Abstract
Growth of Enterobacteriaceae in scombroids such as tuna (Thunnus thynnus) and jack mackerel (Trachurus murphyii) usually promotes aminoacid decarboxylation to biogenic amines. Among these compounds, histamine, produced by histidin-decarboxylase activity, is reported as toxic. The objective of this study was to compare histamine production by a strain, identified as Serratia liquefaciens, isolated from tuna (T. thynnus) caught in the Gulf of Mexico and Morganella morganii isolated from Chilean jack mackerel (T. murphyii). Morganella morganii had higher histamine producing ability in a synthetic medium than S. liquefaciens, presumably as a result of stressing conditions on M. morganii. However, in a medium formulated with muscle protein extracts obtained from tuna S. liquefaciens showed higher histamine production at similar microbial counts. In this medium, histidine was extracted together with proteins from tuna striated muscle, it was assumed that it acted as histamine production promoter.
Introduction
The growth of Enterobacteriaceae, Bacillaceae and some species of Lactobacillus, Pediococcus, and Streptococcus can promote aminoacid decarboxylation in scombroids [tuna (Thunnus thynnus) and jack mackerel (Trachurus murphyii), among others] by enzymatic activity producing biogenic amines. These compounds are biologically active aliphatic organic bases of low molecular weight.Citation1 Among them, histamine, a product of histidine decarboxylation, is reported as toxic for humans and animals. It is related to natural microflora in fish as well as bacteria acquired during catching.Citation2 Citation3 Citation4 High histamine concentrations can be present in products derived from scombroids such as fishmeal or canned tuna, whereas histidine-decarboxylase activity depends on temperature, pH, histidine concentration, oxygen tension, and presence of vitamins and coenzymes, being the most determinant factors temperature and pH.Citation5
Bacteria responsible of histidine decarboxylation are difficult to detect because they are present in a very low proportion of total microflora, although some culture media are specific.Citation6 Several methods have been developed in order to detect histamine concentration in culture media and foods, some are based on fluorometry using o-phtaldehyde or o-diacetylbenzene, or on colorimetry.Citation7 Other methods are based on enzymatic reactions using diaminoxidase and peroxidase together with leucocrystal violet, promoting color development proportional to histamine concentration.Citation8 Faster, more sensitive and reproducible HPLC techniques have also been developed for benzyl-chlorideCitation9 or dansyl-chloride amines derivatives.Citation10 Citation11
The objective of this study was to compare histamine production by an enterobacterium isolated from tropical Mexican tuna (T. thynnus) and Morganella morganii, isolated from Chilean jack mackerel (T. murphyii).
Materials and Methods
Sample Preparation
Three tuna (T. thynnus) of 7–9 kg were obtained from the central seafood deposit in Mexico city. They were caught 24 hours before in Veracruz, Gulf of Mexico, gutted, gilled, and kept in ice during transport to our laboratory. The animals were sectioned in 13 portions, each one divided into two parts (left and right). All sections were vacuum packaged and labeled with randomly assigned numbers and stored at −20°C until analyzed. Samples were taken from the randomized labeled sections. All analysis were carried out in triplicate.
Total Viable Count
Samples were aseptically taken and stored in sterilized 100 mL glass flasks. In order to enrich fish microflora, the flasks were stored at 18°C for 6 days. Total viable count (TVC) was determined in samples stored at 18°C using the method reported by Holt et al.Citation12 in nutrient agar incubated at 25–30°C for 48 h. It was assumed that the higher TVC the more probable the presence of histamine-producing strains.
Histamine-Producing Strain Isolation and Identification
After 6 days of storage of tuna samples at 18°C, assuming that the enriched fish microflora contained histamine-producing microorganisms, isolation of histamine-producing strains was attempted from skin and muscle. One square centimeter tuna skin sample was rubbed with a cotton swab. The swabs were then placed into 10 mL sterile deionized water. Suitable dilutions were inoculated in modified Niven's agar:Citation6 0.5% meat peptone, 0.5% yeast extract, 2% L-histidine· HCl·H2O, 0.5% NaCl, 0.1% calcium carbonate, 2% agar, 1% bromocresol purple solution (0.6% in 95% ethanol); pH was adjusted to 5.3. Petri dishes were incubated at 30°C for 24 h.
