Abstract
Tumor antigen cross-presentation by dendritic cells (DCs) to specific CD8+ T cells is central to antitumor immunity. Although highly efficient in draining lymph nodes, it is defective within the tumor site itself. Importantly, an immunogenic chemotherapy, gemcitabine, reverses this defect, allowing the potential re-stimulation of cytotoxic T lymphocytes within tumor sites.
Introduction
Cross-presentation, which defines how antigen-presenting cells (APCs) such as dendritic cells (DCs) process and present exogenous antigens to CD8+ T cells via major histocompatibility complex (MHC) class I molecules, is pivotal in an effective antitumor immune response.Citation1 Cross-presentation of intracellular tumor antigens by lymph node DCs (LNDCs) is very efficient,Citation1 with levels of tumor antigen as low as 200 nmol/L being detectable.Citation2 It persists throughout tumor growth,Citation1 is an excellent measure of complete tumor resection,Citation3 and is largely localized to the lymph node that drains the tumor site (reviewed in reference 1).
Tumor-infiltrating DCs (TiDCs) may be functionally impaired and/or unable to migrate to tumor-draining lymph nodes (TDLNs),Citation4 but their capacity for cross-presentation has not been clearly defined. We reasoned that there might be a failure of antigen-specific T cells to proliferate in the tumor site due to a lack of TiDCs that could re-stimulate infiltrating T cells, that is, a failure of “post-licensing” – one form of effector site failure.Citation5 Such a defect might prevent antitumor immune attack and explain the failure of endogenous, vaccine-derived, or adoptively transferred CD8+ T cells to kill tumors ().Citation1 There is some evidence that cross-presentation within tissues may be important for CD8+ T-cell effector function or toleranceCitation6; therefore, we examined the tumor and lymph node DC cross-presentation of a tumor neoantigen, hemagglutinin (HA), stably transduced into the murine mesothelioma tumor model AB1-HA.
Figure 1. Tumor antigens are cross-presented by DCs residing in tumor-draining lymph nodes (DLNs) leading to expansion of endogenous tumor-specific CTLs. However, the inability of tumor-infiltrating DCs (TiDCs) to cross-present tumor antigen may lead to a lack of efficient proliferation and activation of infiltrating antigen-specific CD8+ T cells at the effector site. This failure of post-licensing within the tumor microenvironment may limit the generation of an effective antitumor immune response, explaining the failure of endogenous, vaccine-derived, or adoptively transferred CD8+ T cells to kill tumors.
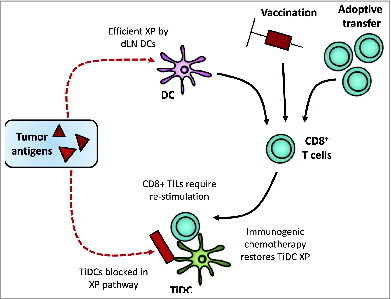
We found that CD11b+ DCs isolated from the tumor did not cross-present tumor antigens and were unable to induce tumor-specific T-cell activation or proliferation. This was not a dominant inhibitory effect because the addition of exogenous MHC class I HA peptide to these DCs induced similar levels of proliferation to that from normal LNDCs. This failure to cross-present a tumor antigen was not due to a defect in TiDC maturation as ex vivo exposure to different activation stimuli failed to restore cross-presentation.Citation6 Interestingly, TiDCs were not defective in their ability to ingest or process exogenous cell-bound antigens or cross-present soluble antigens, as assessed using carboxyfluorescein succinimidyl ester (CFSE)-labeled living, ultraviolet-irradiated, or heat-killed tumor cells and by proteolytic cleavage of the exogenous protein DQ-OVA, releasing the fluorescent dye, BodipyFL. Our finding that the uptake and proteolytic cleavage of exogenous antigen was normal suggests that the failure to cross-prime is distal to antigen uptake and processing. As TiDCs were able to cross-present exogenous HA-protein to specific T cells, the problem may occur after the processes of uptake and proteolytic cleavage within the MHC class I loading pathway. The failure of cross-presentation of cell-derived tumor antigens in association with the preserved ability to process and cross-present soluble tumor antigen is consistent with a defect in the processing pathway between intracellular compartments and the cytoplasm. Current studies are investigating where this block occurs and the molecular events that create the block.
