Abstract
Mosquito are well-known vectors that cause diseases particularly malaria and filariasis which are detrimental to human health. These vectors occur mainly in tropical countries where more than 2 billion people live in endemic regions with about one million deaths been claimed yearly from malaria and filariasis. The study is aimed at evaluating the larvicidal activity of Pistia stratiotes fractions on Anopheles mosquitoes (Diptera: Culicidae). The ethyl acetate extract of P. stratiotes was obtained through percolation process and was chromatographed to yield nine fractions. The larvicidal activity of each of the nine fractions was tested in triplicates by exposing the larvae to 500, 250, 125, 62.5 and 31.3 µg/ml, respectively. Phytochemical screening of the nine fractions revealed the presence of alkaloids, flavonoids, glycosides and phlobatannins in varying quantities. The result obtained shows that fraction E has the highest lethal effect on the Anopheles larvae at LC50 =14.81 µg/ml and was weakly effective at 602.03 µg/ml on brine shrimp larvae. The gas chromatography mass spectrometry analysis of fraction E revealed the presence of 35 pre-cursor compounds. Hence, ethyl acetate fractions of P. stratiotes could be an effective larvicide against Anopheles mosquito larvae as it has been found to be harmless to other aquatic organisms. Further work should be done on other aquatic weeds that have larvicidal potential to isolate the bioactive compounds.
Introduction
Malaria is a very rampant and devastating disease in the tropical regions of the world. Numerous efforts have been made in the past to control its morbidity and mortality [Citation1]. Treatment of the vector at the various developmental stages with insecticides has received wide acceptability, though these synthetic products suffer from major disadvantages of resistance and environmental pollution [Citation2,Citation3]. Plants are rich source of alternative agents for control of mosquitoes, because they possess bioactive chemicals, which act against limited number of species including specific target-insects and are eco-friendly [Citation4]. Several secondary metabolites present in plants serve as a defence mechanism against insect attacks. These bioactive compounds may act as insecticides, antifeedants, moulting hormones, oviposition deterrents, repellents, juvenile hormone mimics, growth inhibitors, antimoulting hormones as well as attractants. Important also will be the identification of any toxic element or injurious fractions whose use should be actively discouraged [Citation5]. Plant based pesticides are less toxic, delay the development of resistance because of its new structure and easily biodegradable [Citation6–8]. Plant extracts or oils in general have been recognized as an important natural resource for the control of parasites and pest of public health importance [Citation9]. Many researchers have reported the effectiveness of plant extract against mosquito larvae [Citation10–12]. Insecticidal activities of different plant essential oils have been reported against different mosquito species. For example, [Citation13] reported the larvicidal activity of essential oils of 11 plants grown in the Himalayan region against Aedes aegypti larvae. Likewise [Citation14], screened the larvicidal effects of ten plant species and found three plant essential oils (Kaempferia galanga, Illicium verum and Spilanthes acmella) to have larvicidal properties against Culex quinquefasciatus. Phytochemicals are secondary metabolites which are natural insecticides derived from plants. Applications of phytochemicals in mosquito control were in use since the 1920s [Citation12] but the discovery of synthetic insecticides such as DDT in 1939 side tracked the application of phytochemicals in mosquito control programme. Several groups of phytochemicals such as alkaloids, steroids, terpenoids, essential oils and phenolics from different plants have been reported previously for their insecticidal activities [Citation15–17]. Pistia stratiotes, referred to as the tropical duck weed, is one of the most dominant aquatic weeds in fresh water, polluted water and streams of Nigeria. The plant is raised in fishponds as a shelter for certain edible shrimp species. Its usage as salad in swine feeds as well as its preference as foliage by buffalos has been reported. However, it has been reported to exert a poisonous effect on rabbits [Citation17–19]. This study is aimed at evaluating the larvicidal activity of P. stratiotes fractions on Anopheles mosquitoes (Diptera: Culicidae) ().
