?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Freeze drying has been well applied in the preparation of high-efficiency probiotic powders. However, the process is generally accompanied by probiotic viability deficiency, which is the bottleneck for further application. To improve the viability of Bifidobacterium bifidum BB01 during freeze-drying, we optimized the cryoprotectant of B. bifidum BB01 by response surface methodology (RSM) with a Central Composite Design (CCD). In this study, two values of B. bifidum BB01 with different protectant factors were investigated, including freeze-drying survival rate and the viable counts of per unit weight of freeze-dried powder. The optimized cryoprotectants were obtained as follows: glycine of 5.5%, sodium bicarbonate of 0.8%, xylo-oligosaccharides of 7%, arginine of 4.5% and skim milk of 25%. The survival rate and the viable counts of per unit weight of powder were 90.37 ± 1.9% and (2.78 ± 0.13) × 1011cfu·g−1, respectively, both close to the predicted value (88.58% and 2.71 × 1011 cfu·g−1). Our research demonstrated that RSM was successful in optimizing composite cryoprotectant for freeze-dried powder of B. bifidum which can as well protect the probiotic cells.
Introduction
Recently, microbial preparations have been rapidly developed with the aid of microecology, which greatly encourage the improvement of probiotic preparation. Probiotics have been identified by Fuller as “a live microbial feed supplement which beneficially affects the host animal by improving its intestinal balance” [Citation1(a,b)]. Probiotics significantly contribute to optimize the balance of intestinal microbiota [Citation2], reducing the risk of disorders and associated intestinal and systemic diseases [Citation3,Citation4]. Therefore, they have been widely used in healthy food, especially in fermented dairy products, such as cheese, ice cream and milk slice [Citation5].
The demand for probiotic products is growing rapidly due to the increasing awareness of consumers about the effects of probiotics on health. The quality of probiotic products is evaluated by the number of viable bacteria because the health benefit of probiotic on the host works depend on their concentration [Citation6,Citation7]. Therefore, it is necessary to ensure the viable counts of probiotic in the product. Bifidobacterium bifidum, one of the most widely used probiotics, is sensitive to acid or oxygen which easily leads to inactivation during storage period [Citation8]. Previous researchers tried to improve the bacterial resistance to acid and oxygen through genetic modification or oxygen and acid-tolerant strains screen [Citation9,Citation10].
Moreover, encapsulation of probiotic bacteria has been found to be successful in prolonging the products shelf-life by protecting microorganisms from the adverse food environment during the storage [Citation11,Citation12]. Last but not the least, the selection and design of a suitable preparation technique is very important. For probiotic products sold in liquid form, they are difficult to store and the number of viable bacteria declines rapidly with time.
In contrast, the suitable preparation technique was used and probiotic products in the dry powder form were found to be more convenient to store and transport. Freeze-drying is a conventional technology for preparing bacterial powder in industrial production, which can effectively remove water from the bacterial sample. Vacuum freeze-drying technology is most favorable in probiotic production due to the less inactivation, good rehydration and faster reduction.
Various probiotic strains such as Lactobacillus plantarum, Lactobacillus casei and B. bifidum have been used extensively in health foods. Bifidobacterium spp. is believed to be the main species with probiotic characteristics. Clinical studies prove that bifidobacteria are the most beneficial intestinal microbiota [Citation13–16]. Bifidobacterium is a Gram-positive anaerobic bacterium, a spore-free, non-moving bacillus, which is usually curved, rod-shaped but changes with age and growth environment [Citation17]. It has been reported that B. bifidum has excellent probiotic effects such as maintaining intestinal micro-ecological balance, bio-antagonism, immune regulation and nutrition [Citation18,Citation19].
However, Bifidobacteria are a great challenge during the freeze-drying process. The factors such as freezing, drying and high pressure could result in mechanical, solute and cell membrane damages [Citation20], which reduce the vitality of the probiotic preparation. Therefore, to improve the survival rate of freeze-dried B. bifidum is essential for both probiotic preparation and production efficiency.
