Abstract
Drying curves have been obtained from bread samples using an isothermal drying apparatus designed and developed in our laboratory. Previous work showed that Fick's diffusion equation was shown only to predict drying time and not drying rate. The results showed that proper measurement of effective moisture diffusivity under isothermal conditions did not solve the problem of accurately predicting moisture transfer. A first‐order irreversible kinetic model was developed to predict moisture loss during isothermal drying by modeling evaporation during drying. The drying curves obtained from the isothermal drying experiments were analyzed to determine the first‐order rate constant. The first‐order rate constant at temperatures between 40 and 70°C ranged from 0.04 s−1 to 0.19 s−1, and the activation energy determined by Arrhenius analysis was 48.7 kJ/mol. The rate equation was shown to impressively predict drying of bread samples throughout the entire moisture range, from 0.9 to 0.002 g/g dry solid.
Introduction
In the literature, many mathematical models have been proposed to model drying. It is preferable that the mathematical form(s) represent the physical behavior. Significant effort have taken place by Tong and his associates to better understand the mechanisms which govern the drying of hygroscopic porous materials.Citation1–9 Pre‐gelatinized dough (or bread) samples were used in all these studies.
Tong and LundCitation[4] modeled the simultaneous heat and moisture transfer in bread during microwave heating. The input parameters, including thermal physical properties, dielectric properties, transfer coefficients at the boundary and effective moisture diffusivity were determined experimentally as a function of temperature and moisture content. Fick's second law equation is the most widely used equation to model moisture transfer during drying; however, this diffusion equation failed to fully describe the drying characteristics of bread especially under prolonged heating.
LouvetCitation[2] studied the drying rates of pre‐gelatinized dough samples during microwave heating at different power levels with and without the presence of saturated steam at the surface. No significant difference in drying rate was observed when the remaining drying parameters, e.g., temperature history, sample size, and microwave power were kept constant. Since the drying rate would have been affected by the boundary conditions if the drying had been diffusion limited, it was suggested that there would be another mechanism that dictated the drying of bread under microwave environments. The author further hypothesized that drying of bread could have been limited by the availability of the enthalpy of vaporization since the drying rate increased with increasing microwave power even when the temperature histories of the samples were quite similar.
Tong et al.Citation[1] measured the pressure profiles in bread samples as a function of sample porosity during microwave heating and observed negligible pressure gradient when the porosity was greater than 0.3. GoedekenCitation[5] noticed that there was a minute increase in vapor pressure inside a closed bread dough sample during microwave baking. However, the pressure difference quickly disappeared when the dough ruptured and became bread. Both studies suggested that the rate of vaporization of water is slower than the rate of vapor migration, and therefore the drying of bread is vaporization limited.
Finally, Parent et al.Citation[3] measured the moisture profiles in bread of infinite cylinders during microwave heating along with convective hot air. Unlike the isothermal drying apparatus developed recently,Citation[9] in this study there was no control of the microwave energy input so the samples being dried were at 100°C. Completely different from convective drying, the rate of moisture loss was the same throughout the entire sample, with the rate being slightly greater at the center than at the surface. The most accurate method to determine the mechanism of moisture transfer is to observe the profile of moisture loss throughout the thickness of the sample, with a parabolic moisture profile indicating a diffusion mechanism.Citation[10] So from the uniform moisture loss profiles with time measured during isothermal drying of bread, the diffusion model would not predict such behavior. Using a simplified model with moisture transfer limited by the vaporization process, the authors successfully predicted the moisture profiles in bread.Citation[3] GoedekenCitation[5] also applied the vaporization limited theory to accurately calculate the moisture loss of bread dough during microwave baking. The existence of pseudo‐wet‐bulb temperatures of bread during convective drying suggests that available latent heat of vaporization is the limiting mechanism.Citation[8]
TongCitation[6] proposed a first‐order reaction kinetic model to predict moisture loss during isothermal drying of hygroscopic porous material:
The uniform moisture loss from previous isothermal drying at 100°C was assumed to also occur during isothermal drying at lower temperatures 40–70°C as obtained in a previous work.Citation[9] Since the moisture profiles were uniform throughout the sample during isothermal drying, the mass average drying curves would represent the moisture loss throughout the sample. The objectives of this research were: (i) determine the kinetic parameters from the drying curves; and (ii) to compare moisture loss predicted from the first‐order kinetic model to experimental data.
Materials and Methods
The isothermal apparatus used to obtain drying curves is described in Ref.Citation[9] The drying curves were from 0.6 porosity bread samples with 0.011 m diameter, and the drying conditions were 3.2 m/s air velocity and 40, 50, 60, and 70°C temperature.