Histaminogenic population in tuna muscle was obtained by homogenizing 10 g of muscle with 90 mL sterile deionized water, suitable dilutions were inoculated in modified Niven's agar, and incubated at 30°C for 24 h. Colonies showing a yellow halo, indicative of possible histamine producing ability, were inoculated into modified TSAH medium (tryptose–soy–casein–agar, plus histidine·HCl·H2O, pH 7.0):Citation13 0.5% casein peptone, 0.5% NaCl, 0.25% K2HPO4, 1% L-histidine· HCl·H2O, incubated at 32°C for 48 h. Strains were further identified by the use of API kits (Biomerieux, Marcy L'Etoile, France) and biochemical tests. An API 20E kit for Enterobacteriaceae and gram negative bacilli was used for preliminary identification. Identity confirmation was carried out using the biochemical tests reported by Mac Faddin.Citation14
Histamine production by isolated strains was compared to that obtained from a highly histaminogenic strain, M. morganii (Proteus morganii) isolated from Chilean jack mackerel (T. murphyii) using Niven's differential mediumCitation6 and identified by an API 20E kit for Enterobacteriaceae (Antylab Products Inc, USA) at Universidad de Concepción, Chile.Citation15 Biochemical tests were also carried out with this strain.
Histaminogenic Ability
Histamine producing strains isolated from tuna, as well as M. morganii, were inoculated in Niven's broth and incubated for 48 h at 30°C. A yellow halo was formed surrounding the colonies, the color changed as an effect of pH increase due to the presence of histamine. Histamine production was recorded using an arbitrary scale where the more intense the yellow color the higher the histamine production. It was ranked from (+) to (+++++).
Growth and Histamine Production in a Synthetic Medium
Growth and histamine concentration curves were obtained with M. morganii and the strain isolated from tuna showing the highest histamine-producing ability, identified as Serratia liquefaciens.
Stock cultures were prepared by inoculating an isolated colony of M. morganii or S. liquefaciens in HB mediumCitation8 and incubating at 37°C for 24 h. One percent of these cultures were then inoculated in Erlenmayer flasks containing HB medium at pH 5.3 or 6.0, and incubated at 18 or 37°C. Samples were taken at 0, 2, 7, 11, 17, 27, and 34 h. The response variables were cfu mL−1 and histamine concentration (µmol mL−1).
Growth and Histamine Production in an Extracted Myofibrillar Proteins Medium
Morganella morganii or S. liquefaciens were inoculated in a tuna muscle protein extract mediumCitation16 obtained as described below and supplemented with 1% glucose, pH was adjusted to 5.3. One percent of M. morganii or S. liquefaciens stock cultures were inoculated in this medium and incubated at 37°C for 35 h.
Muscle Protein Extraction
Proteins were extracted from tuna striated muscle by a modification of the method reported by Cofrades.Citation17 Five gram of fish muscle were homogenized with 16 volumes 0.05 M sodium phosphate buffer, pH 7. The mixture was centrifuged at 10,000g for 15 min. The precipitate was resuspended in eight volumes phosphate buffer pH 7.0 and centrifuged in a similar way. The precipitate was homogenized in five volumes 0.8 M NaCl, pH 7.5 in sodium phosphate buffer, stirred overnight at 4°C and centrifuged at 13,500g for 15 min. The supernatant was transferred to a beaker, the precipitate washed with five volumes phosphate buffer+0.8 M NaCl and centrifuged at 13,500g for 15 min. The supernatant was mixed with the one previously obtained and ionic strength adjusted to 0.06 M with cold water, stirred and left standing overnight at 2–5°C. The supernatant was discarded and the precipitate centrifuged at 8500g for 15 min, collected and weighted. Assuming ρ=1, the ionic strength was adjusted at 0.4 M with 0.8 M NaCl, pH 7.
Analysis of Histidine and Histamine
Samples were centrifuged at 4000g for 15 min and filtered through a 0.22 µm Millipore membrane (Bedford, MA). The filtrate was kept at −30°C until analysis. This was carried out according to the method reported by Herman et al.Citation18 using a HPLC Waters equipment WATO 39787 system (Milford, MA) fitted with a photodiode arrangement detector and Millenium software. Histamine was determined using a 5×50 mm CM-15 HR (weak cationic interchange) column, 15 mm particle size, 1000 Å pore size. The mobile phase was 10% sodium, 0.5 M phosphate buffer (pH 5.0)/90% water (0.25 mL min−1). After 10 min phosphate buffer concentration was exponentially increased up to 60% during 15 min, isocratic conditions were kept for 25 min. Histamine was detected at λ=210 nm. The retention time for the histamine standard was 33.89 min.
