?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To investigate the effects of miR-15a on proliferation and apoptosis of human knee articular chondrocytes and explore its underlying mechanism.
Methods
qRT-PCR was used to detect the expression of miR-15a in normal chondrocytes and knee arthritic chondrocytes; miR-con (transfected miR-con), miR-15a (transfected miR-15a mimics), anti-miR-con group (transfected anti-miR-con), anti-miR-15a group (transfected anti-miR-15a mimics), pcDNA group (transfected pcDNA), pcDNA-SMAD2 group (transfected pcDNA-SMAD2), the miR-15a + pcDNA group (co-transfected miR-15a and pcDNA), miR-15a + pcDNA-SMAD2 group (co-transfected miR-15a mimics and pcDNA-SMAD2), were transfected into knee articular chondrocytes by liposome method, respectively. The cell proliferation and apoptosis of each group were detected by MTT assay and flow cytometry. The protein expression of SMAD2 was detected by Western blot. The fluorescence activity of each group was detected by dual luciferase reporter gene assay.
Results
The expression of miR-15a in knee arthritis chondrocytes was significantly increased (p < .05) compared with that in normal chondrocytes. Moreover, overexpression of miR-15a and silencing of SMAD2 inhibited proliferation and promoted apoptosis in knee arthritis chondrocyte. MiR-15a targeted SMAD2. Overexpression of SMAD2 reversed the inhibitory effects on proliferation and promotion effects on apoptosis induced by miR-15a in knee arthritis chondrocytes.
Conclusion
miR-15a can inhibit the proliferation and promote apoptosis of knee arthritis chondrocytes. The mechanism may be related to SMAD2, which will provide a new target for the treatment of knee arthritis.
Keywords:
Introduction
Osteoarthritis (OA) is a chronic arthropathy characterized by degeneration and destruction of articular cartilage in middle-aged and elderly people, especially knee arthritis [Citation1]. The chondrocytes maintain the integrity of cartilage, weight bearing and repair of damaged cartilage [Citation2]. miRNA is a microRNA molecule that plays a vital role in gene silencing and translational inhibition by binding to target mRNA [Citation3]. miRNAs have been discovered since 1993 and are found in all eukaryotic cells throughout the species . In recent years, miRNAs have been extensively studied in the regulation of the occurrence and development of various human diseases such as retinal diseases, neurodegenerative diseases, cardiovascular diseases, and cancer including osteoarticular cartilage diseases [Citation4]. However, studies have reported that miR-15a played a protective role in osteoarticular cartilage [Citation5,Citation6], but its specific molecular mechanism remains undefined. TGF-beta/SMAD pathway played an important role in chondrocyte [Citation7,Citation8], in which Smad 2/3 played a key role [Citation9,Citation10]. However, the regulation mechanism of SMAD2 in osteoarthritic chondrocytes is not fully understood. In this study, OA chondrocytes were used as the research sample to detect the expression of miR-15a and SMAD2 in OA chondrocytes, and observe the effects of overexpression of miR-15a, silencing SMAD2 and overexpression of SMAD2 on proliferation and apoptosis of OA chondrocytes. Its mechanism may be related to the targeted negative regulation of SMAD2, which will lay the foundation for the treatment of osteoarthrosis.
Materials and methods
Materials
Tissue specimens were collected from 2 cases of abandoned articular cartilage undergoing total knee arthroplasty and 1 case of abandoned hip and knee cartilage in the lower limb in our hospital from April 2017 to November 2018. This study was approved by the Medical Ethics Committee of our hospital. All patients and their families signed the informed consent. DMEM medium, fetal bovine serum, MTT and trypsin were purchased from Sellect Company, USA. LipofectamineTM2000. BCA protein quantification kit and reverse transcription kit were brought from Dalian Takara Company. The PVDF membrane was obtained from Roche, Germany; the SDS-PAGE kit, ECL luminescent solution and RIPA protein lysate were acquired from Bieyotime Biotechnology Company; Dual luciferase reporter gene detection kit was purchased from Promega Company, USA, and Annexin V-FITC/PI apoptosis detection kit was obtained from Solarbio Company, Beijing, China.