The hypothesis of this research is that a reaction kinetic equation can accuarately predict isothermal drying based on internal evaporation as the rate limiting step in the drying process as illustrated below:
For drying, the rate of vapor formation is difficult to measure. The rate of moisture loss is much easier and more practical to measure than vapor formation, so the kinetic equations will be in terms of moisture loss:
EquationEquations (3) and Equation(4) represents a chemical reaction that has gone to completion, meaning the reactant M is zero at infinite time; however, if the reaction does not go to completion but goes to some equilibrium value the equation is:
The D‐value is the time it takes for the unaccomplished moisture content to reduce by 90%, one log‐cycle, at a specific temperature as shown by the following equation:
Table 1. D‐Values calculated from isothermal experiments.Footnotea
The moisture loss as a function of time was calculated from the following equation:
The rate constant is temperature dependent through an Arrhenius relationship:
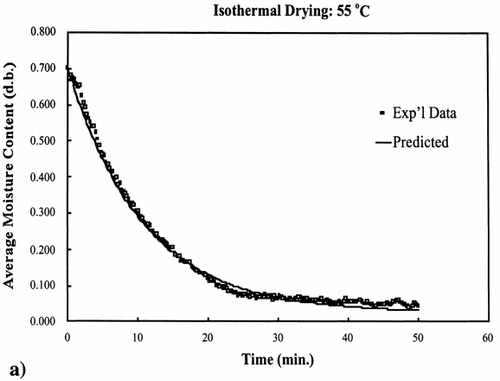
Two additional isothermal drying experiments were conducted: one drying experiment at 55°C, which is a temperature within the 40–70°C temperature range used to determine the kinetic parameters (interpolate), and the other drying experiment at 85°C, which is a temperature above the temperature range (extrapolate). These isothermal experiments were conducted under the same procedure as described for the 40, 50, 60 and 70°C experiments.Citation[9]
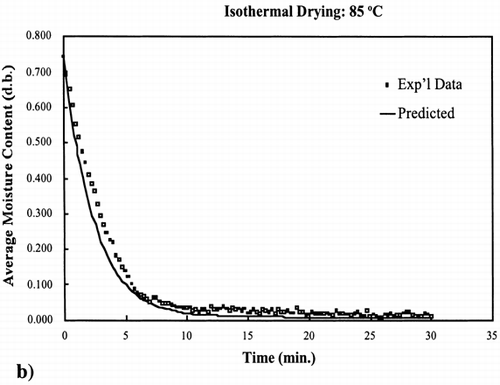
EquationEquation (10) was used to calculate the rate constant for 55°C and 85°C. The equilibrium moisture content was experimentally determined for these two temperatures by allowing the drying of the samples continue for a time where moisture loss was no longer occurring. Using the same time increments as used to collect the experimental data and knowing the input parameters, EquationEq. (8) was used to calculate the moisture content at each time interval.
Results and Discussion
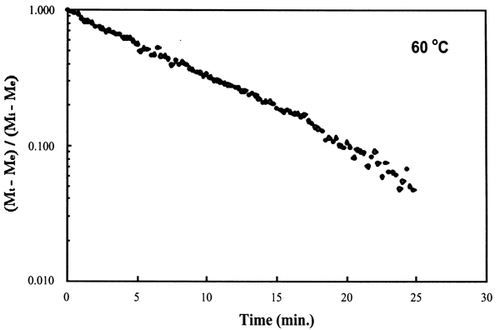
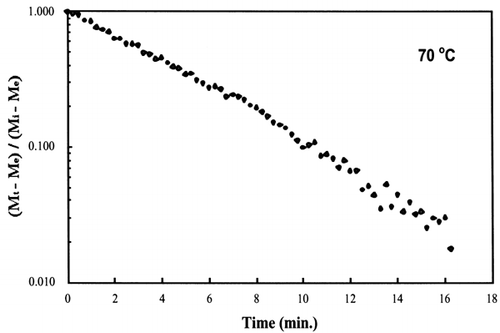
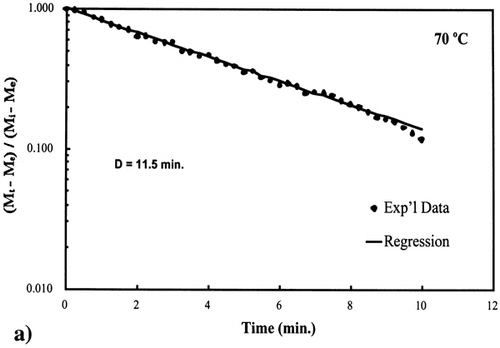
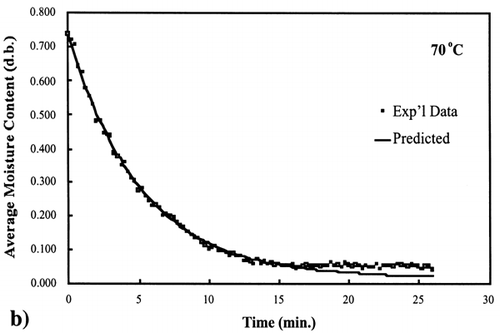
A first‐order kinetic equation was hypothesized as a model to predict isothermal drying. shows that a straight line is observed in the plot of the log of the unaccomplished moisture content vs. time beyond one log cycle reduction. The first‐order kinetic model was used to predict moisture loss and was compared to experimental moisture loss. show graphic representation of the D‐value and the predicted moisture loss using the first‐order kinetic model vs. experimental data at each temperature. The prediction was shown to be in good agreement with experimental data.