Materials and methods
Collection and identification of Pistia stratiotes
P. stratiotes was collected from Kumbotso borrow pit in Kumbotso Local Government area, Kano State. The plant was handpicked with the aid of hand gloves, identified based on [Citation10,Citation20] protocols and confirmed in the herbarium at the Department of Plant Biology, Bayero University, Kano and was given Accession number BUKHAN 0353 on 17 March 2017. The leaves of plant were washed with tap water and dried under a shade for 2 weeks and powdered by a mechanical grinder (mortar and pestle). Eight hundred grams [800 g] of the powdered sample was dispensed in 8 litre ethyl acetate for 2 weeks with shaking at regular intervals after which the content was filtered using Whatmann filter paper (No. 1). The extract was kept in sterile bottles [Citation21].
Isolation of compounds from P. stratiotes
Isolation of pure compounds was performed by column chromatography of the extract on silica gel. Silica gel with 60–200 mesh size (product of Lobachemie Laboratory Reagents and Fine chemicals, Mumbai, India) was used as a stationary phase in the column. To prepare the column, a glass tube of 5.0-cm diameter and 87-cm length was clamped vertically. The lower end of the column was fitted with a stopper and plugged with cotton wool as a support. Slurry of silica gel was prepared in solvent used for separation (250 g silica gel in 500 ml chloroform). The slurry was added to the column gradually and with gentle tapping to avoid cracks. This process was continued till a uniform column of desired length was obtained. The crude extract (40.42 g) was mixed with 25 g of silica gel to obtain homogenous mixture. This mixture was poured into the column and different fractions were eluted with different solvents viz. chloroform, ethyl acetate and formic acid. The extracted fractions were collected separately and the solvent of the collected fractions was evaporated at room temperature [Citation3,Citation22–24].
Thin layer chromatography
Small of the fractions was applied on Merck Aluminum plate pre-coated with silica gel 60F254 of 0.2-mm thickness using capillary tube. The plate was developed in solvent system of chloroform: ethyl acetate: formic acid. The plate then air dried and visualized in UV 254 and 366 nm to detect Spot of different compounds [Citation19,Citation22,Citation25].
Preliminary phytochemical screening
Phytochemical tests were carried out on each fraction for the qualitative determination of phytochemical constituents as described by [Citation3,Citation26,Citation27].
The breeding and identification of larvae of Anopheles spp
The larvae were reared in the insectary of the Department of Biological Sciences, Bayero University Kano. During the rearing period, at least three groups of mosquito larvae were identified accurately in a container by direct visualization based on their position on water surface and the size of their head. The identification was done by following the recommendations in Medical entomology for students, [Citation28]. The larvae were then exposed to logarithmic concentration of the extract of Pistia stratiotes thus the LC50 was determined according to probit regression line.
Preparation of stock and standard solution
The method described by [Citation29] was employed in preparing the stock solution. Ten milligrams each of the fractions was added to 100 ml of dimethylsulphoxide (DMSO) to make the stock solution. It was expressed in weight per volume (w/v). The standard solution was made by serial dilutions. Three replicates of each dose were made per ethyl acetate fractions at different dilutions. The concentrations are shown in the . To make the first dose which was in ratio of 1:1, 25 ml of stock was added to 25 ml of distilled water in a 50-ml container. The second dose (ratio of 1:2), 12.5 ml of stock was added to 37.5 ml of distilled water in a 50 ml container. The third dose (ratio of 1:3), 6.25 ml of extract was added to 43.75 ml of water. The fourth dose (ratio 1:4), 3.13 ml of extract was added to 46.87 ml of tap water. The fifth dose (ratio 1:5), 1.56 ml of extract was added to 48.44 ml of tap water and 10 mosquito larvae species were introduced to each of solution. The 0 µg/ml served as control [Citation3,Citation30].
Table 1. Physical properties of fractions of ethyl acetate extract.
Brine shrimp bioassay
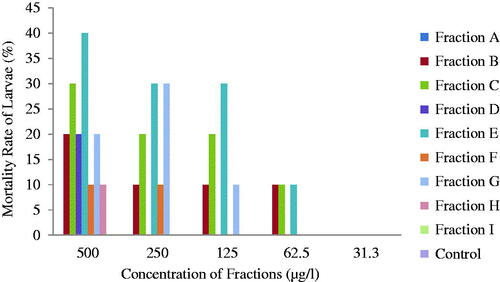
Brine shrimp (Artemia salina) eggs were hatched in hypertonic solution. The hypertonic solution was prepared by dissolving 38 g of sea salt in 1 litre of distilled water for hatching the shrimp eggs. The seawater was put in a 500-ml beaker (hatching chamber). Shrimp eggs were added into the hatching chamber while the lamp above (light) attracted the hatched shrimps. The shrimps were left for 2 d to hatch and mature as nauplii (larva) and were used for the bioassay and the standard solution was made by serial dilutions [Citation31–33] ().
