Abstract
The minced meat from oil sardine (Sardinella longiceps) was subjected to once, thrice, and five water washings. The changes in proximate composition of meat, physico-chemical and functional properties of proteins as affected by number of washings were investigated. A positive correlation (p < 0.05) existed between number of washings and fat content, water absorption capacity, gel strength, expressible water content and emulsion capacity of meat. A reduction in low molecular weight components of proteins during different washings was evident from gel filtration profile. The sodium dodecyl sulfate polyacrylamide gel electrophoresis patterns of total proteins revealed that increase in myosin heavy chain content and decrease in the concentration of 55 and 36 kD components with number of washings. The dynamic visco-elastic behavior of once washed sardine meat in the temperature range of 30–90°C had higher ability to form elastic component (G′) than thrice and five times washed meat.
Introduction
Water washing of separated fish flesh is an important operation in surimi (separated fish flesh, water washed, mixed with cryoprotectants, frozen, and stored) production.Citation1 Citation2 The primary objective of water washing the fish flesh in surimi production is not only to remove fat, sarcoplasmic proteins, undesirable matter including blood, pigments and odorous substances but also to concentrate myofibrillar proteins so as to enhance gel-forming ability.Citation3 Surimi based gel products represent the most suitable food application for underutilized fish species.Citation4 Though the choice of fish species for surimi production is lean variety and white-fleshed fish, various researchers have shown that acceptable surimi from pelagic fishes such as horse mackerel, sardine, and herring can be obtained.Citation5 Citation6 Citation7 Citation8 Citation9
The number of washing cycles and volume of water employed in surimi production will vary with fish species, initial condition of fish, and type of processing unit that is continuous or batch operation.Citation1 There is a need to standardize appropriate number of washing cycles depending on composition and nature of fish species meant for surimi production.
The oil sardine (Sardinella longiceps) is a pelagic shoaling fish with high fat content and had high in dark meat component.Citation10 The average annual landings of oil sardine in India in the last five years were in the range of 200,000–250,000 t.Citation11 The fish is consumed in fresh form and during heavy landings the catches are diverted for reduction purposes. The surimi production in India is of recent origin and during the year 2000–2001 nearly 11,000 t of surimi production have been achieved.Citation12 At present, croaker and threadfin breams are being used as raw material for surimi production in India. Because of its seasonal abundance, oil sardine from Indian waters can be made use for surimi production provided appropriate technologies are followed. It is important that effect of number of washing cycles on physico-chemical and functional properties of sardine meat needs to be established so as to modify the process line in surimi production. In this direction, the present study is related to establishing the number of washing cycles on the physico-chemical and functional properties of oil sardine.
Materials and Methods
Sample Preparation
Fresh oil sardine (Sardinella longiceps) caught by purse seiner along the coast of Mangalore, India, were used for the present study. The average length of fish used for the study was in the range of 12–15 cm and the mass of fish was in the range of 30–40 g. The fish was washed in chilled water and transported to the laboratory in iced condition (covered with ice in the ratio of 1:1 fish:ice) and used for the experiment within 2 h. The fish was dressed to remove scales, head, and viscera. Dressed fish was washed in chilled water and filleted. Meat was separated manually and minced in a prechilled pestle and mortar. The minced meat was used for washing experiment. The minced fish meat was mixed with chilled potable water (4°C) in the ratio of 1:3 (fish:water) and stirred gently using magnetic stirrer for 5 min and allowed to settle. The slurry was decanted through muslin cloth and excess water was removed by manual squeezing. The washing, decantation, and squeezing was carried out five times. The unwashed, once, thrice, and five times washed meat was used for the experimental analysis. The washing experiment was conducted in three different batches and from each batch representative sample was taken for analysis.
Preparation of Heat Induced Gel
The minced meat (100 g) was grinded manually with 2.5% of salt in a prechilled pestle and mortar for 10 min. The paste was packed in krehalon casing (260 mm long, 48 mm diameter, and 200 gauge thickness) without air pockets. The stuffed casings was sealed with aluminium wire using ringer machine and it was kept at 90 ± 2°C for 45 min in a constant water bath (Haake, model K10, Germany) and cooled in chilled water (4–6°C) for 20 min. The gel was kept in refrigerator overnight. The gel strength and expressible water content of the gels were measured after keeping at ambient temperature for 1 h.
