Abstract
A theoretical model (ideal condition) to predict porosity in foods during drying is developed based on conservation of mass and volume principle, and assuming that volume of pores formed is equal to the volume of water removed during drying. As expected the ideal model may not be valid in many practical cases. The ideal model is then extended for non‐ideal conditions, when there is either shrinkage, collapse or expansion, by defining a shrinkage–expansion coefficient. Experimental porosity data from the literature was used to estimate the shrinkage–expansion coefficient for selected food materials.
Introduction
Pores occur in diverse food products, such as cakes, breads, and biscuits, breakfast cereals, extruded, fried, and dried products and the issues of pores in foods are generic. The process of pore formation or collapse during drying is very complex in nature, and it is a challenge to the food engineers and scientists to predict characteristics of pores. In most cases, the porosity is predicted by empirical correlations as a function of water content, such as linear,Citation1–3 quadratic,Citation[2],Citation4–8 exponential forms,Citation[9] and power law.Citation[10],Citation[11] The drawbacks of the empirical models are: (i) they are not generic and limited to the products and the experimental conditions used to develop the correlation, and (ii) they are without theoretical basis, and the parameter cannot be related to the physics.Citation[12] Lozano et al.Citation[13] developed the geometric model to predict the porosity of fruits during drying, which needs apparent shrinkage coefficient, cellular shrinkage coefficient, and density values of cell components. In addition to that the model requires the geometric characteristics of the cell, which is not easy to measure or estimate in a complex structure like food. They also used linear correlation of shrinkage coefficient with water content, which is valid only for early stage of drying. Later Lozano et al.Citation[14] modified the original model without geometric characteristic of cell, but still needs apparent shrinkage coefficient, apparent and solid densities. However, their model does not consider the variability of the material density as a function of water content, i.e. negligible interaction of the component (i.e., ϵ ex = 0). Krokida et al.Citation[15] also developed a model based on apparent shrinkage coefficient and solids density at zero moisture content. Again they also assumed there was no interaction between the components when moisture content was varied. His model was tested for selected food materials. The objective of this study was to develop a theoretical model to predict the porosity in foods during drying based on the conservation of mass and volume principle including a shrinkage–expansion coefficient.
Physics of Pore Formation
In this section a review on the factors, which affect pore formation is discussed. The collapse is a result of decrease in porosity, and caused shrinkage of the product. The factors affecting formation of pores can be grouped as: (i) intrinsic and (ii) extrinsic factors. The extrinsic factors are: temperature, pressure, relative humidity, gas atmosphere, air circulation, and electromagnetic radiation applied in the process, whereas the intrinsic factors are chemical composition and initial structure.Citation[16] The formation of pores in foods during drying can be grouped into two generic types:Citation[16] one with an inversion point and another without an inversion point ( and ). These generic trends are proposed based on the experimental evidence published in the literature. shows that initially pores are collapsed and reached a critical value, and further decrease of water content during drying causes the formation of pores until completely dried. Opposite conditions exists in . shows that pores are increased or decreased as a function of water content. However pattern of the curve changes on the basis of moisture content. For example the porosity of apple is plotted as a function of moisture content with wet basis and dry basis in and . These figures show the variation of the curvature although both graphs show that increase of pores with the decrease of moisture content.
Figure 1. Changes of porosity as a function of water content (with an inversion point).Citation[16]
![Figure 1. Changes of porosity as a function of water content (with an inversion point).Citation[16]](/cms/asset/f7d5ce0c-be4f-44e4-b28d-b08404bfc28d/ljfp_a_10345092_o_f0001.gif)
Figure 2. Change of porosity as a function of water content (no inversion point).Citation[16]
![Figure 2. Change of porosity as a function of water content (no inversion point).Citation[16]](/cms/asset/75821f0a-6bed-4c38-8cef-a788940e087b/ljfp_a_10345092_o_f0002.gif)
Figure 3. Porosity as a function of water content (wet basis) for apple during air drying. Source: Ref. Citation[15].
![Figure 3. Porosity as a function of water content (wet basis) for apple during air drying. Source: Ref. Citation[15].](/cms/asset/7cc661ee-c42c-446d-9e24-14d61c4f8c1d/ljfp_a_10345092_o_f0003.gif)
Figure 4. Porosity as a function of water content (dry basis) for apple during air drying. Source: Ref. Citation[15].
