- Simunovic M, Voth GA, Callan-Jones A, Bassereau P. When physics takes over: BAR proteins and membrane curvature. Trends Cell Biol 2015; 25:780-92; PMID:26519988; http://dx.doi.org/10.1016/j.tcb.2015.09.005
- Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, Haft CR, Whistler J, Schwartz TW. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP). J Biol Chem 2004; 279:54291-303; PMID:15452121; http://dx.doi.org/10.1074/jbc.M406169200
- Nisar S, Kelly E, Cullen PJ, Mundell SJ. Regulation of P2Y1 receptor traffic by sorting Nexin 1 is retromer independent. Traffic 2010; 11:508-19; PMID:20070609; http://dx.doi.org/10.1111/j.1600-0854.2010.01035.x
- Gonzalez-Granado JM, Navarro-Puche A, Molina-Sanchez P, Blanco-Berrocal M, Viana R, Font de Mora J, et al. Sorting nexin 6 enhances lamin a synthesis and incorporation into the nuclear envelope. PLoS One 2014; 9:e115571; PMID:25535984; http://dx.doi.org/10.1371/journal.pone.0115571
- Zhu CH, Morse LR, Battaglino RA. SNX10 is required for osteoclast formation and resorption activity. J Cell Biochem 2012; 113:1608-15; PMID:22174188
- Xu J, Xu T, Wu B, Ye Y, You X, Shu X, Pei D, Liu J. Structure of sorting nexin 11 (SNX11) reveals a novel extended phox homology (PX) domain critical for inhibition of SNX10-induced vacuolation. J Biol Chem 2013; 288:16598-605; PMID:23615901; http://dx.doi.org/10.1074/jbc.M112.449306
- Kang DS, Tian X, Benovic JL. Role of beta-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr Opin Cell Biol 2014; 27:63-71; PMID:24680432; http://dx.doi.org/10.1016/j.ceb.2013.11.005
- Vilardaga JP, Gardella TJ, Wehbi VL, Feinstein TN. Non-canonical signaling of the PTH receptor. Trends Pharmacol Sci 2012; 33:423-31; PMID:22709554; http://dx.doi.org/10.1016/j.tips.2012.05.004
- Claing A, Chen W, Miller WE, Vitale N, Moss J, Premont RT, Lefkowitz RJ. beta-Arrestin-mediated ADP-ribosylation factor 6 activation and beta 2-adrenergic receptor endocytosis. J Biol Chem 2001; 276:42509-13; PMID:11533043; http://dx.doi.org/10.1074/jbc.M108399200
- Macia E, Partisani M, Paleotti O, Luton F, Franco M. Arf6 negatively controls the rapid recycling of the beta2 adrenergic receptor. J Cell Sci 2012; 125:4026-35; PMID:22611259; http://dx.doi.org/10.1242/jcs.102343
- Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, et al. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell 2005; 123:833-47; PMID:16325578; http://dx.doi.org/10.1016/j.cell.2005.09.011
- Scott MG, Le Rouzic E, Perianin A, Pierotti V, Enslen H, Benichou S, Marullo S, Benmerah A. Differential nucleocytoplasmic shuttling of beta-arrestins. Characterization of a leucine-rich nuclear export signal in beta-arrestin2. J Biol Chem 2002; 277:37693-701; PMID:12167659; http://dx.doi.org/10.1074/jbc.M207552200
- DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 2000; 148:1267-81; PMID:10725339; http://dx.doi.org/10.1083/jcb.148.6.1267
- Mosca TJ, Schwarz TL. The nuclear import of Frizzled2-C by Importins-beta11 and alpha2 promotes postsynaptic development. Nat Neurosci 2010; 13:935-43; PMID:20601947; http://dx.doi.org/10.1038/nn.2593
- Valdehita A, Bajo AM, Fernandez-Martinez AB, Arenas MI, Vacas E, Valenzuela P, Ruíz-Villaespesa A, Prieto JC, Carmena MJ. Nuclear localization of vasoactive intestinal peptide (VIP) receptors in human breast cancer. Peptides 2010; 31:2035-45; PMID:20691743; http://dx.doi.org/10.1016/j.peptides.2010.07.024
- Savard M, Barbaz D, Belanger S, Muller-Esterl W, Bkaily G, D'Orleans-Juste P, Coté J, Bovenzi V, Gobeil F Jr. Expression of endogenous nuclear bradykinin B2 receptors mediating signaling in immediate early gene activation. J Cell Physiol 2008; 216:234-44; PMID:18264983; http://dx.doi.org/10.1002/jcp.21398
- Charo DN, Ho M, Fajardo G, Kawana M, Kundu RK, Sheikh AY, Finsterbach TP, Leeper NJ, Ernst KV, Chen MM, et al. Endogenous regulation of cardiovascular function by apelin-APJ. Am J Physiol Heart Circ Physiol 2009; 297:H1904-13; PMID:19767528; http://dx.doi.org/10.1152/ajpheart.00686.2009