Gas chromatography mass spectrometry GC/MS analysis
The gas chromatography mass spectrometry (GC/MS) analysis of the fraction with the lowest LC50 value was performed using Agilent GC 7890B, MSD 5977 A (Agilient Technologie, Santa Clara, California, USA. Library: NIST14.L SOFTWARE MASSHUNTER) equipped with AB innowax column (60 × 0.25 mm id, film thickness 0.25 μm). For GC-MS detection, an electron ionization system with ionization energy of 70.007 eV was used. Helium gas was used as a carrier gas at a total flow rate of 7.4206 ml/min. Injector and mass transfer line temperature were set at 270 and 280 °C, respectively. The oven temperature was programmed from 50 to 180 °C at 3 °C min−1 with hold time of min−1 and from 180 to 250 °C at 6 °C min−1 with hold time 20 min, respectively. Diluted samples (prepared methanol) of 0.2 μl were manually injected in the split less mode. Identification of compounds of the samples was based on GC retention time on AB inno-wax column; Total GC running time was 30 min [Citation3,Citation34].
Statistical analysis
Estimation of LC50 was done using probit analysis by employing SPSS software (version 16.0) statistical software so as to obtain 50% lethal concentration per each fraction at p ≤ .15. The effect of different P. stratiotes Fractions treatments were compared through one way analysis of variance followed by Tukey test, by using IBM SPSS software (version 16.0). A p < .05 was considered statistically significant.
Result and discussion
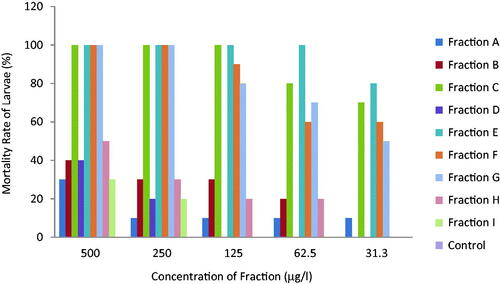
The physical properties of the fractions of P. stratiotes are summarized in . Fraction A and C are dark brown in colour, where fraction A is odourless and oily, fraction C has a tea smell and smooth texture. Fraction B is orange in colour, odourless and smooth while fraction D is light brown, has a stinky smell and smooth texture. Fraction E is blue black in colour, odourless and sticky. Fraction F and G are dirty green in colour, odourless and have smooth texture while fraction H and I are light green in colour, odourless and smooth. In contrast to other mosquitoes, Anopheles larvae lack a respiratory siphon and as such breathe through spiracles located on the eighth abdominal segment and must therefore come to the surface frequently to breathe. Fraction E which has the lowest LC50 () when diluted, formed a mass of cloudy substance on the surface of the test tubes; hence, the cloudy substance could hinder the penetration of air, resulting in the suffocation and eventual death of the larvae. Fraction A which is second most toxic fraction is oily, this oil could block the spiracles, resulting in suffocation and death of the larvae. Phytochemicals are non-nutritive plant chemicals that have protective or disease preventive properties [Citation35–37]. Different phytochemicals have been found to possess a wide range of activities. Phytochemical analysis of P. stratiotes fractions indicates the presence of alkaloids, flavonoids, phlobatannins and steroid glycosides in the fractions in varying quantities while tannins, saponins and resins were absent () [Citation29] reported the presence of alkaloids and phlobobatannin in the ethanolic extract of P. stratiotes [Citation38] reported the absence of tannins and saponins in the ethyl acetate extract of P. stratiotes. Among the compounds isolated from P. stratiotes, alkaloids appeared to be of great interest in pharmacological studies. In Ghana, extracts of roots of Cryptolepis sanguinolenta in which cryptolepine the main alkaloid, have been used clinically to treat malaria, colic and stomach ulcers [Citation39]. Cryptolepine itself is found to produce many pharmacological effects such as antimicrobial, antiprotozoal, antihyperglycemic and cytotoxic effects through GC-rich DNA sequence intercalation that provides basis for design for new anticancer drug [Citation25,Citation30]. Also alkaloids compounds extracted from the skin of poison frog (dentrobatids) from the Smithsonian Institution, Virginia, were found to repel mosquitoes and