Methods
Cell isolation and culture
Human normal chondrocytes and osteoarthritic articular cartilage were isolated and labeled as normal chondrocyte group and OA chondrocyte group.
Cell transfection
OA chondrocyte cells were randomly divided into miR-con (transfected miR-con), miR-15a (transfected miR-15a mimics), anti-miR-con (transfected anti-miR-con), anti-miR-Group 15a (transfected anti-miR-15a mimics), pcDNA group (transfected pcDNA), pcDNA-SMAD2 group (transfected pcDNA-SMAD2), miR-15a + pcDNA group (co-transfected with miR-15a and pcDNA), miR-15a + pcDNA-SMAD2 group (co-transfected with miR-15a and pcDNA-SMAD2). The cells were transfected into OA chondrocytes according to the instructions of the liposome LipofectamineTM 2000 kit. After transfection for 48 h, the transfection efficiency was detected by qRT-PCR. Then, it was used for subsequent experiments.
QRT-PCR experiment
The appropriate amount of cells in each group at logarithmic growth stage were extracted and quantified according to the instructions of RNA extraction kit, and then the cDNA was synthesized according to the instructions of reverse transcription kit. Finally, the miR-15a detection was performed according to the instructions of the qRT-PCR kit. The relative expression of miR-15a was calculated using 2–ΔΔCt.
MTT experiment
Take appropriate amount of 1.2.2 cells in each group, add 20 µl 5 g/L MTT solution, incubate for 3.5/4 h, then discard the supernatant. 150 µl of DMSO was added to each well, shaken to dissolve the crystals, and the absorbance of the cells was measured at a wavelength of 570 nm. The proliferative ability of the cells is directly proportional to the absorbance.
Annexin V-FITC/PI flow cytometry experiment
The cells in each transfection group were suspended in 500 µl buffer solution, and 5 µl Annexin V-/FITC and PI were added respectively. The cells were evenly blended and kept silent at room temperature for 15 min. The results of the assay were analyzed by flow cytometry.
Western blot experiment
The cells of each group in logarithmic growth stage were taken for quantitative analysis by BCA after RIPA lysis, and the supernatant was taken for protein sampling after denaturation and centrifugation. Electrophoresis-transmembrane-blocking-I-incubation-II anti-incubation-development exposure was performed according to the routine procedure of Western blot. Image J analyzed the gray value of the target strip, and analyzed the expression of the target protein by the ratio of the gray value of the target strip to the gray value of β-actin.
Dual luciferase reporter gene assay
The luciferase reporter vector (psiCHECK2-SMAD2-WT, psiCHECK2-SMAD2-MUT) was transfected into articular chondrocyte cells with miR-15a mimics or miR-NC. After 6 h of culture, fresh culture medium was replaced and further cultured for 48 h. Operate according to the instructions of dual luciferase reporter gene detection kit. The firefly luciferase activities were normalized by Renilla luciferase activities.
Statistical analysis
All data in the experiment were analyzed using SPSS 21.0 software (IBM, Armonk, NY, USA). Measurement data from three independent experiments were presented as mean ± standard deviation (). Data comparison between groups was performed by one-way analysis of variance (ANOVA). Pairwise comparison was performed by Student’s t-test. The data were considered statistical significance at p values < .05.
Results
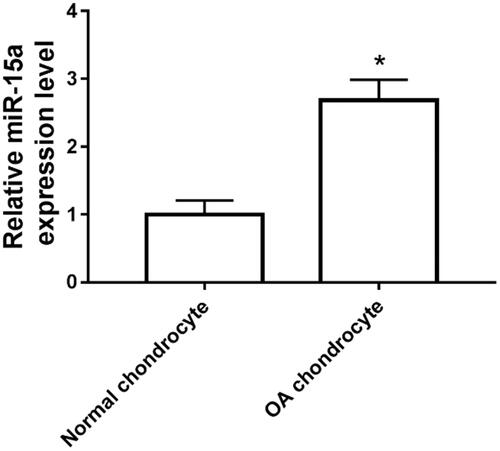
Expression of miR-15a in human osteoarthritic chondrocytes
As shown in , the expression of miR-15a in the osteoarthritic chondrocytes was significantly increased compared with that in normal chondrocyte group.