Cross-presentation by TiDCs is important in potentially re-stimulating TILs.Citation1,7 We, therefore, reasoned that immunogenic chemotherapies inducing strong CD8+ T–cell-dependent effector responses in vivo might reverse this block. We found that TiDCs from gemcitabine-treated animals spontaneously induced proliferation of tumor-specific T cells and increased their interferon gamma production, but only at the tumor site. Gemcitabine is a chemotherapeutic agent known to be immunogenic and facilitates the accumulation of T cells within tumorsCitation1,2; therefore, the fact that it could reverse the failure of TiDCs to cross-present antigens could be part of its immunogenic role. It may induce a quantitative change, such as increased antigen load consequent upon drug-induced cell death or a qualitative change, through overcoming the block in cross-presentation or by altering TiDC populations or function. Chemotherapy can act to alter the immunogenicity of dying tumor cells or regulate immune suppression.Citation1,8 Anthracycline chemotherapeutics induce recruitment of CD11c+CD11b+Ly6hi DCs to the tumor bed, resulting in enhanced cross-presentation.Citation9 Our data show that gemcitabine, a drug that is widely used in human breast, pancreatic, ovarian, and lung cancers, also alters the cross-presenting capacity of TiDCs.
Gemcitabine-induced apoptotic destruction of tumor cells potentially loads the immune system with large amounts of tumor antigen, but this is not enough to initiate a protective antitumor response.Citation1 Adjuvant immunotherapy is required, and activating anti-CD40 antibody treatment following gemcitabine chemotherapy can induce long-term cures in this tumor model and has recently been translated into a clinical trial.Citation1 Our studies support the notion that this immune-priming effect of gemcitabine is important and suggest this immunogenic property can be exploited therapeutically, for example, through combination with immune-checkpoint blockade.Citation10
Our data clearly show that tumor antigen-specific T-cell activation, even when CD8+ T cells are delivered directly into the tumors, does not occur within the tumor microenvironment. These tumor-specific CD8+ T cells may fail to engage with antigen within the tumor environment rather than being blocked by local immune suppression. This may be a reason why antitumor CTLs, even when delivered as adoptive cell therapy, fail to eradicate tumors. These data increase our understanding of how some chemotherapeutic drugs exert immunogenic effects. It is not known which other chemotherapeutic drugs can also induce this phenomenon. These results have implications for anticancer therapy, specifically in the use of immunotherapy in conjunction with cytotoxic chemotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- McDonnell AM, Robinson BW, Currie AJ. Tumor antigen cross-presentation and the dendritic cell: where it all begins? Clin Dev Immunol 2010; 2010: 539519; PMID:20976125; http://dx.doi.org/10.1155/2010/539519
- Anyaegbu CC, Lake RA, Heel K, Robinson BW, Fisher SA. Chemotherapy enhancescross-presentation of nuclear tumor antigens. PLoS One 2014; 22: 9(9).
- Brown MD, van der Most R, Vivian JB, Lake RA, Larma I, Robinson BW, Currie AJ. Loss of antigen cross-presentation after complete tumor resection is associated with the generation of protective tumor-specific CD8(+) T-cell immunity. Oncoimmunology 2012; 1: 1084–94; PMID:23170256; http://dx.doi.org/10.4161/onci.20924
- McDonnell AM, Currie AJ, Brown M, Kania K, Wylie B, Cleaver A, Lake R, Robinson BW. Tumor cells, rather than dendritic cells, deliver antigen to the lymph node for cross-presentation. OncoImmunology 2012; 1: 840–6; PMID:23162751; http://dx.doi.org/10.4161/onci.20493
- Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol 2000; 165: 6047–55; PMID:11086036; http://dx.doi.org/10.4049/jimmunol.165.11.6047
- McDonnell AM, Lesterhuis WJ, Khong A, Nowak AK, Lake RA, Currie AJ, Robinson BW. Tumor-infiltrating dendritic cells exhibit defective cross-presentation of tumor antigens, but is reversed by chemotherapy. Eur J Immunol doi:10.1002/eji.201444722
- Boissonnas A, Licata F, Poupel L, Jacquelin S, Fetler L, Krumeich S, Théry C, Amigorena S, Combadière C. CD8+ tumor-infiltrating T cells are trapped in the tumor-dendritic cell network. Neoplasia 2013; 15: 85–94; PMID:23359264
- McDonnell AM, Nowak AK, Lake RA. Contribution of the immune system to the chemotherapeutic response. Semin Immunopathol 2011; 33: 353–67; PMID:21274535; http://dx.doi.org/10.1007/s00281-011-0246-z
- Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013; 38: 729–41; PMID:23562161; http://dx.doi.org/10.1016/j.immuni.2013.03.003
- Lesterhuis WJ, Salmons J, Nowak AK, Rozali EN, Khong A, Dick IM, Harken JA, Robinson BW, Lake RA. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PLoS One 2013; 23: 8(4): e61895; http://dx.doi.org/10.1371/journal.pone.0061895