that very little amount was required to have toxic effect [Citation20]. Also, flavonoids, extracted from Poncirus trifoliate i.e. rhoifolin, provided maximum of 365 + 12.0 min protection and also 100.0% + 0.00 repellency against mosquito bites [Citation40]. It is also possible that toxicity of the fractions from the plant might arise through odour. Other compounds like saponins, flavonoids and tannins have larvicidal effect on mosquitoes [Citation26]. Probably, certain compounds not exploited in this investigation because of time and material constraints might play role in repelling the larvae. The toxicity studies of mosquito larvae after being exposed to all the fractions for 24 h is shown in . According to [Citation22] and [Citation41], classification of plant larvicidal activities is considered as nontoxic when the LC50 is >750 µg/ml, weakly effective (LC50 is between 200 and 750 µg/ml), moderate (LC50 is between 100 and 200 µg/ml), effective (LC50 is between 50 and 100 µg/ml), and highly effective (LC50 is <50 µg/ml). Ethyl acetate fraction was highly effective against Anopheles larvae (LC50 = 14.8 µg/ml) (fraction E) and weakly effective (LC50 = 702.8 µg/ml) (fraction I). In the present study, most of the water lettuce fractions exhibited high larvicidal activities against the larvae (E, A, F and G) with few fractions showing weak activities (H, D, B and I). The larvicidal effect was dose dependent. The bioassay showed that fraction E of the ethyl acetate extract of P. stratiotes LC50 = 14.81 µg/ml exerted highest lethal activity on Anopheles. This was followed by fraction A with LC50 = 28.48 µg/ml while the least LC50 (702.8 µg/ml) was observed in fraction I. This could be due to the fact that, fraction E contains high amount of secondary metabolites while fraction I contains trace amount. The presence of certain secondary metabolites in the plant like alkaloids might be responsible for the toxicity of the fractions. Low larvicidal activities suggest presence of many compounds which may provoke each other and reduce the activity as established by [Citation42]. According to [Citation15], mosquito larvicidal efficacies of the purified compounds were observed to be relatively higher than that of the crude extracts. This proposes that purification of the extracts is important to enhance the larvicidal activity of compounds. This indicates that bioactivity guided fractionation and purification may lead to the isolation of active chemical compounds responsible for the larvicidal activity [Citation43]. The fractions of P. stratiotes have low activity on Brine shrimp larvae. The fraction E was weakly effective at 602.03 µg/ml (. This confirmed some few reported works, which stated that the crude fraction of P. straiotes L. was moderately toxic to brine shrimp [Citation44]. Perhaps, this may partly be the reason why some shrimps prefer to associate with the plant in their sea environment [Citation45]. Additionally, an investigation carried out on the toxicity of extracts of P. stratiotes in rats has shown adverse effects but no lethality was recorded [Citation40]. The GC-MS analysis of fraction E of ethyl acetate extract of P. stratiotes leaves revealed the presence of 35 compounds (). Among the identified phytochemicals, Linolenic acid (17-octadecacynoic acid), ethyl ester found to act as Insectifuge [Citation1].
Studies conducted by [Citation47] on some Thai plants conclude that plants with LC50 less than 50, 100 and 750 can be classified as active, moderate and effective larvicide respectively while plants with LC50 of more than 750 are classified as inactive larvicide. The present study shows that all the fractions of Pistia stratiotes consider for this study fall under either active, moderate or effective larvicides.
Figure 3. Larvicidal effect of fractions of P. stratiotes and ethyl acetate fractions on A. salina and Anopheles mosquitoes, respectively.
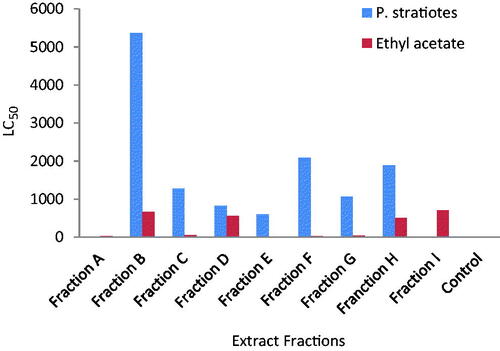
Table 2. Qualitative preliminary phytochemical screening of fractions of P. stratiotes.