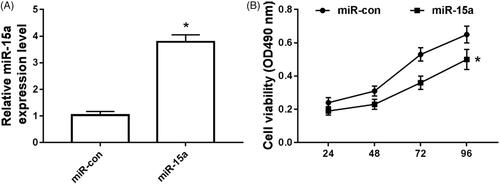
Overexpression of miR-15a inhibits chondrocyte proliferation
The results are shown in . Compared with the miR-15a expression in miR-NC group, its expression was significantly increased in the osteoarthritic chondrocytes transfected with miR-15a (), and the cell activity was significantly decreased ().
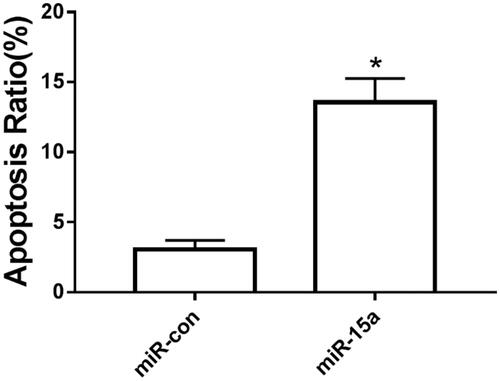
Effect of overexpression of miR-15a on chondrocyte apoptosis
The results are shown in . The apoptotic rate of osteoarthritic chondrocytes in the miR-15a group was dramatically increased compared with that in the miR-NC group.
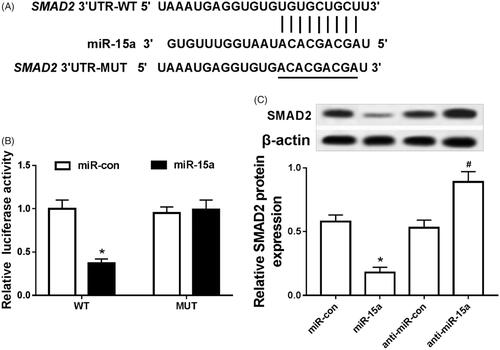
MiR-15a targets SMAD2
The binding sites between miR-15a and SMAD2 3′UTR were predicted by using the microcode database (). The results of dual luciferase activity assay showed that the fluorescence activity of SMAD2-WT cells in miR-15a group was apparently decreased compared with that in miR-NC group, and the fluorescence activity in SMAD2-WT cells was not affected (). Compared with the expression of SMAD2 in the miR-NC group, its expression was strikingly decreased in the miR-15a group. SMAD2 was notably up-regulated in the anti-miR-15a group compared with its matched group ().
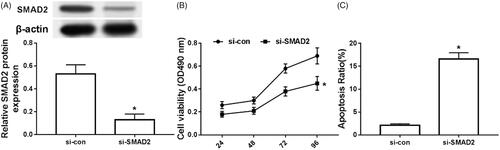
Silencing SMAD2 inhibits chondrocyte proliferation and induces apoptosis in arthritis
The results are shown in . SMAD2 was obviously decreased in the articular chondrocytes of the si-SMAD2 group compared with that in the si-con group (), and the cell activity was remarkably decreased (), and the apoptosis was markedly increased ().
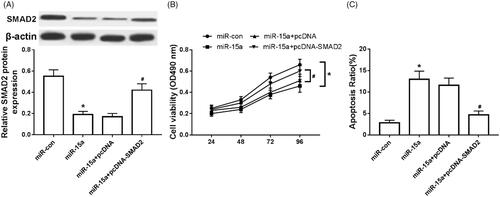
Overexpression of SMAD2 partially reverses the inhibitory effect of miR-15a overexpression on chondrocytes
Compared with the expression of SMAD2 in the miR-con group, SMAD2 expression was obviously decreased in the miR-15a group (). Also, the cell activity was significantly decreased (), while the apoptosis was evidently increased (). Moreover, The expression of SMAD2 was apparently increased in the miR-15a + pcDNA-SMAD2 group compared with that in the miR-15a + pcDNA group (). The cell activity was significantly increased (), and the apoptosis was significantly decreased ().