Proximate Composition
Total protein (Nx6.25), moisture, ash content, and fat content were determined according to the method as described in AOAC.Citation13 The nonprotein nitrogen content in the TCA extract (20%) of minced meat was determined by the method as described by Velankar and Govindan.Citation14
pH
The pH of the meat samples was measured using Systronic 324 pH meter. Five grams of meat ground with 45 mL distilled water using a pestle and mortar for 5 min and pH was measured.
Viscosity
The apparent reduced viscosity (η red) of total proteins from oil sardine meat was measured using Oswald's viscometer at 25 ± 1°C using extraction buffer (phosphate buffer 0.05 M, pH 7.5 containing 1.0 M NaCl) as solvent. The reduced viscosity at different protein concentrations was calculated by the method described by Yang.Citation15 The reduced viscosity at single protein concentration (3 × 106 mg/m3) was derived from the plot of protein concentration vs. η red.
Gel Filtration Profile
Gel filtration profile of total proteins extracted from oil sardine meat was carried out using a sepharose 6B gel packed in a column of 1.5 × 80 cm (dia. × height) using extraction buffer as solvent at ambient temperature (28 ± 1°C). The bed volume of the column was 154 mL and the void volume as determined by using blue dextran was 50 mL. A protein concentration of 3.5–4.0 mg/mL was loaded to the column and eluted with extraction buffer at a flow rate of 30 mL/h. Fractions of 3 mL were collected manually and the concentration of the proteins in the eluent was monitored by measuring the absorbance at 280 nm using Bausch and Lomb UV/VIS spectrophotometer. A plot of elution volume vs. absorbance at 280 nm for each fraction was obtained.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of total proteins from unwashed and washed oil sardine meat was carried out under reducing conditions according to the method as described by Laemmli.Citation16 Electrophoresis was carried out using polyacrylamide gel slabs of 10 × 8 cm (length × width) in a vertical gel electrophoresis unit (model Mighty small II, SE 250, Hoefer-Pharmacia Biotech, USA). A discontinuous gel of acrylamide concentration of T% = 10, C% = 2.5 (for resolving gel) and T% = 4, C% = 2.5 (for stacking gel) was used. The thickness of the gel was 0.75 mm and number of wells in each gel was 10. The run was carried out in a constant current mode at 2 mA per well using Hoefer-Pharmacia Biotech, power pack (model PS 3000). Meat samples were mixed with treatment buffer (1:1), macerated well and heated in boiling water bath for 2 min. The slurry was centrifuged at 8000 × g using Remi T-8 centrifuge. A clear protein solution of 25–30 µg was loaded to the well. The standard molecular weight markers of wide range (205–14.2 KD) procured from Sigma Chemical Co, USA were loaded separately. After the run, the gels were stained using coomassie brilliant blue R-250 overnight. The gels were destained using acetic acid–methanol mixture till the protein bands were clearly visible. The molecular weight of the bands obtained in the sample was approximated by measuring the relative mobility of the standard proteins in the molecular weight markers.
Water Absorption Capacity, Fat Absorption Capacity and Emulsion Capacity
Water absorption capacity was determined by the method as described by SosulkiCitation17 and expressed as gram water/g dried material. Fat absorption capacity was estimated by following the method described by Lin et al.Citation18 and expressed as gram oil/g dried material. Emulsion capacity of total proteins was determined according to the method of Swift et al.Citation19 and expressed as mL oil/mg protein.
Gel Strength Measurement
The gel strength of heat-induced gel was determined by using Okada gellometer (Saitama Keki Seisa Kuso Co. Ltd., Tokyo, Japan) as described by Suzuki.Citation20 The expressible water content of the heat-induced gel was measured according to the method described by Okada.Citation21
Dynamic Visco-Elastic Behavior (DVB)
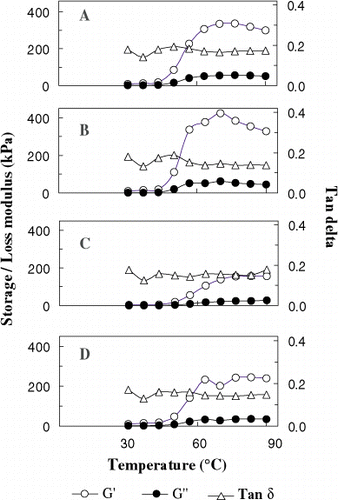
The dynamic visco-elastic behavior (DVB) of unwashed and washed oil sardine meat in the temperature range of 30–90°C was measured using Controlled Stress Rheometer (model CSR-500, Carri-Med brand, UK) under oscillatory mode using 4 cm parallel plate measuring geometry. Four grams of meat was ground with 2.5% of sodium chloride and used for DVB measurements. The gap between measuring geometry and peltier plate was adjusted to 2 mm.Temperature sweep was carried out by applying small amplitude oscillation with a frequency of 1 Hz, which was in linear visco-elastic region. A heating rate of 1°C/min was achieved through peltier plate system during heating regime. The applied sinusoidal stress was compared with resultant sinusoidal strain. The two sine waves had a phase difference δ, which gave elastic (storage modulus G′) and viscous (loss modulus G′′) element of the gel. These two values along with tan δ were recorded simultaneously by the instrument. The average of three measurements was used for plotting.