Figure 3. Isothermal drying of 0.6 porosity bread sample at 40°C: (a) semi‐logarithm plot of unaccomplished moisture content vs. time; (b) moisture loss vs. time.
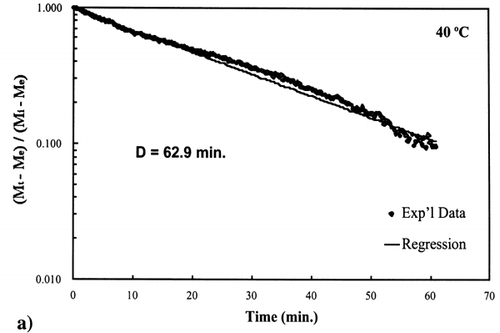
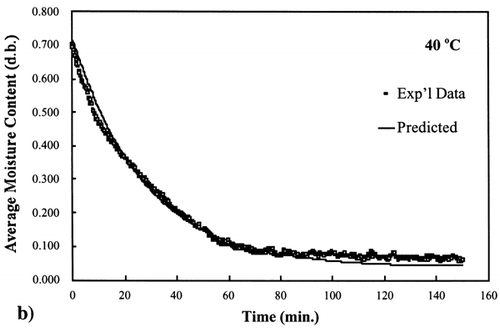
Figure 4. Isothermal drying of 0.6 porosity bread sample at 50°C: (a) semi‐logarithm plot of unaccomplished moisture content vs. time; (b) moisture loss vs. time.
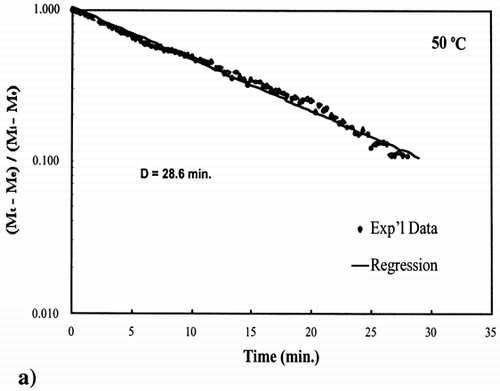
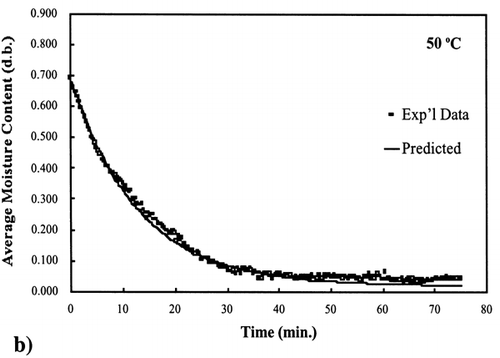
Figure 5. Isothermal drying of 0.6 porosity bread sample at 60°C: (a) semi‐logarithm plot of unaccomplished moisture content vs. time; (b) moisture loss vs. time.
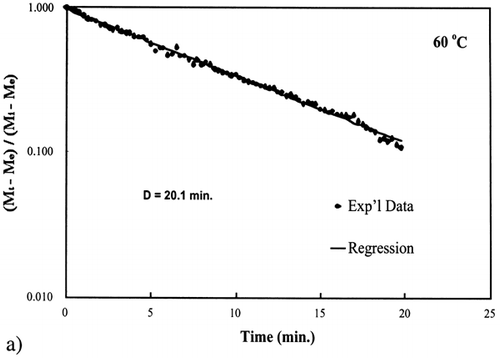
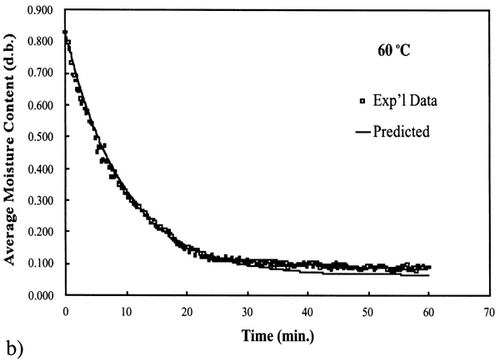
Figure 6. Isothermal drying of 0.6 porosity bread sample at 70°C: (a) semi‐logarithm plot of unaccomplished moisture content vs. time; (b) moisture loss vs. time.