Figure 6. Overexpression of SMAD2 partially reversed the inhibitory effect of overexpression of miR-15a on chondrocytes. (A) Overexpression of SMAD2 on the expression of SMAD2 in arthritis chondrocytes; (B) Overexpression of SMAD2 on the activity of arthritis chondrocytes; (C) Effect of overexpression of SMAD2 on the apoptosis of arthritis chondrocytes. *p < .05.
Discussion
The function of miRNA in OA has been attracted more and more attention [Citation11]. miRNA is a short, small, non-coding RNA group molecules involved in various human diseases [Citation12]. miRNAs regulate gene expression by inhibiting messenger RNA (mRNA) translation and promoting mRNA degradation [Citation13]. Previous studies have confirmed that many miRNAs, such as miR-21, miR-23a, ha-miR-24, ha-miR-27, miR-93, ha-miR-100, ha-miR-140, ha-miR-145, Ha-miR-146a and hsa-miR-675 are associated with the development of OA [Citation14–16]. It was clarified in the study of osteoarthritis that over-expression of miR-15a could significantly promote osteoarthritis chondrocyte cells apoptosis and inhibit osteoarthritis chondrocyte cells proliferation. In the study of osteoarthritis, Chen et al. measured the expression of miR-15a-5p in OA chondrocytes increased significantly, while the expression of VEGFA decreased significantly [Citation17]. Functional analysis based on luciferase activity assay, CCK-8 assay and flow cytometry revealed that miR-15a-5p directly targeted the 3'-UTR of VEGFA and inhibited its expression, VEGFA overexpression or miR-15a-5p inhibition promoted cell proliferation, suppressed apoptosis and reduced matrix degradation in OA chondrocytes. Furthermore, restoration experiments of VEGFA and miR-15a-5p demonstrated that miR-15a-5p promoted apoptosis and matrix degradation by inhibiting VEGFA. We further provide evidence that a variety of proteins involved in matrix synthesis were regulated by miR-15a-5p and VEGFA, elucidating the potential mechanism by which miR-15a-5p regulated OA activity and matrix degradation. In this study, qRT-PCR was used to detect the expression of miR-15a in normal chondrocytes and osteoarthritic chondrocytes. It was found that miR-15a was highly expressed in osteoarthritic chondrocytes, and overexpression of miR-15a could inhibit osteoarthritis chondrocyte proliferation and promote cell apoptosis, which is consistent with previous studies. Further in-depth studies revealed that miR-15a and SMAD2 had a targeted binding site, and dual luciferase reporter assay was used to verify that miR-15a targeted SMAD2.
Transforming growth factor beta (TGF-β) in articular cartilage can be signaled by two pathways: ALK5/Smad2/3P and ALK1/Smad1/5/8P pathway, the former is protective and the latter is beneficial for chondrocyte terminal differentiation [Citation18,Citation19]. Madej et al. found that excessive stress can up-regulate the expression of Smad2/3 P response genes bSerpine1, bSmad 7 and bAlk5 in articular cartilage, but down-regulate the expression of Smad1/5/8P response gene bId1 [Citation20]. When ALK4/5/7 inhibitor blocked the ALK5/Smad2/3 pathway, it prevented the effect of excessive mechanical compression on the expression of bSmad 7 and bAlk5, suggesting that mechanical compression is not only related to physiology, but also related to excessive pressure-activated Smad2/3P signaling, which protects articular cartilage and blocks the differentiation of cartilage cell ends. Shi et al. reported that SMAD family member 2 (SMAD2) mRNA expression levels were lower in OA patients. The interaction between miR-486-5p and SMAD2 was verified by dual luciferase reporter gene assay. The expression level of miR-486-5p increased significantly in patients with OA. Overexpression of miR-486-5p inhibited the proliferation and migration of CHON-001 human chondrocytes, and also inhibited the expression level of type II collagen and aggregating proteoglycan. On the contrary, treatment with miR-486-5p inhibitor promoted proliferation and migration, as well as expression of type II collagen and aggrecan. Silencing SMAD2 reversed the up-regulation of miR-486-5p-induced up-regulation of proliferation and migration, as well as the expression levels of type II collagen and aggrecan, suggesting that miR-486-5p is up-regulated in OA, possibly inhibiting the proliferation and migration of chondrocytes by inhibiting SMAD2 [Citation21]. In this study, we examined the expression of SMAD2 in arthritic chondrocyte tissues and cells. Restoration experiments presented that SMAD2 silencing could inhibit the proliferation and promote apoptosis in arthritic chondrocytes by negatively regulated miR-15a. In-depth studies indicated that overexpression of SMAD2 partially reversed the proliferation inhibition and apoptosis-promoting effects of miR-15a on arthritic chondrocytes.
