ABSTRACT
Four recent publications reported the role of PD-L1 expression on host versus malignant cells within the tumor for PD-1/PD-L1 checkpoint blockade therapy. All four research groups harmoniously report: PD-L1 expressed by both host as well as tumor cells are capable of suppressing T cell functions. Thus, checkpoint therapy can be effective, if malignant cells do not express PD-L1.
Within a time window of 2 mo in early 2017, four independent research groups published papers in high impact journals advocating a similar message using gene silencing technologies in vivo. All groups investigated the role of PD-L1 expression on different cell types within the tumor-microenvironment, in terms of T cell inhibition and/or immune-checkpoint blockade therapy (). Not only is this a tribute to the importance of the PD-1/PD-L1 axis in tumor immunology, but also illustrates the speed at which the oncoimmunology field is progressing.
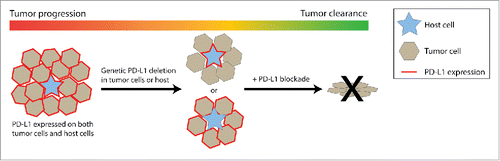
Figure 1. PD-L1 expression on tumor cells and host cells jointly support tumor outgrowth. Left: in a typical immunogenic tumor, both tumor cells and intratumoral host cells express PD-L1, resulting in progressive tumor growth. Middle: experimental genetic deletion of PD-L1 on tumor cells or the host results in reduced tumor growth, mediated by CD8 T cells. Right: complete removal of PD-L1 expression by addition of blocking antibodies results in tumor eradication.
In Cancer Immunology Research, Noguchi et al. used two variants of an MCA-induced mouse tumor model that either grow progressively or regress spontaneously.Citation1 By overexpressing or knocking out PD-L1, using CRISPR-Cas9, they described how PD-L1 on tumor cells determines whether tumors progress or regress, by inhibiting the antitumor T cell response. Tumors that were otherwise too immunogenic to establish, were growing out rapidly by overexpression of PD-L1. Conversely, tumors growing out in immune competent mice were spontaneously eradicated or slowed in their tumor-outgrowth when PD-L1 was knocked out from tumor cells. In all these cases, blocking PD-L1 with a therapeutic antibody had additional effect, indicating a role of PD-L1 on stromal cells.
In Nature Communications, Lau et al. used both PD-L1−/− tumor cells, generated with CRISPR-Cas9, and a newly generated PD-L1 knockout mouse as a host, to study T cell inhibition by PD-L1 in two commonly used mouse tumor models, MC38 and CT26.Citation2 PD-L1 knockout variants of these tumors spontaneously regress or grow slower than WT counterparts, while therapeutic PD-L1 blockade further extends survival, indicating an additive role of PD-L1 on both tumor and host cells. Gene expression analysis showed the strongest enrichment for T cell immunity-related genes when PD-L1 was lacking on both tumor cells and host cells. The authors describe several alternative immune escape mechanisms in outgrowing PD-L1 knockout tumors, including reduced MHC-I expression and increased PD-L2 expression.
In the Journal of Experimental Medicine, Juneja et al. emphasize that while both tumor and host cell PD-L1 expression can play a crucial role in T cell suppression and response to blockade therapy, their exact relative contribution is context-dependent, as it differed per tumor model in their experiments.Citation3 The authors used three different mouse tumor models in host mice deficient for either PD-1 or for both PD-L1 and PD-L2, as compared with WT hosts. Whereas B16 and BRAF.PTEN tumors grew slower in both PD-1 deficient mice and PD-L1 deficient mice, MC38 tumors only benefited from the absence of host PD-1, indicating no relevant role for host PD-L1 in MC38 tumor growth. The strong effect of therapeutic PD-L1 blockade was therefore largely dependent on tumor cell-expressed PD-L1, and could be mimicked by knocking out PD-L1 selectively on tumor cells. The authors further argue that PD-1 expression on T cells in tumors or tumor-draining lymph nodes may well reflect recent activation and not necessarily a dysfunctional state, especially when PD-1 ligands are lacking or blocked at the target site.
Finally, in OncoImmunology, we have described a non-redundant role of PD-L1 expression on tumor cells and host cells.Citation4 Using CRISPR-Cas9 technology, we created PD-L1 knockout variants of MC38 and CT26, the two most widely used pre-clinical tumor models in tumor immunotherapy research, which both grew out more slowly than WT tumors. In straight-forward experiments, we show that blocking PD-L1 or PD-1 with therapeutic antibodies still has tumor-eradicating effects on these tumors, indicating an additional role for PD-L1 on immune infiltrating cells within the tumor microenvironment. T cell depletion studies emphasized the crucial role of CD8 T cells in the antitumor effects of both the lack of PD-L1 on tumor cells and of blocking antibody therapy.
Each study reveals, from a different angle, that PD-L1 on tumor and host cells is involved in suppressing the antitumor T cell response. All manuscripts show that in immunocompetent mice, tumor cells grow out slower or regress spontaneously when PD-L1 is genetically knocked out, an effect mediated by T-cell responses (). There was, however, a minor discrepancy between the studies. Juneja et al. concluded that PD-L1 expression on MC38 tumor cells was fully responsible for inhibiting antitumor T cell responses, with no additional role for PD-L1 on host cells, whereas the papers of Lau and ourselves both showed that PD-L1 blocking antibody therapy of MC38 PD-L1 knockout tumors still gives a therapeutic response. These two conclusions were based on slightly different experimental setups, which may explain the differences. In Lau et al. and our paper, the role of host PD-L1 expression was shown by therapeutic PD-L1 blockade in WT mice-bearing PD-L1 knockout MC38 tumors. Although in Juneja's study, outgrowth of untreated PD-L1 knockout MC38 tumors was much more hampered compared with the other studies, an additional role of PD-L1 on host cells cannot be fully excluded, since they did not treat these mice with PD-L1 blocking antibody.
The knowledge gained by the four studies contributes greatly to our understanding of tumor immunology. By investigating the topic from different angles and using various techniques and pre-clinical models, the four studies complement and validate each other. The combined outcomes signify an important biomarker for the use of PD-1 and PD-L1 blocking antibody therapeutics. Expression of PD-L1 within the tumor, but not necessarily on tumor cells, is sufficient for a therapeutic effect of PD-1/PD-L1 blocking antibodies, meaning that absence of PD-L1 expression on tumor cells does not disqualify patients for treatment.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Noguchi T, Ward JP, Gubin MM, Arthur CD, Lee SH, Hundal J, Selby MJ, Graziano RF, Mardis ER, Korman AJ et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res 2017; 5:106-117; PMID:28073774; https://doi.org/10.1158/2326-6066.CIR-16-0391
- Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun 2017; 8:14572; PMID: 28220772; https://doi.org/10.1038/ncomms14572
- Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, Freeman GJ, Sharpe AH. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 2017; 214:895-904; PMID:28302645; https://doi.org/10.1084/jem.20160801
- Kleinovink JW, Marijt KA, Schoonderwoerd MJA, van Hall T, Ossendorp F, Fransen MF. PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. Oncoimmunology 2017; e1294299; PMID:28507803; https://doi.org/10.1080/2162402X.2017.1294299