The addition of cryoprotectants can reduce cell damage and cell death rate [Citation21]. Single protective agent is not enough to protect the B. bifidum effectively; therefore, complex protective agents are required to improve the bacterial survival rate. It has been reported that 10 or 20% polysaccharide mixed with 10% skim milk exhibits the best protective effect against Bifidobacterium MYL16 [Citation22]. Generally, the formulation of the protective agent depends on the cell structure. Response surface methodology (RSM) can be used to design, optimize and improve the protective agent combination which is time-saving and low cost [Citation23,Citation24]. Center composite design (CCD) is appropriate to study factors with three and/or five levels. The obtained results could fit with a nonlinear mathematical model and the equation expresses the relationship between the independent factors and the response values. The response values finally show the optimized cryoprotectant combination [Citation25].
In our previous study, the carbon and nitrogen sources of the MRS (lactose) medium were optimized [Citation26]. The composition of anti-freezing factors was also optimized. The combined cryoprotectants included the prebiotic cryoprotectant and the amino acid cryoprotectant, which effectively improved the viable counts and the survival rate of B. bifidum BB01 during the freeze-drying process. The freeze-drying survival rate increased with the addition of anti-freeze factors but powder yield did not. However, adding multiple components cost high. Therefore, the combination of various cryoprotectants for BB01 was further optimized by the CCD of RSM in this study. Suitably combined cryoprotectants are essential for the application and preservation of B. bifidum.
Materials and methods
Microorganism and cultivate preparation
Starter bacteria of B. bifidum BB01 was provided by School of Food and Biological Engineering, Shaanxi University of Science and Technology. The optimized MRS medium was used for bacterial activation and fermentation. MRS agar medium (Hope Biotechnology CO. LTD, Qingdao, China) was used for the determination of viable counts.
The freeze-dried bacterial powder was freshly prepared in optimized MRS medium and incubated for 24 h at 37 °C. After microscopic examination, 5% (v/v) inoculum was subsequently inoculated into MRS twice and finally cultured at 37 °C for 18 h. The fully activated strains were stored at 4 °C for further study.
Preparation of cryoprotectants and vacuum freeze-drying
The cryoprotectant factors were dissolved in distilled water and formulated into various concentrations. Then, all of them were sterilized at 108 °C for 15 min, while saccharides sterilized at 115 °C for 15 min.
Bifidobacterium bifidum cells were grown in MRS medium at 37 °C for 18 h, harvested by a high-speed centrifuge LG10-2.4 (Beijing Medical Centrifuge Company, Beijing, China) at 8000 r/min for 15 min. After adding equal volume cryoprotectants, these cells were first pre-frozen at −40 °C for 24 h and then at −50 °C, 7.23 Pa for 24 h by a vacuum freeze dryer FD-1D-50 (Biocool Science Instrument Beijing Co., Ltd, Beijing, China).
Viable counts determination
Plate counting method was used to measure the viable counts of B. bifidum BB01 during the freeze-drying process. The sample was sequentially diluted 10 times to the appropriate gradient, then 0.1 ml of solution was spread to the MRS agar medium before coating uniformly. The plates were incubated at 37 °C for 48 h. The freeze-dried bacterial powder was returned to its original pre-freeze-dried volume by adding sterile saline solution and the viable counts were determined. Experiments were performed in triplicate. The survival rate of B. bifidum during freeze-drying and the viable counts of freeze-dried powder under the effect of the cryoprotectant were calculated by the following equation:
(1)
(1)
(2)
(2)
where A1 is the viable counts of cell suspension after freeze-drying, A0 is the viable counts of cell suspension before freeze drying, V is the volume of cell suspension, m is the weight of freeze-dried powder and the unit of viable counts is cfu/mL.
Plackett–Burman experimental design
The Plackett–Burman (P–B) design is an efficient statistical method, which can screen the important factors for response values among multiple variables and predict their optimum values. The anti-freeze factors (NaCl of 0.74%, sorbitol of 0.08%, glutamic acid of 0.02%) obtained in previous studies (data unpublished) were taken into the optimized MRS medium and the various cryoprotectants were selected.