Statistical Analysis
All the results expressed are the mean of three measurements. Each measurement from a single batch and mean values were reported. Simple correlation was used to relate the number of washing with WAC, FAC, EC, gel strength, and expressible water. Kruskals wallis test was used to find out the significance of difference due to washing.Citation22
Results and Discussion
The proximate composition of fresh, unwashed, and washed oil sardine meat is given in Table . The washed meat had lower levels of fat, protein, and ash, and higher moisture content compared to unwashed meat.Citation23 The increase in moisture content of meat after five washings is mainly due to absorption of water by hydrophilic residues of myofibrillar proteins. The reduction in fat content due to water washing was comparable to the values obtained by Roussel and CheftelCitation24 in washing of sardine meat. The decrease in ash content after washing may be due to removal of water-soluble mineral constituents from the meat.Citation25
Table 1 Proximate composition of fresh unwashed and washed oil sardine meat based on wet weight basis (kg/100 kg mince)
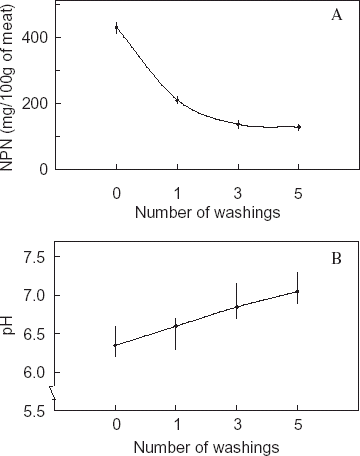
The nonprotein nitrogen content (NPN) of the meat reduced by 74% from initial value upon five washings (Fig. A). This reduction in NPN with washings has reduced the total proteins of washed meat. The pH value showed an increase with number of washing cycles (Fig. B). The increase in pH by 0.7 units in five times washed meat is due to removal of free nitrogen, free fatty acids, free acidic amino acids, or other water soluble acidic compounds during washing process.Citation25 The surimi prepared from pelagic fish like herring and horse mackerel have shown a higher pH of 7.0–7.26.Citation26
Figure 1. Effect of number of water washing cycles of fresh sardine meat on A—nonprotein nitrogen constituents; B—pH of meat.
The reduced viscosity value at single protein concentration (3 mg/mL) showed (Table ) a slightly higher value with number of washing and this increase in the viscosity value is mainly due to extensive hydration of protein molecule.Citation27
Table 2 Properties of proteins from fresh unwashed and washed oil sardine meat
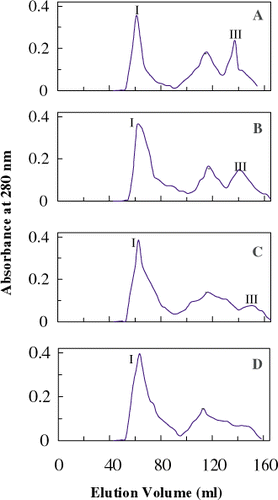
The gel filtration profile of proteins from unwashed meat revealed three fractions (Fig. A), one high molecular weight (peak I) and two low molecular weight fractions (peak II and III). With increase in number of washings there was a reduction in low molecular weight fraction (peak II and III) (Figs. B and D). The reduction in peak III is attributable to removal of free amino acids, peptides, and other low molecular weight components. This was further evidenced by reduction in NPN content with increase in number of washings. This removal of low molecular weight components will concentrate the myofibrillar proteins and may alter the properties because of increase in concentration of myofibrillar proteins.Citation5 Citation28
Figure 2. Gel filtration profile of total proteins from unwashed and washed fresh oil sardine meat in extraction buffer (PB 50 mM, pH 7.5, containing 1.0 M NaCl. A—unwashed meat; B—once washed meat; C—thrice washed meat; D—five times washed meat.