Experimental Design and Statistical Analysis
Samples were randomly allocated to a 22×6 complete factorial design (pH 5.3 and 6.0; 18 and 37°C; 0, 2, 3, 11, 27, and 34 h). Data were subjected to analysis of variance using a SAS package.Citation19
Results and Discussion
Total viable counts were significantly higher in tuna skin than in the muscle, in agreement with other authorsCitation20 who concluded that fish skin is directly contaminated by the intestinal content expelled during catching, therefore the anal region usually shows higher counts. Contamination also occurs due to handling during catching, transportation, gutting, and gulling. Table shows total viable counts in samples stored at 18°C as well as histaminogenic bacteria counts on the skin, determined by Niven's medium. Four decarboxylase-producing strains, labeled C1, C2, C3, and C4, were isolated from tuna skin. No histamine-producing strains were isolated from muscle. Presence of histaminogenic strains in fish has been reported by several authors.Citation8 Citation21 Citation22 Citation23 It was assumed that histaminogenic population growth, as well as decarboxylase production, require suitable conditions such as temperature, pH, free histidine concentration, oxygen tension, and presence of vitamins and coenzymes.Citation5 Sodium chloride also affects histamine production.Citation24 Nonetheless, bacteria responsible of histamine production are difficult to detect as they are present a very small percentage of total microflora.Citation25 API kits gave between 85% and 99% confidence identity of the following strains: Hafnia alvei (C1), Serratia rubidae (C2), S. liquefaciens (C3), and Enterobacter agglomerans (C4). Identification was confirmed by biochemical testsCitation12 Citation14 (Table ). Morganella morganii was also tested as a means of comparison. None of these strains produced H2S.
Table 1 Total viable counts and histaminogenic bacteria (log cfu cm−2) in tuna stored at 18°C
Table 2 Biochemical tests for strains isolated from tuna skin and M. morganii
Table shows the halo intensity produced by the four isolated and identified strains. From these test strain C3, identified as S. liquefaciens was selected for further studies.
Table 3 Histidine-decarboxylase production of strains isolated from tuna
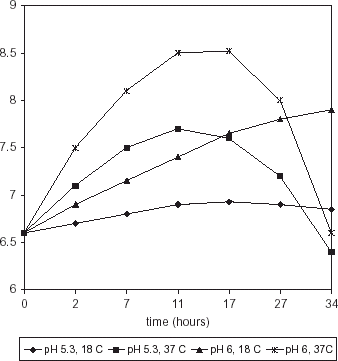
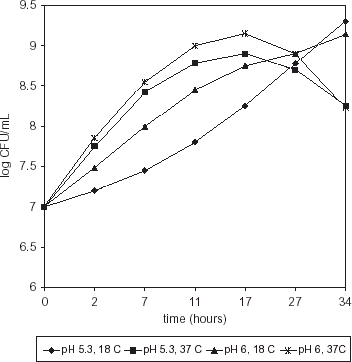
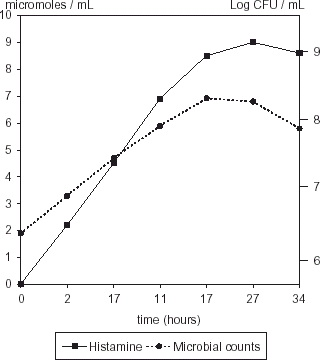
Maximum growth of M. morganii was observed at 17 h (pH 6, 37°C) with a further decrease; however when incubated at 37°C, pH 5.3, bacterial counts were significantly lower (P>0.002) (Fig. ). Serratia liquefasciens growth showed a similar trend with a maximum value at 17 h (pH 6, 37°C) but, conversely as with M. morganii, population at pH 5.3, 37°C was not significantly different (P>0.275) (Fig. ). Incubation of both strains at 18°C, pH 5.3 or 6.0, resulted in a steady increase throughout the study time. Temperature had a highly significant effect on growth rate (P>0.0001) whereas pH effect was less significant (P>0.0121).