In conclusion, miR-15a can inhibit the proliferation and promote apoptosis of arthritic chondrocytes. The miR-15a/SMAD2 axis regulatory pathway may provide new target for the treatment of osteoarthritis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Li YP. Osteoarthritis genetic factors animal models mechanisms and therapies. Front Biosci. 2012;E4:74–100.
- Kim HA, Blanco FJ. Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets. 2007;8:333–345.
- Chan WC, Lin WC. MetaMirClust: discovery and exploration of evolutionarily conserved miRNA Clusters. Methods Mol Biol. 2016;1375:75–89.
- Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol. 2017;1509:1–10.
- Seeliger C, Karpinski K, Haug AT, et al. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J Bone Miner Res. 2014;29:1718–1728.
- Lu X, Lin J, Jin J, et al. hsa-miR-15a exerts protective effects against osteoarthritis by targeting aggrecanase-2 (ADAMTS5) in human chondrocytes. Int J Mol Med. 2016;37:509–516.
- Lo RS, Massagué J. Ubiquitin-dependent degradation of TGF-beta-activated smad2. Nat Cell Biol. 1999; 1:472–478.
- Tsukazaki T, Chiang TA, Davison AF, et al. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 1998;95:779–791.
- Zhu Y, Gu J, Zhu T, et al. Crosstalk between Smad2/3 and specific isoforms of ERK in TGF-β1-induced TIMP-3 expression in rat chondrocytes. J Cell Mol Med. 2017;21:1781–1790.
- Qureshi HY, Ricci G, Zafarullah M. Smad signaling pathway is a pivotal component of tissue inhibitor of metalloproteinases-3 regulation by transforming growth factor beta in human chondrocytes. Biochim Biophys Acta. 2008;1783:1605–1612.
- Le LT, Swingler TE, Clark IM. Review: the role of microRNAs in osteoarthritis and chondrogenesis. Arthritis Rheum. 2013;65:1963–1974.
- Sethupathy P, Collins FS. MicroRNA target site polymorphisms and human disease. Trends Genet. 2008;24:489–497.
- Djuranovic S, Nahvi A, Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336:237–240.
- Yu C, Chen WP, Wang XH. MicroRNA in osteoarthritis. J Int Med Res. 2011;39:1–9.
- Dudek KA, Lafont JE, Martinez-Sanchez A, et al. A. Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J Biol Chem. 2010;285:24381–24387.
- Swingler TE, Wheeler G, Carmont V, et al. The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum. 2012;64:1909–1919.
- Chen H, Tian Y. MiR-15a-5p regulates viability and matrix degradation of human osteoarthritis chondrocytes via targeting VEGFA. Bst. 2016;10:482–488.
- Davidson ENB, Remst DFG, Vitters EL, et al. Increase in alk1/alk5 ratio as a cause for elevated mmp-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;182:7937–7945.
- Caam AV, Davidson EB, Vitters E. AB0119 BMP-9 induces both SMAD1/5/8 and SMAD2/3 phosphorylation, and specific response genes, in chondrocytes. Osteoarthritis Cartilage. 2012;20:S133–S133.
- Madej W, Caam AV, Davidson ENB, et al. Physiological and excessive mechanical compression of articular cartilage activates Smad2/3P signaling. Osteoarthritis Cartilage. 2014;22:1018–1025.
- Shi J, Guo K, Su S, et al. miR‑486‑5p is upregulated in osteoarthritis and inhibits chondrocyte proliferation and migration by suppressing SMAD2. Mol Med Rep. 2018;18:502–508.