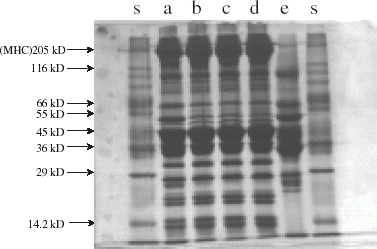
The SDS-PAGE pattern of total proteins from unwashed and washed meat showed a clear distinction with concentration of 205 kD component (myosin heavy chain) in washed meat (Fig. , lane a, b, c, d). The increase in concentration of 205 kD band was proportional to number of washings. A marginal reduction in 55 and 36 kD components was observed in washed meat.
Figure 3. SDS-PAGE pattern of total proteins from unwashed and washed oil sardine meat. Lane s—standard markers; a—unwashed meat; b—once washed meat; c—thrice washed meat; d—five times washed meat.
The water absorption capacity, fat absorption capacity, and emulsion capacity of washed meat were higher than unwashed meat (Table ). A positive correlation (p < 0.05) between number of washings with WAC and EC were recorded (Table ). The water absorption capacity is mainly related to number of polar residues that the protein molecule carries. The mechanism of fat absorption is attributed to the physical entrapment of oil by protein molecule.Citation29 The ability of protein molecule to emulsify the given volume of oil mainly depends on the surface hydrophobic groups, which can orient readily in aqueous phase lowering the interfacial free energy.Citation30 It is well documented that myofibrillar proteins are the major contributors for emulsification property.Citation31 Repeated washing of oil sardine meat could concentrate myofibrillar proteins and hence there is an increase in emulsion capacity.
Table 3 Correlation coefficient (r) between number of washings and different parameters analyzed
The gel strength of gels prepared from unwashed meat is comparatively low in relation to gels prepared from washed meat. The gel strength increased with number of washings and the gels from five times washed meat had a gel strength value of 0.035 N.M. (Table ). The strength of the gels prepared from fish meat depends on the amino acid composition, protein conformation, preprocessing conditions, pH, rate of heating, and the type of ingredients used in the preparation of gel.Citation32 Increase in gel strength values for washed meat from pacific mackerel has been reported.Citation9 The gel strength values in the present study were lower in all the samples in comparison to values from other Indian fishes like pink perch reported by Ratnakumar and Shamasundar.Citation33
The expressible water content of the prepared gels from unwashed and washed oil sardine meat was found to have an inverse relation with the gel strength (Table ). The water inside the gel is in free form and can be expelled out by application of pressure. The retention of water inside gels is related to the strength of network formed and the other ingredients used.Citation32 The high expressible water contents in gels from unwashed and washed meat are because of the poor gel forming ability of proteins from oil sardine.
In order to provide information about gelation and structure formation, a nondestructive small strain test with continuous data recording is required.Citation34 The dynamic visco-elastic behavior of fresh unwashed and washed oil sardine meat in the temperature range of 30–90°C is given in Fig. (A–D). The elastic component (G′) value increased with increase in temperature. The rate of increase was maximum between 50–56.7°C for all the samples. The unwashed meat showed a maximum G′ value of 337.1 KPa at 76.6°C and once washed meat had higher G′ values than unwashed meat at any given temperature in the experimental range. The thrice and five times washed meat showed lower G′ values than once washed meat and this may be due to high moisture content in the sample. Further, the maximum G′ values obtained for thrice and five times washed meat were lower than the unwashed meat. It is likely the higher moisture content has hindered the development of elastic component (G′′ values) in thrice and five times washed meat. The temperature at which sol–gel transition occurred as indicated by tan δ values showed three transitions regions in the temperature range of 36.7–70°C for all the four samples. This three transition region is due to the involvement of different sub fragments of fish proteins.Citation35 Citation36 The gelation profile of sardine meat indicates its inability to form dense network in comparison to pink perch meat.Citation33 Though the maximum value of G′ was higher in once washed meat, the gel strength value was lower in comparison to thrice and five times washed meat. It is not clear for this difference in large strain and small strain test. LanierCitation37 indicated the possibility that surimi sample may display undesirable traits in indirect test; yet perform quite well in the product application.