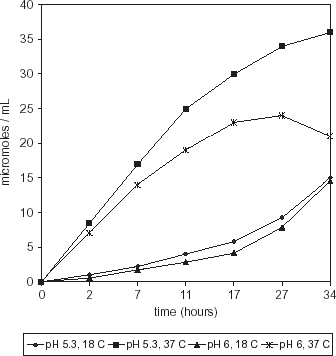
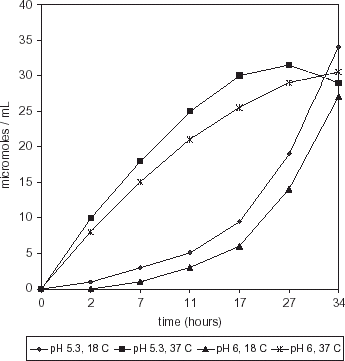
Histamine production was higher (P>0.0004) in a medium inoculated with M. morganii at pH 5.3, 37°C than at pH 6.0, 37°C (Fig. ). At 34 h, histamine concentration was 36 µmol mL−1 and 21 µmol mL−1 at pH 5.3 and 6.0, respectively. Histamine production by S. liquefaciens showed a maximum at 27 h (31.5 µmol mL−1) with a further decrease in a medium incubated at pH 5.3, 37°C (Fig. ). Lower values (P<0.0174) were observed in a medium incubated at pH 6.0, 37°C.
When the population growth of both strains was compared to the respective histamine production, it was observed that M. morganii incubated at pH 5.3, 37°C was able to produce higher histamine concentrations at lower microbial counts, than at pH 6, 37°C (P>0.0001). Similar trend was observed for S. liquefaciens, although the difference in histamine production between media incubated at 37°C, pH 5.3 or 6.0 was not significant (P>0.093). Maximum histamine production by M. morganii was significantly higher (P>0.0018) than by S. liquefaciens in HB medium at the same temperature and pH conditions (pH 5.3, 37°C).
Although M. morganii population decreased from 17 h onwards, constant histamine production could be due to the ability of this strain to produce or liberate histamine during the stationary or decay phase, in agreement with the results reported by Beheling and Taylor.Citation26 It is possible that histamine production is a result of a cell mechanism to obtain energy by carboxyl cleavage.Citation1 Therefore, even though the population decreased, the histamine concentration increased as a means to obtain metabolic energy. This is more evident in stressing conditions, as was the case of M. morganii isolated from a temperate seawater fish and incubated at a relatively high temperature (37°C), whereas at 18°C histamine production was lower.
IenisteaCitation27 concluded that amine production by bacteria is a protection mechanism against an aggressive environment. He reported that pH 5.0–5.5 promotes histamine production, whereas Chen et al.Citation5 indicated that histidine-decarboxylase synthesis and activity could occur at normal pH values of scombroids (5.9–6.8). With the exception of the enzyme produced by Hafnia sp. histidine-decarboxylase activity is not noticeable at alkaline pH.Citation27
Histidine availability in striated muscle acts as inductor promoting a suitable environment for histamine formation.Citation25 Morganella morganii, growing in muscle tissue can synthesize histidine-decarboxylase and accumulate histamine to toxic levels, 5–10 mg/100 g.Citation1
Temperature conditions maximizing either histamine production and bacterial growth are different for each one of these events. Maximum enzyme production occurs at 10°C whereas at 37°C occurs maximum bacterial growth and histidine-decarboxylase production is at a minimum probably as a result of enzyme thermolability.Citation8
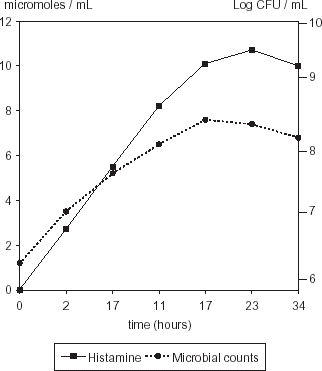
Storage temperature is also a critical factor influencing histamine formation in tuna. The content of biogenic amines in a recently caught fish is around 5 mg histamine/100 g fish. After catching, handling and storage histamine concentration can increase up to 2350 mg kg−1 if histaminogenic microflora is around 108 cfu g−1.Citation21 Maximum growth of M. morganii and S. liquefaciens inoculated in a muscle protein extract medium was observed at 17 h, and maximum histamine production in both cases was at 27 h (Figs. and ). Maximum population growth for M. morganii and S. liquefaciens (log−1 6.92 and log−1 7.6 cfu mL−1, respectively) has no significant difference (P>0.462). Histamine concentration reached values up to 10.7 µmol mL−1 in samples inoculated with S. liquefaciens and 9 µmol mL−1 for those inoculated with M. morganii (P>0.092), with a further decrease. This reduction could be due to chemical detaching of the histamine side chain, leaving the imide ring not detectable by the ionic exchange method employed to analyze histamine. The decrease in amine concentration was also observed by Chávez and GuerreroCitation28 when analyzing cadaverine and putrescine in red meats and by Sanceda et al.Citation29 in fish supplemented with histidine.