![Figure 4. Porosity as a function of water content (dry basis) for apple during air drying. Source: Ref. Citation[15].](/cms/asset/2666159c-bd1a-412d-a5bf-66e8ed8a262a/ljfp_a_10345092_o_f0004.gif)
Slade and LevineCitation[17] first applied the concept of glass transition to identify or explain the physico‐chemical changes in foods during processing and storage. The glass transition theory is one of the concepts that have been proposed to explain the process of shrinkage, collapse, fissuring, and cracking during drying.Citation[16],Citation18–21 The methods of freeze‐drying and hot air drying can be compared based on this theory. In freeze‐drying, with the drying temperature below or close to t ′ g (maximally freeze‐concentrated glass transition temperature, it is independent of solids content) or t g (glass transition as a function of solids content), the material is in the glassy state. Hence shrinkage is negligible. As a result, the final product is very porous. With hot air drying, on the other hand, with the drying temperature above t ′ g or t g , the material is in the rubbery state, and substantial shrinkage occurs causing lower level of pores. During the initial stage of freeze‐drying, the composition of the freeze‐concentrated phase surrounding the ice dictates the t ′ g . In initial or early stage of drying, t ′ g is very relevant and the vacuum must be sufficient to ensure that sublimation is occurring. At the end of initial stage of drying, the pore size and the porosity are dictated by ice crystal size, if collapse of the wall of the matrix that surrounded the ice crystal does not occur. Secondary stage of drying, on the other hand, refers to removal of water from the unfrozen phase. After sublimation is completed, the sample begins to warm up to the shelf temperature. At this stage, t g of the matrix is related to the collapse and no longer to t ′ g . This is due to the t g > t ′ g (t g increases from t ′ g as the concentration of solids increases during the process of drying). In many cases during convection air‐drying, the observations related to collapse are just the opposite the glass transition concept.Citation[22],Citation[23] The mechanism proposed for this was the concept of case hardening.Citation[24],Citation[25] They indicated that at a low drying rate (low temperature), the moisture gradient within the product is small and internal stresses are low and hence the material shrinks down fully onto a solid core, and shrinkage is uniform. At a high drying rate (higher temperature), the surface moisture decreased very fast so that the surface became stiff (i.e. casehardening phenomenon), limiting subsequent shrinkage, thus increase pore formation. RahmanCitation[16] identified that the glass transition theory does not hold true for all products or processes. Other concepts, such as surface tension, pore pressure, structure, environment pressure, and mechanisms of moisture transport also play important roles in explaining the formation of pores. RahmanCitation[16] hypothesized that as capillary force is the main force responsible for collapse, so counterbalancing this force causes formation of pores and lower shrinkage. The counterbalancing forces are due to generation of internal pressure due to vaporization of water, variation in moisture transport mechanism, and pressure outside the material. Other factor could be strength of solid matrix (i.e., ice formation, case hardening, permeability of crust, and matrix reinforcement). The complexity of the pore formation mechanism needs further study with diversified food materials and with a wider variation of processing conditions in order to develop a more unified concept of pore formation.
Model Development
Theoretical Equation for Ideal Condition
Food materials can be considered as multiphase systems (i.e., gas–liquid–solid systems). When the mixing process conserves both mass and volume principle, then the density of the multiphase system can be written as:
Ideal pore formation during drying can be predicted based on the assumption that there is no shrinkage (or collapse) or expansion in the initial volume of the material. If no pores exist and no interaction between the components takes place, from EquationEq. 2 the material density (excluding pores) and true density (excluding pores and interaction of components) at any given water content can be estimated as:
Model for Non‐ideal Conditions
The ideal model could be extended in the cases of non‐ideal conditions by introducing a shrinkage–expansion coefficient as:
Applications of the Model
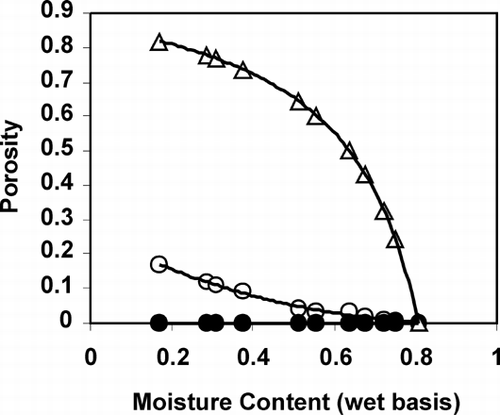
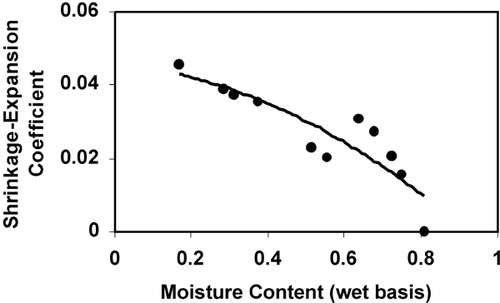
The proposed physical model was applied to the porosity data of apple and potato. shows the experimental values of porosity developed in potato during drying and values from the models considering φ equals to 0 and 1. This shows that it is important to know the values of φ as a function of product's characteristics as well as the processing conditions and types of unit operations. Experimental porosity data from the literature is compiled and the estimated values of φ for selected food materials are given in . The value of ρ s (material density at zero moisture content) is needed to estimate the values of φ. The values of ρ s shown in are estimated from the initial porosity and apparent density using EquationEq. 2 considering only solids and water as a component. The substance density can also be measured experimentally removing all moisture and grinding the material with guarantee that no closed pores present. The substance density can also be estimated from the composition of solids using EquationEq. 3 considering no water in the solids. shows the values of φ with the standard deviations in order to assess the variability with moisture content. The shrinkage–expansion coefficients of apple and potato show that the value of φ is higher when the pore formation is higher as indicated in the theoretical non‐ideal model. In case of potato, the trend is that φ increased with the increase of drying temperature (). In many cases the porosity data in the literature is difficult to use for further analysis due to the missing conditions of drying and processing as well as material's characteristics and composition.