Table 3. Chemical constituents present in fraction E (lowest LC50) using GC-MS analyses.
Conclusion
Many control measures and prevention have been employed to manage the nuisance caused by mosquitoes. Inorganic pest control have however remain the most effective but this tends have much side effects on humans and other living organisms in the environment. The current results obtained in this study have important implications in the practical control of mosquito larvae. The fractions of P. stratiotes were active on Anopheles mosquito larvae. Fraction E of the ethyl acetate extract appeared with the highest potential of acting as anti-mosquito larvae because of its high lethality with LC50 of 14.81 µg/ml than all the other tested fractions and was weakly effective at 602.03 µg/ml on brine shrimp larvae. Hence, ethyl acetate fractions of P. stratiotes could be an effective larvicide against Anopheles mosquito larvae as it has been found to be harmless to other aquatic organisms.
Recommendations
Further work should be done to further purify the biologically active components that are responsible for the larvicidal activity of this plant.
Further studies should be carried out to compare the effectiveness of the crude extract and fractions of P. stratiotes.
Further work should be done on other aquatic weeds that have larvicidal potential to isolate the bioactive compounds.
Ethical approval
This paper has been approved by the review board of College of New Energy and Environment, Jilin University as well as Department of Biological Sciences, Bayero University Kano.
Acknowledgements
We acknowledge the supports rendered to us by the Department of Biological Sciences and Department of Pure and Industrial Chemistry Bayero University Kano, as well as the College of New Energy and Environmental Resources, Jilin University, China.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Karou D, Savadogo A, Canini A, et al. Antibacterial activity of alkaloids from Sida acuta. Afr J Biotechnol. 2005;4(12):1452–1457.
- Vincent S, Kovendan K, Chandramohan B, et al. Swift fabrication of silver nanoparticles using bougainvillea glabra: potential against the Japanese encephalitis vector, Culex tritaeniorhynchus Giles (Diptera: Culicidae). J Clust Sci. 2017;28:37–58.
- Mohammadi Z, Sharif ZM, Majdi H, et al. The effect of chrysin-loaded nanofiber on wound healing process in male rat. Chem Biol Drug Des. 2017;90:1106–1114.
- Shivakumar MS, Srinivasan R, Natarajan D. Larvicidal potential of some Indian medicinal plant extracts against Aedes Aegypti (L.). Asian J Pharm Clin Res. 2013;6(3):77–80.
- Carcamo MC, Carapeto LP, Duarte JP, et al. Larvicidal efficiency of the mushroom Amanita muscaria (Agaricales, Amanitaceae) against the mosquito Culex quinquefasciatus (Diptera, Culicidae). Rev Soc Brasil Med Trop. 2016;49:95–98.
- Govindarajan M, Vijayan P, Kadaikunnan S, et al. One-pot biogenic fabrication of silver nanocrystals using Quisqualis indica: effectiveness on malaria and Zika virus mosquito vectors and impact on non-target aquatic organisms. J Photochem Photobiol B Biol. 2016;162:646–655.
- Guarda C, et al. Larvicidal activity of natural products and assessment of susceptibility to the insecticide temefos in controlling the Aedes aegypti (Diptera, Culicidae). Interciencia. 2016;41:243–247.
- Singh G, Prakash S. Virulency of novel nanolarvicide from Trichoderma atroviride against Aedes aegypti (Linn.): a CLSM analysis. Environ Sci Pollut Res. 2015;22:12559–12565.
- Benelli G, Chandramohan B, Murugan K, et al. Neem cake as a promising larvicide and adulticide against the rural malaria vector Anopheles culicifacies (Diptera: Culicidae): a HPTLC fingerprinting approach. Nat Prod Res. 2017;31:1185–1190.
- Gusain R, Suthar S. Potential of aquatic weeds (Lemna gibba, Lemma minor, Pistia stratiotes and Eichhornia sp.) in biofuel production. Process Saf Environ Prot. 2017;109:233–241.
- Hanan AS, Jazem MA, Hamed GA, et al. Larvicidal activity of synthesized silver nanoparticles using Rhazya strieta leaf extract against mosquito vectors Aedes Aegypti. Res J Biotechnol. 2018;13: 65–72.