- Gobeil F, Jr., Bernier SG, Vazquez-Tello A, Brault S, Beauchamp MH, Quiniou C, Marrache AM, Checchin D, Sennlaub F, Hou X, et al. Modulation of pro-inflammatory gene expression by nuclear lysophosphatidic acid receptor type-1. J Biol Chem 2003; 278:38875-83; PMID:12847111; http://dx.doi.org/10.1074/jbc.M212481200
- Waters CM, Saatian B, Moughal NA, Zhao Y, Tigyi G, Natarajan V, Pyne S, Pyne NJ. Integrin signalling regulates the nuclear localization and function of the lysophosphatidic acid receptor-1 (LPA1) in mammalian cells. Biochem J 2006; 398:55-62; PMID:16716145; http://dx.doi.org/10.1042/BJ20060155
- Estrada R, Wang L, Jala VR, Lee JF, Lin CY, Gray RD, Haribabu B, Lee MJ. Ligand-induced nuclear translocation of S1P(1) receptors mediates Cyr61 and CTGF transcription in endothelial cells. Histochem Cell Biol 2009; 131:239-49; PMID:18936953; http://dx.doi.org/10.1007/s00418-008-0521-9
- Lynch JM, Henson PM. The intracellular retention of newly synthesized platelet-activating factor. J Immunol 1986; 137:2653-61; PMID:3093579
- Wright CD, Wu SC, Dahl EF, Sazama AJ, O'Connell TD. Nuclear localization drives alpha1-adrenergic receptor oligomerization and signaling in cardiac myocytes. Cell Signal 2012; 24:794-802; PMID:22120526; http://dx.doi.org/10.1016/j.cellsig.2011.11.014
- Bkaily G, Sleiman S, Stephan J, Asselin C, Choufani S, Kamal M, Jacques D, Gobeil F Jr, D'Orléans-Juste P. Angiotensin II AT1 receptor internalization, translocation and de novo synthesis modulate cytosolic and nuclear calcium in human vascular smooth muscle cells. Can J Physiol Pharmacol 2003; 81:274-87; PMID:12733826; http://dx.doi.org/10.1139/y03-007
- Wang L, Wang Z, Yang B, Yang Q, Wang L, Sun Y. CXCR4 nuclear localization follows binding of its ligand SDF-1 and occurs in metastatic but not primary renal cell carcinoma. Oncol Rep 2009; 22:1333-9; PMID:19885584
- Bkaily G, Choufani S, Hassan G, El-Bizri N, Jacques D, D'Orleans-Juste P. Presence of functional endothelin-1 receptors in nuclear membranes of human aortic vascular smooth muscle cells. J Cardiovasc Pharmacol 2000; 36:S414-7; PMID:11078437; http://dx.doi.org/10.1097/00005344-200036051-00121
- Boivin B, Chevalier D, Villeneuve LR, Rousseau E, Allen BG. Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. J Biol Chem 2003; 278:29153-63; PMID:12756260; http://dx.doi.org/10.1074/jbc.M301738200
- Jacques D, Descorbeth M, Abdel-Samad D, Provost C, Perreault C, Jules F. The distribution and density of ET-1 and its receptors are different in human right and left ventricular endocardial endothelial cells. Peptides 2005; 26:1427-35; PMID:16042982; http://dx.doi.org/10.1016/j.peptides.2005.03.048
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science 2005; 310:1344-7; PMID:16311339; http://dx.doi.org/10.1126/science.1117051
- Doufexis M, Storr HL, King PJ, Clark AJ. Interaction of the melanocortin 2 receptor with nucleoporin 50: evidence for a novel pathway between a G-protein-coupled receptor and the nucleus. FASEB J 2007; 21:4095-100; PMID:17625072; http://dx.doi.org/10.1096/fj.06-7927com
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol 2009; 5:734-42; PMID:19701185; http://dx.doi.org/10.1038/nchembio.206
- Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria-Gomez E, Matias I, Delamarre A, Metna-Laurent M, Cannich A, et al. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat Neurosci 2012; 15:558-64; PMID:22388959; http://dx.doi.org/10.1038/nn.3053
- Sergin I, Jong Y-JI, Harmon SK, Kumar V, O'Malley KL. Sequences within the C-terminus of the metabotropic glutamate receptor, mGluR5, are responsible for inner nuclear membrane localization. J Biol Chem 2017; pii: jbc.M116.757724; PMID:28096465; http://dx.doi.org/10.1074/jbc.M116.757724