Table 1. Values of φ during air drying of selected food materials
shows the variation of φ as a function of moisture content in order to identify their variation with the extent of drying in case of potato. It shows that there is a specific trend (φ increase with the decrease of moisture) of the variation of φ. The future works need to be targeted to estimate the values of φ for a wide variation of material's characteristics and processing conditions. It is also important to classify or group the values of φ with advanced statistical techniques. Moreover the pattern of shrinkage–expansion coefficient as functions of materials' initial characteristics and drying conditions could be done using hybrid neural network. The hybrid technique could be applied using both regression (statistics), and artificial neural network (machine learning and knowledge development from data mining, KDD). The future works need to be continued in characterizing the shrinkage–expansion coefficient of different foods during different methods of drying. More focus should be given to explore the possibility of identifying a generic pattern or nature of the coefficient as a function of drying conditions and initial characteristics of the foods. The future work of the author is targeting to achieve more in this direction.
Conclusion
A theoretical model is developed based on the conservation of mass and volume principle including a shrinkage–expansion coefficient. Shrinkage–expansion coefficient was found to be 0.174 for air‐drying of apple at 70°C, and the values varied from 0.009 to 0.051 for potato when air drying temperature increased from 30 to 70°C.
Acknowledgments
The project was supported by the Sultan Qaboos University from the internal grants Nos. IG/AGR/FOOD/00/02 and IG/AGR/BIOR/02/03. The comments provided by Dr. Mushtaque Ahmed are acknowledged and appreciated.
| Nomenclature | ||
| M | = |
Mass of sample at any moisture content per unit initial mass (kg/kg) |
| n | = |
Total number of components present in the mixtures |
| t | = |
Drying temperature (°C) |
| X | = |
Mass fraction (wet basis, kg water/kg sample) |
| ρ | = |
Density (kg/m3) |
| φ | = |
Shrinkage–expansion coefficient |
| ϵ | = |
Porosity |
| α | = |
Volume of water per unit mass (m3/kg) |
| β | = |
Volume of water and solids per unit mass (m3/kg) |
| Subscript | ||
| a | = |
apparent |
| ex | = |
excess |
| g | = |
glass transition |
| i | = |
ith component |
| m | = |
material |
| s | = |
substance (dry solids) |
| T | = |
true |
| w | = |
water |
| Superscript | ||
| o | = |
initial state before drying |
| ′ | = |
maximal freeze‐concentration condition in t or ideal condition in case of ϵ a |
| ″ | = |
non‐ideal conditions |
References
- Madamba , P. S. , Driscoll , R. H. and Buckle , K. A. 1994 . Bulk density, porosity and resistance to airflow of garlic slices . Dry. Technol. , 12 ( 4 ) : 937 – 954 .
- Palipane , K. B. , Driscoll , R. H. and Srzednicki , G. 1992 . Density, porosity and composition of macadamia in‐shell nuts . Food Aust. , 44 ( 6 ) : 276 – 280 .
- Joshi , D. C. , Das , S. K. and Mukherjee , R. K. 1993 . Physical properties of pumpkin seeds . J. Agr. Eng. Res. , 54 : 219 – 229 .
- Senadeera , W. , Bhandari , B. R. , Young , G. and Wijesinghe , B. 1998 . Change of physical properties of green beans during drying and its influence on fluidization . IDS '98, Proc. 11th International Drying Symposium IDS '98 . August 19–22 1998 , Halkidiki , Greece.
- McMinn , W. A.M. and Magee , T. R.A. 1998 . Density, shrinkage and porosity of starch gel during convective drying . IDS '98, Proceedings 11th International Drying Symposium IDS'98 . August 19–22 1998 , Halkidiki , Greece.
- Rahman , M. S. and Potluri , P. L. 1990 . Shrinkage and density of squid flesh during air drying . J. Food Eng. , 12 ( 2 ) : 133 – 143 .