These cryoprotectants, namely, xylo-oligosaccharides, inulin, stachyose, glycine, arginine, sodium bicarbonate, ascorbic acid and skim milk were further optimized by a two-level-eleven-factor P–B design. The survival rate of B. bifidum was taken as the response value. The factors and coded levels of the P–B design were shown in . X2, X5 and X9 are three virtual terms to estimate the error.
Table 1. The factor levels coding table of P–B design.
Steepest ascent experiment
According to the effect of three significant factors on the response value, their step size and direction of change were set. The steepest ascent experiment was subsequently performed to determine the center point of the following response surface analysis.
Central composite design of RSM
Zero level was set for the center point, five levels were set for each factor. The survival rate (Y1) and viable counts (Y2) of B. bifidum were used as the response values. A five-level-three-factor CCD was conducted to determine the optimal composition of cryoprotectant in the B. bifidum BB01 freeze-drying experiment (). The value of alpha was determined by the formula:
(3)
(3)
Table 2. The factors level coding table of central composite design.
Experimental runs in CCD can be estimated by:
(4)
(4)
where k is the number of independent variables and cp is the replicate number of the central point [Citation25].
The viable counts of per unit weight of freeze-dried powder were selected as one of the two response values in the subsequent experiments, which was on account of the low content of optimized additive and there was little difference between the experimental groups. Since the yield of the powder was basically stable, two indicators of viable counts and survival rate were considered as response values.
Date analysis
All P–B experimental design, CCD design, data analysis and model establishment in this paper were operated by statistical software SAS 9.1 (SAS Institute Inc., Cary, NC, USA).
Results and discussion
P–B experimental design and results
The experimental design and the results of P–B are listed in , including eight independent factors and three virtual items. The response value Y1 indicates the freeze-drying survival rate of B. bifidum, expressed as a percentage.
Table 3. The experimental design and results of Plackett–Burman.
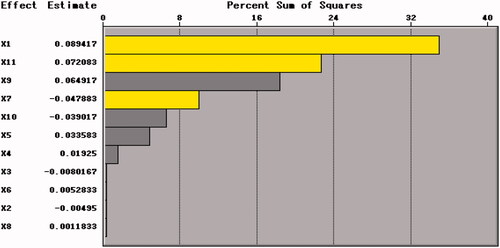
The effect of each factor to the survival rate of BB01 (Y value) was analyzed by statistical software SAS 9.1. The analysis results showed that several factors, such as X1, X7, X9 and X11 had reached a higher value in Pareto charts (. Among them, X9 was used to estimate the error which could be removed. Meanwhile, among the several factors with less influence, X6 and X8 were selected as fixed additives and added with maximum concentrations because their effects on Y1 were positive but not significant.
Similarly, also showed that X1, X7 and X11 made up for a large proportion of the percent sum of squares, which suggested that these three factors had a great influence on the freeze-drying effect of B. bifidum. As the concentration of these two factors increased, the response value Y1 increased accordingly. Therefore, xylo-oligosaccharides (X1), arginine (X7) and skim milk (X11) were selected as the main factors for further analysis. Meanwhile, glycine (X6) and sodium bicarbonate (X8) were also selected as fixed additives.
Steepest ascent experimental design and results
The three significance factors (X1, X7, X11) selected by the P–B design was used to conduct the steepest ascent experiment to determine the center point which was regarded as level 0. The test design and results are shown in . The value Y1 (%) represents the survival rate of B. bifidum during freeze-drying and Y2 (1011 cfu/g) represents viable counts of freeze-dried powder.
Table 4. Design and results of the path of steepest ascent experiment.
As shown in , the survival rate of B. bifidum during freeze-drying and viable counts of freeze-dried powder reached the maximum when the three factors of X1, X7 and X11 were near the second step. Therefore, the level of each factor in the second step, that is, 7% xylo-oligosaccharide, 4.5% arginine and 25% skim milk were selected as the center point of the subsequent CCD.