Conclusions
The results from various parameters studied suggest that the meat from sardine can be a potential alternative source for production of gel-based products. The properties of proteins from fresh (unwashed) and washed oil sardine meat can make considerable difference in the quality of final surimi. Keeping in view the loss of nutrients as well as the ease of processing and effluent cost effectiveness, three times water washing of sardine meat is found to be optimum for high quality surimi. The physico-chemical, functional, and rheological properties of proteins from sardine enhanced significantly upon water washing and the number of cycles of such washes have no noticeable effect on the proteins. These are correlated by both gel filtration and gel electrophoretic data suggesting water washing can improve the desired characteristics of meat mince without addition of any external additives and chemicals to the mince.
Acknowledgments
The authors gratefully acknowledge the funding received from Indian Council of Agricultural Research, New Delhi, India. (Grant No. F 4(6)/99-ASR-1) for carrying out this work.
References
- Lee , C.M. 1984 . Surimi process technology . Food Technol. , 38 : 69 – 80 .
- Chumreang , S. , Sangjindavong , M. and Pluksawanich , K. 1999 . Use of potassium bromate and egg white for increasing gelforming ability of surimi . Int. J. Food Prop. , 2 ( 2 ) : 163 – 173 .
- Mendes , R. and Nunes , M.L. 1992 . “ Characterization of sardine (Sardina pilchardus) protein changes during surimi preparation ” . In Quality Assurance in the Fish Industry Edited by: Huss , I.M. , McKeth , F.K. and Lan , Y.H. 63 – 71 . London : Elseiver Science Publishers .
- Lanier , T.C. 1986 . Functional properties of surimi . Food Technol. , 40 : 107 – 114 .
- Chen , H.-H. , Chiu , E.-M. and Huang , J.-R. 1997 . Color and gel forming properties of horse mackerel (Trachurus japonicus) as related to washing conditions . J. Food Sci. , 62 : 985 – 991 .
- Roussel , H. and Cheftel , J.C. 1990 . Mechanisms of gelation of sardine proteins: influence of thermal processing and of various additives on the texture and protein solubility of kamaboko gels . Int. J. of Food Sci. Technol. , 25 : 260 – 280 . [CSA]
- Mendes , R. , Batista , I. , Kandado , R. and Howell , N. 1998 . Influence of washing parameters on the characteristics of horse mackerel (Trachurus trachurus) mince . J. Food Biochem. , 22 : 511 – 528 .
- Reppond , K.D. , Babbitt , J.K. , Bernsten , S. and Tsuruta , M. 1995 . Gel properties of surimi from pacific herring . J. Food Sci. , 60 : 707 – 710, 714 .
- Shimizu , Y. , Toyohara , H. and Lanier , T.C. 1992 . “ Surimi production from fatty and dark fleshed fish species ” . In Surimi Technology Edited by: Lanier , T.C. and Lee , C.M. 181 – 208 . New York : Marcel Decker .
- Gopakumar , K. 1997 . Tropical Fishery Products New Delhi : Oxford and R & H Publishing Co. Pvt. Ltd. .
- CMFRI Central Marine Fisheries Research Institute , Annual Report . 1999–2000; Kochi, India, 2000 pp. 1 – 161 . .
- Shamasundar , B.A. Proceedings of National Seminar on Sustainable Fisheries for Nutritional Security . Fish Processing in India , Edited by: Pandian , T.J. pp. 250 – 271 . New Delhi : National Academy of Agricultural Sciences .
- AOAC. 1995 . Official Methods of Analysis, , 16th Ed. Virginia, , USA : Association of Official and Analytical Chemists International .
- Velankar , N.L. and Govindan , T.K. 1958 . A preliminary study of the distribution of non-protein nitrogen in some marine fishes and invertebrates . Proc. Indian Acad. Sci. , 47 : 202 – 209 .
- Yang , J.T. 1961 . The viscosity of macromolecule in relation to molecular conformation . Advances in Protein Chemistry , 16 : 323 – 400 . [PUBMED] [INFOTRIEVE]
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head bacteriophage . T4 Nature , 227 : 680 – 685 .
- Sosulki , F.W. 1962 . The centrifuge method for determining water absorption in hard red spring wheats . Cereal Chem. , 39 : 344 – 350 .