- Saleh MS, Abuzinadah OA, Al-Ghamdi KM, et al. Effectiveness of slow-release tablet formulations of the IGR diflubenzuron and the bioinsecticide spinosad against larvae of Aedes aegypti (L.). Afr Entomol. 2013;21:349–353.
- Massebo F, Tadesse M, Bekele T, et al. Evaluation on larvicidal effects of essential oils of some local plants against Anopheles arabiensis Patton and Aedes aegypti Linnaeus (Diptera, Culicidae) in Ethiopia. Afr J Biotechnol. 2009;8:4183–4188.
- Pitasawat B CW, Kanjanapothi D, Panthong A, et al. Screening for larvicidal activity of ten Carminative plants: South east asia. J Trop Med Public Health. 1998;29:660–662.
- Haq S, Singh SP, Kumar G, et al. Evaluation of mosquito larvicidal efficacy of different parts of Dalbergia sissoo plant. Res J Pharm Biol Chem Sci. 2016;7:463–467.
- Igbal J, Ishtiag F, Alqarni A, et al. Evaluation of larvicidal efficacy of indigenous plant extracts against Culex quinquefasciatus (Say) under laboratory conditions. Turk J Agric For. 2018;42:207–215.
- Mukhtar MD. Cytotoxicity of fractions of Pista stratiotes L. on larvae of Culex mosquito and A. salina. Anim Res Int. 2004;1(2):95–99.
- Ishwarya R, Vaseeharan B, Kalyani S, et al. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J Photochem Photobiol B Biol. 2018;178:249–258.
- Jazem MA, Khalid A-GM, Abdulaziz BBM. Toxicological and histological effects of selected bacterial biopesticides on Aedes aegypti (L.), the vector of dengue in Saudi Arabia. Res J Biotechnol. 2018;13:1–7.
- Hanafiah MM, Mohamad NHSM, Abd Aziz NIH. Salvinia molesta and Pistia stratiotes as phytoremediation agents in sewage wastewater treatment. Sains Malaysiana. 2018;47:1625–1634.
- Chantawee A, Soonwera M. Efficacies of four plant essential oils as larvicide, pupicide and oviposition deterrent agents against dengue fever mosquito, Aedes aegypti Linn. (Diptera: Culicidae). Asian Pac J Trop Biomed. 2018;8:217–225.
- Jiraungkoorskul W. Efficiency of Tinospora crispa against Culex quinquefasciatus larva. Environ Sci Pollut Res Int. 2018. doi:10.1007/s11356-018-2429-9.
- Rajakumar G, Rahuman AA, Jayaseelan C, et al. Solanum trilobatum extract-mediated synthesis of titanium dioxide nanoparticles to control Pediculus humanus capitis, Hyalomma anatolicum anatolicum and Anopheles subpictus. Parasitol Res. 2014;113:469–479.
- Patil SV, Patil CD, Narkhede CP, et al. Phytosynthesized gold nanoparticles-Bacillus thuringiensis (Bt-GNP) formulation: a novel photo stable preparation against mosquito larvae. J Clust Sci. 2018;29:577–583.
- Osanloo M, Sereshti H, Sedaghat MM, et al. Nanoemulsion of Dill essential oil as a green and potent larvicide against Anopheles stephensi. Environ Sci Pollut Res. 2018;25:6466–6473.
- Kalimuthu K, Panneerselvam C, Chou C, et al. Predatory efficiency of the copepod Megacyclops formosanus and toxic effect of the red alga Gracilaria firma-synthesized silver nanoparticles against the dengue vector Aedes aegypti. Hydrobiologia. 2017;785:359–372.
- Ola-Davies OE, Ajani, O.S. Semen characteristics and sperm morphology of Pistia stratiotes Linn. (Araceae) protected male Albino rats (wistar strain) exposed to sodium arsenite. Faseb J. 2016;30:
- Ola-Davies O, Ajani OS. Semen characteristics and sperm morphology of Pistia stratiotes Linn. (Araceae) protected male albino rats (Wistar strain) exposed to sodium arsenite. J Complement Integr Med. 2016;13:289–294.