Histamine production reported by other authors is very variable. Ababouch et al.Citation22 reported 11.75 to 17.43 µmol histamine mL−1 produced by several M. morganii strains in a culture medium made of sardine muscle protein supplemented with histidine and incubated at 35°C during 24 h, whereas Eitenmiller et al.Citation25 reported 0.3–1.0 µmol histamine mL−1 by M. morganii in a synthetic medium also supplemented with histidine. However, 2.3–24 µmol histamine mL−1 was found by the same authors in a medium formulated with tuna proteins and incubated during 12 h at 35°C. This variation is possibly due to histamine-decarboxylase activity depending on the culture media and incubation conditions, as well as to the analytical methods used.
Conclusions
Histaminogenic strains were isolated only from skin tuna, but not from muscle. Although M. morganii growth rate was lower than S. liquefaciens in HB medium, histamine production was significantly higher (P>0.0018) at the same temperature and pH conditions (pH 5.3, 37°C), probably as an effect of stressing conditions upon this temperate seawater strain. Histamine production in a medium formulated with a muscle protein extract reached higher values (P>0.092) in samples inoculated with S. liquefaciens. The strain, isolated from the same source as the proteins, probably was adapted to the environment promoting higher decarboxylase activity at lower microbial counts than a strain isolated from a different environment, such as M. morganii.
Acknowledgments
Author Guillén-Velasco thanks the National Council of Science and Technology (CONACYT, Mexico) for a graduate scholarship. The authors also thank Cristina Martí and Silvia Torres, Universidad de Concepción, Chile for supplying the M. morganii strain as well as for their suggestions and recommendations.
References
- Halász , A. , Bárat, , A. , Simon-Sarkadi , L. and Holzapfel , W. 1994 . Biogenic amines and their production by microorganisms in food . Trends Food Sci. Technol. , 55 ( 2 ) : 42 – 49 .
- Osuna , R. 1984 . Toxicología Aviar: vómito negro. La histidina y la mollerosina en la harina de pescado . Agricultura Profesional , 2 : 111 – 115 .
- Castro , E. 1987 . Erosiones de la molleja y vómito negro aviar: su prevención a través del control de calidad a las harinas de pescado . Avicultura Profesional , 5 ( 2 ) : 55 – 56 .
- Castro , E. 1991 . “ Relación entre el procesamiento de la harina de pescado, su contenido de aminas biogénicas y sus posibles efectos sobre la salud avícola y animal ” . In In III Simposio de Avances Tecnológicos Modernos Santiago, , Chile : Fundación Chile .
- Chen , C.M. , Wei , C.I. , Koburger , J.A. and Marshal , M.R. 1989 . Comparison of four agar media for detection of histamine-producing bacteria in tuna . J. Food Protec. , 52 ( 11 ) : 808 – 813 .
- Niven , C.F. , Jeffrey , M.B. and Corlett , D.A. 1981 . Differential plating medium for quantitative detection of histamine-producing bacteria . Appl. Environ. Microbiol. , 41 ( 1 ) : 321 – 322 . [PUBMED] [INFOTRIEVE]
- Taylor , S.L. and Lieber , E.R. 1977 . Specificity and sensitivity of seven histamine detection methods . J. Food Sci. , 42 ( 6 ) : 1584 – 1586 .
- López-Sabater , E.I. , Rodríguez-Jerez , J.J. , Hernández-Herrero , M. and Mora-Ventura , M.T. 1994 . Evaluation of histidine decarboxylase activity of bacteria isolated from sardine (Sardina pilchardus) by enzymatic method . Lett. Appl. Micro. , 19 : 70 – 75 . [CSA]
- Hwang , D. , Chang , S. , Shiau , C. and Cheng , C. 1995 . Biogenic amines in the flesh of sailfish (Istiophorus platypterus) responsible for scombroid poisoning . J. Food Sci. , 60 ( 5 ) : 926 – 928 .