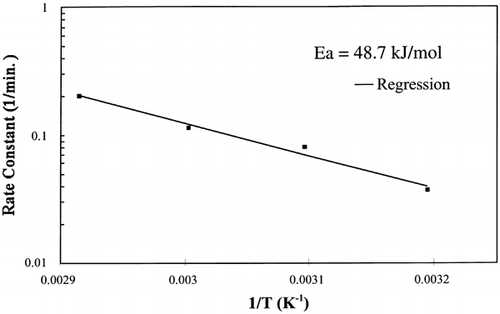
The slope of the Arrhenius plot shown in was used in EquationEq. (9) to calculate the activation energy, 48.7 kJ/g mol, and is very close to the activation energy of the rate constant for flour dough, 47.8 kJ/g mol.Citation[7] This activation energy is greater than the latent heat of vaporization, 40.7 kJ/g mol, which was expected since the water being vaporized is bound to the solid matrix and requires more energy to vaporize.
The regression of the Arrhenius plot was used to further test the kinetic model at any temperature below the boiling point of water, 100°C. Based on the regression analysis of the Arrhenius relationship between the rate constant and temperature, EquationEq. (10), the rate constants for 55°C and 85°C were calculated. shows the interpolated and extrapolated moisture loss prediction using the first‐order kinetic model compared to experimental moisture loss data obtained at the respective temperatures. The model predicted accurate moisture loss at each temperature in the moisture range 0.002–0.9 kg/kg solids.
Conclusion
Previous results in this laboratory suggest that the drying mechanism in hygroscopic porous material is heat transfer controlled. A model based on evaporation of bound water was introduced, and this first‐order irreversible kinetic model accurately predicted moisture loss during isothermal drying. The advantages of this model were in its simplicity and ability to predict moisture loss from initial to equilibrium moisture content using only one parameter. Further investigation is needed for non‐isothermal drying, which is often the case when only convective hot air is used.
Nomenclature
| D | = |
decimal reduction time, min |
| E a | = |
activation energy, kJ/mol |
| k 1 | = |
first‐order rate constant, min−1 |
| k 0 | = |
reference rate constant, min−1 |
| M | = |
moisture content, kg H2O/kg dry solids |
| M i | = |
initial moisture content, kg H2O/kg dry solids |
| M t | = |
moisture content at time t, kg H2O/kg dry solids |
| M ∞ | = |
effective moisture content, kg H2O/kg dry solids |
| R | = |
sample radius, m |
| R c | = |
universal gas constant, 0.008314 kJ/g mol K |
| s | = |
slope of unaccomplished moisture content vs. time, min−1 |
| T | = |
temperature, K |
| t | = |
time, min |
References
- Tong , C. H. , Fu , Y. C. and Lund , D. B. 1990 . Temperature, Pressure, and Moisture Content of Porous and Non‐Porous Materials During Microwave Heating . IDS '90 Seventh International Drying Symposium . Aug 26–30 1990 , Prague , Czechoslovakia.
- Louvet , P. 1991 . Comprehension des mecanisms de sechage pour les produits hygroscopiques et poreux . Memoire de fin d'etudes, ENSBANA . Sept 25 1991 , Dijon , France.
- Parent , A. , Tong , C. H. and Lund , D. B. 1993 . Temperature and moisture distributions in porous food materials during microwave heating . Sixth International Congress on Engineering and Food . May 23–27 1993 .
- Tong , C. H. and Lund , D. B. 1993 . Microwave heating of baked dough products with simultaneous heat and moisture transfer . J. Food Eng , 19 : 319 – 339 .
- Goedeken , D. L. 1994 . Microwave Baking of Bread Dough with Simultaneous Heat and Mass Transfer Rutgers : Dept. Food Sci., The State University of New Jersey . Ph.D. Dissertation
- Tong , C. H. 1995 . Personal Communication
- Fu , Y. C. 1996 . Microwave‐Assisted Heat and Mass Transfer in Food Rutgers : Dept. Food Sci., The State University of New Jersey . Ph.D. Dissertation
- Roberts , J. S. , Tong , C. H. and Lund , D. B. 2001 . Drying kinetics and time‐temperature distribution of pregelatinized bread . J. Food Sci. , 67 ( 3 ) : 1080 – 1087 .
- Roberts , J. S. and Tong , C. H. 2003 . The development of an isothermal drying apparatus and the evaluation of the diffusion model on hygroscopic porous material . Int. J. Food Prop. , 6 ( 1 ) : 165 – 180 .
- Doulia , D. , Tzia , K. and Gekas , V. 2000 . A knowledge for the apparent mass diffusion coefficient (D EFF ) of foods . Int. J. Food Prop. , 3 ( 1 ) : 1 – 14 .