CCD experimental design and results
The survival rate and viable counts of per unit weight of powder are the evaluation indices of the freeze-drying effect of cryoprotectants on B. bifidum, which can directly reflect the lyophilization protection effect of the cryoprotectant on B. bifidum. Normally, the larger the two values, the better the freeze-drying effect of the powder. A five-level-three-factor response surface analysis experiment with N = 23 was performed according to the design of CCD. The survival rate and the viable counts in the powder after lyophilization are shown in .
Table 5. Design and results of the path of steepest ascent experiment.
Response surface analysis was performed on the above results by using SAS software. The multiple regression equation could be obtained after quadratic fitting:
(5)
(5)
(6)
(6)
where Y1 and Y2 are the predicted values of the freeze-drying survival rate and the viable counts of per unit weight of freeze-dried powder and X1, X2 and X3 are the coded values of the independent variables xylo-oligosaccharides, arginine and skim milk, respectively.
According to the regression equation obtained above, analysis of variance (ANOVA) was used to test the significant level. The results of the ANOVA for the quadratic model are shown in .
Table 6. ANOVA of the response variables for the hydroxyl free radical scavenging rate (Y1) and the DPPH radical scavenging rate (Y2).
showed that the p values of the two response surface models (p<.001) revealed the extreme significance of the model, while the values of lack of fit were not significant (p>.05), which indicated that the model was extremely significant and the regression analysis was effective. Furthermore, the complex correlation coefficient (R2) of Y1 model was 94.96% and the adjusted complex correlation coefficient (adjR2) was 91.46%, while the R2 of Y2 was 97.74% and adjR2 is 96.18%, which indicated that the regression equation can accurately predict the effect of different factors on the two responded values of freeze-drying survival rate and the viable counts of B. bifidum freeze-dried powder, which were represented by Y1 (%) and Y2 (1011 cfu/g), respectively. In addition, there was not a simple linear correlation between the xylo-oligosaccharides, arginine and skim milk because the result that the quadratic of the two equations were all extremely significant (pY1 = pY2 = .0001 < .001).
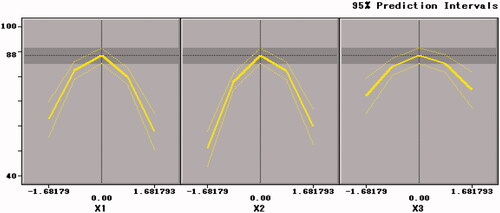
As shown in , the freeze-drying survival rate of B. bifidum (Y1) was significantly affected by the curvature effects of three factors and both extreme values emerged at the inflection point in the three graphs (. Among them, survival rate (Y1) increased with increasing of X1, X2 and X3 until it reached extreme values, then Y1 decreased with increasing of X1, X2, and X3.
The interaction between survival rate of B. bifidum (Y1) and significant factors containing xylo-oligosaccharides (X1), arginine (X2) and skim milk (X3) can be reflected by the contour plots and response surface analysis in the following , Citation5, and Citation6.
Figure 4. Contour plots and response surface of xylo-oligosaccharides (X1) and arginine (X2) for the survival rate of BB01 (Y1).
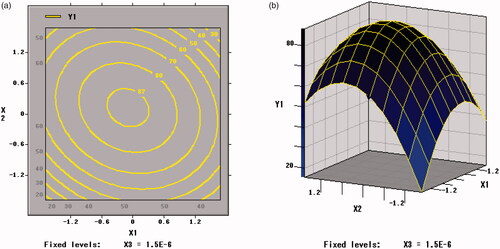
showed the response surface of xylo-oligosaccharide and arginine to the freeze-dried survival rate of B. bifidum. The contour plot corresponding to the three-dimensional figure was an ellipse that approximates to a circle, so the xylo-oligosaccharide and arginine had a mutual effect on the freeze-drying survival rate, and the influence was significant (p=.0334 < .05). Therefore, the selection of an appropriate ratio between xylo-oligosaccharide and arginine can increase the value of the freeze-drying survival rate (Y1).
showed the response surface of xylo-oligosaccharides and skim milk to the freeze-dried survival rate of B. bifidum. The contour plot of the surface response figure was approximately circular, indicating that the interaction between the two factors was weak and the influence between each other was small (p = .9477 > .05). The circular shape in implied that there was a weak interaction between arginine and skim milk (p = .2381 > .05) on the freeze-dried survival rate of B. bifidum. This result is similar to that in .