- Imam T, Um T. Qualitative phytochemical screening and larvicidal potencies of ethanolic extracts of five selected macrophyte species against Anopheles mosquitoes (diptera: culicidae). J Res Environ Sci Toxicol. 2013;2:121–125.
- Murugan K, Suresh U, Panneerselvam C, et al. Managing wastes as green resources: cigarette butt-synthesized pesticides are highly toxic to malaria vectors with little impact on predatory copepods. Environ Sci Pollut Res. 2018;25:10456–10470.
- Deng SQ, Deng MZ, Chen JT, et al. Larvicidal activity of recombinant Escherichia coli expressing scorpion neurotoxin AaIT or B.t.i toxin Cyt2Ba against mosquito larvae and formulations for enhancing the effects. J South Med Univ. 2017;37:750–754.
- Uragayala S, Verma V, Natarajan E, et al. Adulticidal and larvicidal efficacy of three neonicotinoids against insecticide susceptible and resistant mosquito strains. Indian J Med Res. 2015;142:64–70.
- Subramaniam J, Murugan K, Panneerselvam C, et al. Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: towards an integrative approach? Environ Sci Pollut Res. 2015;22:20067–20083.
- Dhanker R, Kumar R, Hwang JS. How effective are Mesocyclops aspericornis (Copepoda: Cyclopoida) in controlling mosquito immatures in the environment with an application of phytochemicals? Hydrobiologia. 2013;716:147–162.
- Fukruksa C, Yimthin T, Suwannaroz M, et al. Isolation and identification of Xenorhabdus and Photorhabdus bacteria associated with entomopathogenic nematodes and their larvicidal activity against Aedes aegypti. Parasit Vectors. 2017;10:440.
- Subarani S, Sabhanayakam S, Kamaraj C. Studies on the impact of biosynthesized silver nanoparticles (AgNPs) in relation to malaria and filariasis vector control against Anopheles stephensi Liston and Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res. 2013;112:487–499.
- Soni N, Prakash S. Silver nanoparticles: a possibility for malarial and filarial vector control technology. Parasitol Res. 2014;113:4015–4022.
- Tulika TaMA, Tulika T, Mala A. Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. J Pharm Phytochem. 2017;6:195–206.
- Rai PK, Kumar V, Lee S, et al. Nanoparticle-plant interaction: Implications in energy, environment, and agriculture. Environ Int. 2018;119:1–19.
- Madhumitha G, Rajakumar G, Roopan SM, et al. Acaricidal, insecticidal, and larvicidal efficacy of fruit peel aqueous extract of Annona squamosa and its compounds against blood-feeding parasites. Parasitol Res. 2012;111:2189–2199.
- Kovendan K, Chandramohan B, Govindarajan M, et al. Orchids as sources of novel nanoinsecticides? Efficacy of Bacillus sphaericus and Zeuxine gracilis-fabricated silver nanoparticles against dengue, malaria and filariasis mosquito vectors. J Clust Sci. 2018;29:345–357.
- Chelela BL, Chacha M, Matemu A. Larvicidal potential of wild mushroom extracts against Culex quinquefasciatus Say, Aedes aegypti and Anopheles gambiae Giles S.S. Am J Res Commun. 2014;2(8):105–114.
- Murugan K, Dinesh D, Nataraj D, et al. Iron and iron oxide nanoparticles are highly toxic to Culex quinquefasciatus with little non-target effects on larvivorous fishes. Environ Sci Pollut Res. 2018;25:10504–10514.
- Murugan K, Jaganathan A, Rajaganesh R, et al. Poly(styrene sulfonate)/poly(allylamine hydrochloride) encapsulation of TiO2 nanoparticles boosts their toxic and repellent activity against zika virus mosquito vectors. J Clust Sci. 2018;29:27–39.
- Kumar VA, Ammani K, Jobina R, et al. Larvicidal activity of green synthesized silver nanoparticles using Excoecaria agallocha L. (Euphorbiaceae) leaf extract against Aedes aegypti. Iet Nanobiotechnology. 2016;10:382–388.
- Komalamisra N, Trongtokit Y, Rongsriyam Y, Apiwathnasorn C. Screening for larvicidal activity in some Thai plants against four mosquito vector species. The Southeast Asian Journal of Tropical Medicine and Public Health. 2005;36:1412–1422.
- Service M. W. (2012). Medical entomology for students. Cambridge: Cambridge University Press.