- Rahman , M. S. , Perera , C. O. , Chen , X. D. , Driscoll , R. H. and Potluri , P. L. 1996 . Density, shrinkage and porosity of calamari mantle meat during air drying in a cabinet dryer as a function of water content . J. Food Eng. , 30 : 135 – 145 .
- Rapusas , R. S. and Driscoll , R. H. 1995 . Thermophysical properties of fresh and dried white onion slices . J. Food Eng. , 24 : 149 – 164 .
- Mattea , M. , Urbicain , M. J. and Rotstein , E. 1986 . Prediction of thermal conductivity of vegetables foods by the effective medium theory . J. Food Sci. , 51 ( 1 ) : 114 – 115 . 134
- Perez , M. G.R. and Calvelo , A. 1984 . Modeling the thermal conductivity of cooked meat . J. Food Sci. , 49 : 152 – 156 .
- Wildegger‐Gaissmaier , A. E. and Agarwal , P. K. 1990 . “ Shrinkage during drying and devolatillization of wet coal ” . In Drying '89 Edited by: Mujumdar , A. S. and Roques , M. A. 320 – 325 . NY : Hemisphere Publishing Corporation .
- Rahman , M. S. 1994 . The accuracy of prediction of the freezing point of meat from general models . J. Food Eng. , 21 : 127 – 136 .
- Lozano , J. E. , Rotstein , E. and Urbicain , M. J. 1980 . Total porosity and open‐pore porosity in the drying of fruits . J. Food Sci. , 45 : 1403 – 1407 .
- Lozano , J. E. , Rotstein , E. and Urbicain , M. J. 1983 . Shrinkage, porosity and bulk density of foodstuffs at changing moisture contents . J. Food Sci. , 48 : 1497 – 1553 .
- Krokida , M. K. , Zogzas , N. P. and Maroulis , Z. B. 1997 . Modelling shrinkage and porosity during vacuum dehydration . Int. J. Food Sci. Technol. , 32 : 445 – 458 .
- Rahman , M. S. 2001 . Toward prediction of porosity in foods during drying: A brief review . Dry. Technol. , 19 ( 1 ) : 1 – 13 .
- Slade , L. and Levine , H. 1991 . “ A food polymer science aroach to structure property relationships in aqueous food systems: Non‐equilibrium behavior of carbohydrate‐water systems ” . In Water Relationships in Food Edited by: Levine , H. and Slade , L. 29 – 101 . New York : Plenum Press .
- Cnossen , A. G. and Siebenmorgen , T. J. 2000 . The glass transition temperature concept in rice drying and tempering: Effect on milling quality . T. ASAE , 43 ( 6 ) : 1661 – 1667 .
- Karathanos , V. , Anglea , S. and Karel , M. 1993 . Collapse of structure during drying of celery . Dry. Technol. , 11 ( 5 ) : 1005 – 1023 .
- Karathanos , V. T. , Anglea , S. A. and Karel , M. 1996 . Structural collapse of plant materials during freeze drying . J. Therm. Anal. , 46 ( 5 ) : 1541 – 1551 .
- Krokida , M. K. , Karathanos , V. T. and Maroulis , Z. B. 1998 . Effect of freeze‐drying conditions on shrinkage and porosity of dehydrated agricultural products . J. Food Eng. , 35 : 369 – 380 .
- Wang , N. and Brennan , J. G. 1995 . Changes in structure, density and porosity of potato during dehydration . J. Food Eng. , 24 : 61 – 76 .
- Del Valle , J. M. , Cuadros , T. R.M. and Aguilera , J. M. 1998 . Glass transitions and shrinkage during drying and storage of osmosed apple pieces . Food Res. Int. , 31 ( 3 ) : 191 – 204 .
- Ratti , C. 1994 . Srinkage during drying of foodstuffs . J. Food Eng. , 23 : 91 – 105 .
- Achanta , S. and Okos , M. R. 1996 . Predicting the quality of dehydrated foods and biopolymers–research needs and opportunities . Dry. Technol. , 14 ( 6 ) : 1329 – 1368 .
- Miles , C. A. , Beek , G. V. and Veerkamp , C. H. 1983 . “ Calculation of thermophysical properties of foods ” . In Physical Properties of Foods Edited by: Jowitt , R. , Escher , F. , Hallstrom , B. , Meffert , H. F.T. , Spiess , W. E.L. and Vos , G. 269 – 312 . New York : Alied Science Publishers .
- Choi , Y. and Okos , M. R. 1983 . “ Effect of temperature and composition on the thermal properties of foods ” . In Food Engineering and Process Alications Edited by: Maguer , M. and Jelen , P. Vol. 1 , 93 – 101 . London : Elsevier Alied Science . Transport Phenomena
- Rahman , M. S. 1995 . Handbook of Food Properties 87 – 122 . Boca Raton , FL : CRC Press .