Figure 5. Contour plots and response surface of xylo-oligosaccharides (X1) and skim milk (X3) for the survival rate of BB01 (Y1).
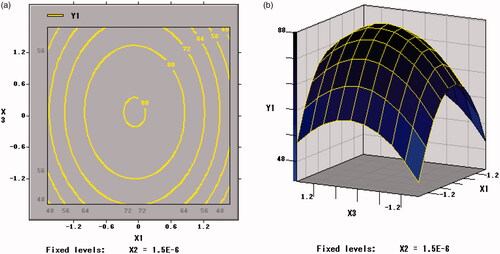
Figure 6. Contour plots and response surface of arginine (X2) and skim milk (X3) for the survival rate of BB01 (Y1).
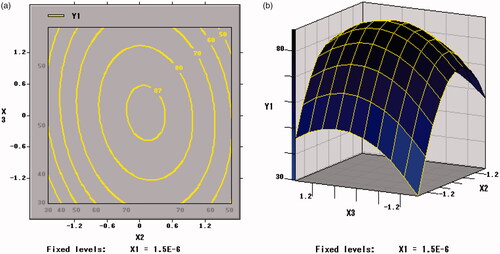
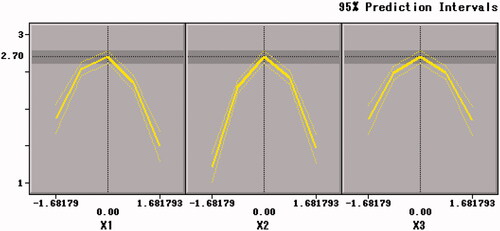
The trends of Y2 with factors are shown in . The X1 × X1, X2 × X2 and X3 × X3 were extremely significant (p < .001). The viable counts of per unit weight of freeze-dried powder of B. bifidum (Y2) was significantly affected by the curvature effects of xylo-oligosaccharides, arginine and skim milk and both extreme values were obtained at the inflection point. The change tendency of the three factors on Y2 was in accordance with that of Y1 in .
As shown in , Citation9, and Citation10, the contour plots corresponding to the three-dimensional figures were nearly a circle, so the interaction between X1 and X2 (p = .1137 > .05), X1 and X3 (p = .9277 > .05), X2 and X3 (p = .1023 > .05) were weak, thus the influence between each other was insignificant. Therefore, an appropriate ratio between xylo-oligosaccharide and arginine can increase the value of the freeze-drying survival rate (Y1).
Figure 8. Contour plots and response surface of xylo-oligosaccharides (X1) and arginine (X2) for the viable counts of per unit freeze-dried powder of BB01 (Y2).
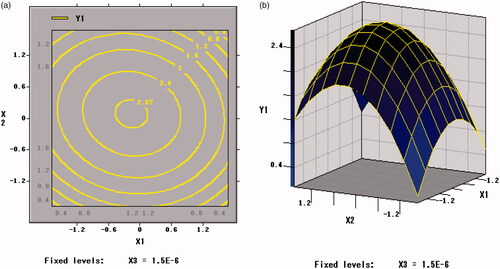
Figure 9. Contour plots and Response surface of xylo-oligosaccharides (X1) and skim milk (X3) for the viable counts of per unit freeze-dried powder of BB01 (Y2).
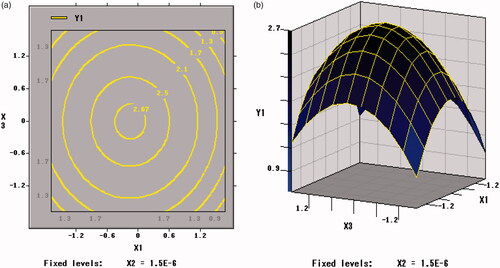
Figure 10. Contour plots and Response surface of arginine (X2) and skim milk (X3) for the viable counts of per unit freeze-dried powder of BB01 (Y2).