- Friedman , M. and Noma , A.T. 1981 . Histamine analysis on a single column aminoacid analyzer . J. Chromat. , 219 : 343 – 348 . [CROSSREF]
- Maijala , R. , Eerola , S. , Lievonen , S. , Hill , P. and Hirivi , T. 1995 . Formation of biogenic amines during ripening of dry sausages as affected by starter culture and thawing time of raw materials . J. Food Sci. , 60 ( 6 ) : 1187 – 1190 .
- Holt , J.G. , Krieg , N.R. , Sneath , P.H. , Stanley , J.T. and Williams , S.T. 1994 . Bergey's Manual of Determinative Bacteriology 175 – 248 . Baltimore, MA : Williams and Wilkins .
- International Commission on Microbiological Specifications for Foods . 1980 . “ Fish and shellfish, and their products ” . In InMicrobial Ecology of Foods. Food Commodities 567 – 605 . London : Academic Press . Chap. 20, Vol. 2
- MacFaddin , J. 1993 . Pruebas Bioquímicas para la Identificación de Bacterias de Importancia Clínica; Mexico City : Editorial Panamericana .
- Torres , S. 1999 . “ Cinética de formación de histamina oir la degradación bacteriana de histidina en la industria procesadora de pescado ” . Chile : Universidad de Concepción . M.Sc. Thesis
- Omura , Y. , Price , R.J. and Olcott , H.S. 1978 . Histamine-forming bacteria isolated from spoiled skipjack tuna and jack mackerel . J. Food Sci. , 43 : 1779 – 1781 .
- Cofrades , S. 1994 . “ Funcionalidad y características fisicoquímicas de actomiosina procedente de distintas especies. Influencia de la congelación y conservación en estado congelado ” . Madrid, , Spain : Universidad Complutense . Doctoral Thesis
- Herman , K. , Frank , G. and Ring , J. 1995 . High performance liquid chromatography for the separation of histamine, its precursor and metabolites: application to biological samples . J. Liquid Chrom. , 18 ( 1 ) : 189 – 204 .
- SAS Institute . 1995 . JMP Statistics and Graphics Guide,Version 3.1 Cary, NC : SAS Institute .
- Chichester , C.O. and Graham , H.D. 1973 . Microbial Safety of Fishery Products New York : Academic Press .
- Stratton , J.E. , Hutkins , R.W. and Taylor , S. 1991 . Biogenic amines in cheese and other fermented foods: a review . J. Food Protec. , 54 ( 6 ) : 460 – 470 .
- Ababouch , L. , Afilal , M.E. , Rhafiri , S. and Busta , F.F. 1991 . Identification of histamine-producing bacteria isolated from sardine (Sardina pilchardus) stored in ice and at ambient temperature (25°C) . Food Microbiol. , 8 : 127 – 136 .
- López-Sabater , E.I. , Rodríguez-Jerez , J.J. , Hernández-Herrero , M. , Roig-Sagués , A.X. and Mora-Ventura , M.T. 1995 . Sensory quality and histamine formation during controlled decomposition of tuna (Thunnus thynnus) . J. Food Protec. , 59 ( 2 ) : 174 – 176 .
- Taylor , S. and Woychik , N. 1982 . Simple medium for assessing quantitative production of histamine by Enterobacteriaceae . J. Food Protec. , 45 ( 8 ) : 747 – 751 . [CSA]
- Eitenmiller , R.R. , Orr , J.H. and Wallis , W.W. 1981 . Production of histidine decarboxylase and histamine by Proteus morganii . J. Food Protec. , 44 : 815 – 820 .
- Beheling , A.R. and Taylor , S.L. 1982 . Bacterial production as a function of temperature and time of incubation . J. Food Sci. , 47 : 1311 – 1314, 1317 .
- Ienistea , C. 1973 . “ Significance and detection of histamine in food ” . In In The Microbiological Safety of Food Edited by: Hobbs , B.C. and Christian , J.B. London : Academic Press .
- Chávez , A.M. and Guerrero , M.I. 1991 . Detection of biogenic amines as meat spoilage indicators . J. Muscle Foods , 2 : 263 – 278 .
- Sanceda , N.G. , Suzuki , E. , Ohashi , M. and Kurata , T. 1984 . 1999Histamine behavior during the fermentation processing the manufacture of fish sauce . J. Agric. Food Chem. , 47 : 3596 – 3600 . [CSA] [CROSSREF]