- Lin , M.Y.J. , Humert , E.S. and Sosulki , F.W. 1974 . Certain functional properties of sunflower meal products . J. Food Sci. , 39 : 368 – 370 .
- Swift , C.E. , Lockett , C. and Fryer , A.J. 1961 . Comminuted meat emulsion, the capacity of the meat to emulsify fat . Food Technol. , 15 : 468
- Suzuki , T. 1981 . Fish and Krill protein: Processing Technology Barking, Essex : Applied Science Publishers .
- Okada , M. 1963 . Elastic properties of kamaboko (fish meat jelly) . Bulletin of Takai. Regional Fisheries , 36 : 26 – 126 .
- Hays , W.L. 1981 . Statistics Tokyo, , Japan : Hold-Saunders .
- Chuapoehuk , P. , Raksakulthani , N. and Worawattanamateekul , W. 2001 . Process development of fish sausage . Int. J. Food Prop. , 4 ( 3 ) : 523 – 529 . [CSA] [CROSSREF]
- Roussel , H. and Cheftel , J.C. 1988 . Characteristics of surimi and kamaboko from sardines . Int. J. Food Sci. Technol. , 23 : 607 – 623 . [CSA]
- Suvanich , V. , Jahncke , M.L. and Marshall , D.L. 2000 . Changes in selected chemical quality characteristics of channel catfish frame mince during chill and frozen storage . J. Food Sci. , 65 : 24 – 29 .
- Hastings , R.J. , Keay , J.N. and Young , K.W. 1990 . The properties of surimi and kamaboko gels from nine British species of fish . Int. J. Food Sci. Technol. , 25 : 281 – 294 . [CSA]
- Jimmez-Colmenero , F. and Borderias , A.J. 1983 . A study of effects of frozen storage on certain functional properties of meat and fish protein . J. Food Sci. Technol. , 18 : 731 – 737 .
- Lin , T.M. and Park , J.W. 1996 . Extraction of proteins from pacific whiting mince at various washing conditions . J. Food Sci. , 61 : 431 – 438 .
- Kinsella , J.E. 1982 . “ Relationship between structure and functional properties of food proteins ” . In Food Proteins Edited by: Fox , P.F. and Condon , J.J. 51 – 103 . New York : Applied Science Publishers .
- Kato , A. and Nakai , S. 1980 . Hydrophobicity determined by fluorescence probe method and its correlation with surface properties of proteins . Biochem. Biophys. Acta , 624 : 13 – 20 . [PUBMED] [INFOTRIEVE]
- Gordon , A. and Barbut , S. 1992 . Mechanism of meat latter stabilization: a review, CRC . Critical Reviews in Food Science and Nutrition , 32 : 299 – 332 . [PUBMED] [INFOTRIEVE]
- Niwa , E. 1992 . “ Chemistry of surimi gelation ” . In Surimi Technology Edited by: Lanier , T.C. and Lee , C.M. 389 – 427 . New York : Marcell Dekker Inc. .
- Ratnakumar , K. and Shamasundar , B.A. 1998 . “ Visco-elastic properties of pink perch (Nemipterus japonicus) meat during setting and gelation ” . In Advances and Priorities in Fisheries Technology Edited by: Balachandran , K.K. , Iyer , T.S.G. , Madhavan , P. , Joseph , J. , Perigreen , P.A. , Raghunath , M.R. and Varghese , M.D. 251 – 256 . Cochin : Society of Fisheries Technoligists (India) .
- Hamann , D.D. 1987 . Methods for measuring rheological changes during thermally induced gelation of proteins . Food Technol. , 41 : 100 – 108 .
- Sano , T. , Noguchi , S.F.T. , Suchiya , T. and Matsumoto , J.J. 1988 . Dynamic visco-elastic behavior of natural actomyosin and myosin during thermal gelation . J. Food Sci. , 53 : 224 – 228 .
- Zhang , J. , Farkas , B.E. and Hale , S.A. 2001 . Thermal properties of skipjack tuna (Katsuwonus pelamis) . Int. J. Food Prop. , 4 ( 1 ) : 81 – 90 . [CSA] [CROSSREF]
- Lanier , T.C. 1992 . “ Measurement of surimi composition and functional properties ” . In Surimi Technology Edited by: Lanier , T.C. and Lee , C.M. 115 – 151 . New York : Marcel Dekker, Inc. .