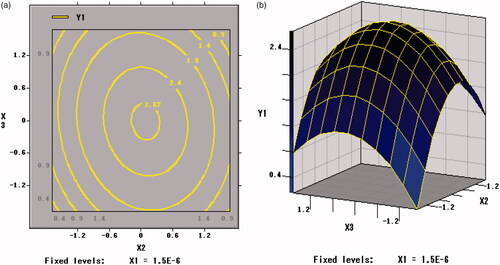
The regression equation was analyzed by SAS software. The two maximum points of X1, X2, and X3 obtained under the two response values were same, which were the central points that is xylo-oligosaccharides of 7%, arginine of 4.5% and skim milk of 25%. The predicted freeze-dried survival rate of B. bifidum under this condition was 88.58% and viable counts of per unit weight of freeze-dried powder of B. bifidum were 2.71 × 1011 cfu/g.
Verification experiment
The anti-freeze factors (NaCl of 0.74%, sorbitol of 0.08%, glutamic acid of 0.02%) were added to the optimized medium and the bacterial mud was prepared according to the method described in section “Preparation of cryoprotectants and vacuum freeze-drying”. The experimental group added the optimized composite cryoprotectant while the control group only added the phosphate buffer protectant. Three confirmatory experiments were carried out to obtain the final freeze-dried survival rate and the viable counts of per unit weight of freeze-dried powder of the experimental group (90.37 ± 1.9%, (2.78 ± 0.13) × 1011 cfu/g). The predicted values of both responses are within this range, which proves that it is feasible to use the response surface method to optimize the cryoprotectant of B. bifidum BB01. The survival rate and the viable counts of per unit weight of powder were increased by 22.8 and 51.91%, respectively, after optimization by RSM.
Discussion
The Central Composite Design of RSM optimized the composition of composite cryoprotectant for BB01. The survival rate and the viable counts of per unit weight of powder were increased by (90.37 ± 1.9%) and (2.78 ± 0.13) × 1011 cfu/g with the addition of the optimal compound protectants, which were respectively increased by 22.80 and 51.91% in comparison with the control group. It suggested that RSM was successfully used to optimize composite cryoprotectant for freeze-dried powder of B. bifidum, which can as well protect the probiotic cells. Yu et al. [Citation27] found that sucrose alone cannot prevent low levels of damage during freezing and pre-freezing. Wang et al. [Citation28] reported that using skim milk powder of 7.06%, maltose of 6.46% and sodium glutamate of 6.70% as a composite cryoprotectant, the survival rate of Lactobacillus can reach to 92.95%. This also illustrates that the combination of several protectants has a better effect on the survival rate of B. bifidum compared with a single protectant.
The compound freeze-protectant formulations we optimized were as follows: glycine of 5.5%, sodium bicarbonate of 0.8%, xylo-oligosaccharides of 7%, arginine of 4.5%, and skim milk of 25%. In this condition, the freeze-drying survival rate of B. bifidum and the number of per unit weight of powder is significantly improved because of the complementary effects of these components. The compounds are differently permeable to the cells, thus affecting the mechanism of their protective effect. Skim milk mainly acts as a protective layer on the cell surface, while other components have different permeability to the cell membrane or may penetrate the cell wall or the cell interior [Citation29]. Tymczyszyn et al. [Citation30] found that sugars replace water and protein to maintain protein structure stability and reduce intracellular ice crystal formation.
Under the same conditions, there are differences in the characteristics of period underlying freezing, drying, and subsequent storage between different microorganisms [Citation31,Citation32]. The study showed that [Citation31] the morphology of cells influences the survival rate of lyophilization and live bacteria. For example, spherical bacteria are significantly more resistant than rod-shaped bacteria. This is due to the reason that the damage of the formed cell ice crystal to its cell membrane increases with the larger cell-specific surface area during the freezing process. Culture conditions of the Bifidobacterium are also optimized, which is based on the characteristics of the strain, to provide a culture medium having a high viable count for the preparation of the high-activity B. bifidum powders. Huang et al. [Citation33] added sorbitol, skim milk, etc. to the culture medium and found that the survival rate of L. burglarious cells was lyophilized to 86.53%.
Several factors such as bacteria itself, culture conditions, freeze-drying process and protective effect of various protectants are the significant parts in the quality of biological products under lyophilization conditions [Citation34]. Apart from the cryoprotectants studied in this paper and the methods mentioned above, the improvement of the anti-freeze ability of the bacteria itself is crucial to the production and storage of high-activity powders. Some research had been conducted in our previous study, especially on the prebiotics anti-freeze factors. However, the mechanism of cell changes and damage in the process of freeze-drying has not been fully explained yet. Therefore, further research on the self-resistance abilities of B. bifidum is needed, such as sublethal processing. Besides, an effective and low-cost composite cryoprotectant formulation was obtained in this study and we also made some effort to gain several anti-freezing factors in our previous study. Further studies are needed on the appropriate ratio of the anti-freezing factors studied earlier and the freeze-drying protective agent to meet the economic and efficient requirements.
Conclusions
In this study, RSM was employed to optimize the cryoprotectant of B. bifidum BB01, the optimized formula of composite cryoprotectant was obtained: glycine of 5.5%, sodium bicarbonate of 0.8%, xylo-oligosaccharides of 7%, arginine of 4.5%, skim milk of 25%. The survival rate and the number of viable cells per unit weight of powder were (90.37 ± 1.9)% and (2.78 ± 0.13) × 1011 cfu/g with the addition of the optimal compound protectants, which were not significantly different with the predicted value (88.58% and 2.71 × 1011 cfu/g). Therefore, the results demonstrate that it is feasible to use the RSM to optimize the cryoprotectant of B. bifidum BB01.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- (a) Capela P, Hay TKC, Shah NP. Effect of homogenisation on bead size and survival of encapsulated probiotic bacteria. Food Res Int. 2007;40:1261–1269. (b) Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378.
- Huang J, Xin X, Pang M, et al. Application progress of prebiotics in global dairy products. Shandong Food Ferment. 2010;1:40–43.
- Roberfroid M, Gibson GR, Hoyles L, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:S1–S63.
- Ku S, Park M, Ji G, et al. Review on Bifidobacterium bifidum BGN4: functionality and nutraceutical applications as a probiotic microorganism. IJMS. 2016;17:1544.
- Zhou H, Bo G, Zhao R. Preparation of probiotics Koumiss tablets. Chi Dai Ind. 2009;1:47–49.
- Gandhi A, Shah NP. Effect of salt on cell viability and membrane integrity of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium longum as observed by flow cytometry. Food Microb. 2015;49:197.
- Sanders ME. Probiotics: considerations for human health. Nutr Rev. 2003;61:91–99.
- Li J, Shigwedha N, Mwandemele OD. Use of Dacid-, Dbile-, zacid-, and zbile- values in evaluating bifidobacteria with regard to stomach ph and bile salt sensitivity. J Food Sci. 2010;75:14–18.
- Piumi DA, Abeysundara1 ND, Ramakrishna N. Influence of cold stress on the survival of Listeria monocytogenes Bug600 and ScottA in lethal alkali, acid and oxidative stress. LWT. 2019;100:40–47.
- Soni KA, Nannapaneni R, Tasara T. The contribution of transcriptomic and proteomic analysis in elucidating stress adaptation responses of Listeria monocytogenes. Foodborne Pathog Dis. 2011;8:843–852.
- Mokhtari S, Jafari SM, Khomeiri M. Survival of encapsulated probiotics in pasteurized grape juice and evaluation of their properties during storage. Food Sci Tech Int. 2018;25(2).
- Mokhtari S, Jafari SM, Khomeiri M, et al. WITHDRAWN: The cell wall compound of Saccharomyces cerevisiae as a novel wall material for encapsulation of probiotics. Food Res Int. 2017;96:19–26.
- Sharma M, Devi M. Probiotics: a comprehensive approach toward health foods. Crit Rev Food Sci Nutri. 2014;54:537–552.
- Furrie E. Is Bifidobacterium a more effective probiotic therapy than Lactobacillus for patients with irritable bowel syndrome? Nat Rev Gastroenterol Hepatol. 2005;2:304–305.
- Bezirtzoglou E, Stavropoulou E. Immunology and probiotic impact of the newborn and young children intestinal microflora. Anaerobe. 2011;17:369–374.
- Liu Z, Ma Y, Wen D. Treatment of obesity and its mechanisms by probiotics and prebiotics. Chin J Microecol. 2018;9:1096–1101.
- Zhang Y, Ma Y. Biocharacteristics and health effect of Bifidobacterium. J Prevent Med Chin PLA. 2005;23:386–389.
- Wang Y, Huang Y. Effect of Lactobacillus acidophilus and Bifidobacterium bifidum supplementation to standard triple therapy on Helicobacter pylori eradication and dynamic changes in intestinal flora. World J Microbiol Biotechnol. 2014;30:847–853.
- Sadeq H, Amin I, Mohd Y. Hypocholesterolaemic effect of yoghurt containing Bifidobacterium pseudocatenulatum G4 or Bifidobacterium longum BB536. Food Chem. 2012;135:356–361.
- Chen H, Chen S, Shu G, et al. Response surface optimization of lyoprotectant for Lactobacillus bulgaricus during vacuum freeze-drying. Prep Biochem Biotech. 2015;45:463.
- Hubalek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003;46:205–229.
- Yi-Heng C, Chu-Chyn O, Hsiao-Ching F. Abelmoschus esculentus (L.) Moench polysaccharide enhances the resistance of Bifidobacterium longum MYL16 to freeze-drying and artificially digestive processes. J Funct Food. 2014;11:172–177.
- Ghorbannezhad P, Bay A, Yolmeh M, et al. Optimization of coagulation–flocculation process for medium density fiberboard (MDF) wastewater through response surface methodology. Desalination Water Treatm. 2016;1–16.
- Kaushik R, Saran S, Isar J, et al. Statistical optimization of medium components and growth conditions by response surface methodology to enhance lipase production by Aspergillus carneus. J Molec Catal B. 2006;40:121–126.
- Yolmeh M, Jafari SM. Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 2017;10:413–433.
- Shu G, Lei N, Chen H, et al. Application of central composite design to optimize the amount of carbon source and prebiotics for Bifidobacterium bifidum BB01. Food Tech. 2016;20:41–52.
- Yu J, Anchordoquy TJ. Synergistic effects of surfactants and sugars on lipoplex stability during freeze-drying and rehydration. J Pharm Sci. 2009;98:3319–3328.
- Wang S, Hong Y, Yuan X. Optimization of cryoprotectants for Bacillus coagulans and Lactobacillus plantarum by response surface methods. J Sichuan Uni Sci Eng. 2013;5:23–26.
- Pu L, Liu N. The research development of cryoprotector of lactic acid bacteria and its protective mechanism. Mod Food Sci Tech. 2005;1:147–149.
- Tymczyszyn EE, Gómez ZA, Disalvo EA. Effect of sugars and growth media on the dehydration of Lactobacillus delbrueckii ssp. bulgaricus. J Appl Microbiol. 2007;102:845–851.
- Fonseca F, Beal C, Corrieu G. Method for quantifying the loss of acidification activity of lactic acid starters during freezing and frozen storage. J Dairy Res. 2000;67:83–90.
- Gardiner GE, O’Sullivan E, Kelly J. Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L. salivarius strains during heat treatment and spray drying. App Envl Microb. 2000;66:2605–2612.
- Huang L, Lu Z, Yuan Y, et al. Optimization of a protective medium for enhancing the viability of freeze-dried Lactobacillus delbrueckii subsp. Bulgaricus based on response surface methodology. J Ind Microbiol Biotechnol. 2006;33:55–61.
- Tripathi MK, Giri SK. Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods. 2014;9:225–241.