Abstract
Despite declining global malaria incidence, the disease continues to be a threat to people living in endemic regions. In 2015, an estimated 214 million new malaria cases and 438,000 deaths due to malaria were recorded. Plasmodium vivax is the second most common cause of malaria next to Plasmodium falciparum. Vivax malaria is prevalent especially in Southeast Asia and the Horn of Africa, with enormous challenges in controlling the disease. Some of the challenges faced by vivax malaria-endemic countries include limited access to effective drugs treating liver stages of the parasite (schizonts and hypnozoites), emergence/spread of drug resistance, and misperception of vivax malaria as nonlethal. Primaquine, the only 8-aminoquinoline derivative approved by the US Food and Drug Administration, is intended to clear intrahepatic hypnozoites of P. vivax (radical cure). However, poor adherence to a prolonged treatment course, drug-induced hemolysis in patients with glucose-6-phosphate dehydrogenase deficiency, and the emergence of resistance make it imperative to look for alternative drugs. Therefore, this review focuses on data accrued to date on tafenoquine and gives insight on the potential role of the drug in preventing relapse and radical cure of patients with vivax malaria.
Introduction
Among Plasmodium species causing malaria in humans, Plasmodium falciparum and Plasmodium vivax are the most common causes of malaria. Despite progress in reducing global malaria incidence by 37%, an estimated 214 million new cases of malaria and 438,000 deaths caused by the disease were recorded in 2015. Africa was the most severely affected region, with 88% of global malaria incidence followed by Southeast Asia where 10% of the global incidence was reported.Citation1–Citation3
P. vivax is the second most common cause of malaria in humans next to P. falciparum. In 2015, an estimated 13.8 million new cases were reported globally. The highest vivax incidence was recorded in Southeast Asia (74%), followed by the Eastern Mediterranean Region (11%) and Africa (10%).Citation2,Citation3 Surprisingly, 80% of global vivax malaria cases were reported from three countries, Ethiopia, India, and Pakistan.Citation1 Even though P. vivax is widely distributed, its global incidence rate is wrongly perceived to be low. Some factors that explain underestimation of vivax malaria incidence include microscopic misreading as falciparum in co-endemic areas and presence of undetectable parasitemia among symptomatic patients and liver hypnozoites among asymptomatic patients.Citation2,Citation4–Citation11
Hypnozoites and relapse of malaria
Human infection with Plasmodium species starts when sporozoites are injected into the blood circulation while female Anopheles mosquito feeds on human blood. These sporozoites then migrate shortly to the liver hepatocytes and enter the exoerythrocytic cycle, in which high numbers of schizonts are produced mitotically. Within 5–15 days of liver infection, thousands of merozoites are released and enter blood circulation. The merozoites then infect red blood cells (RBCs), and this constitutes the erythrocytic cycle that repeats many times in a course of single malaria episode. The erythrocytic cycle produces an average of 8–32 new merozoites and gametocytes per infected RBC. When female Anopheles mosquitoes feed on blood of infected individuals, these gametocytes are ingested and begin sporogonic phase in the gut of mosquitoes, thereby perpetuating vivax infection ().Citation8,Citation9,Citation12–Citation17
Figure 1 Life cycle of P. vivax and hypnozoite.
Abbreviation: P. vivax, Plasmodium vivax.
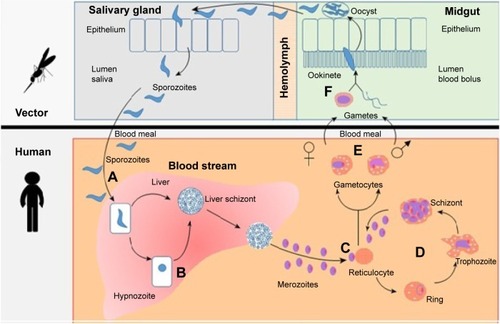
However, a few hepatic schizonts of P. vivax and Plasmodium ovale hibernate instead of migrating to the vascular RBCs. These dormant stages, called hypnozoites, are capable of reactivation and could cause relapse of malaria weeks, months, or even years after the first malaria episode.Citation8,Citation9,Citation12 The underlying mechanisms for reactivation are not thoroughly understood. However, factors related to relapse of malaria include the adaptive trait of the parasites, geographical variations, the presence of other febrile illness, and dose of the injected sporozoites.Citation9,Citation18,Citation19 A pooled analysis of data from studies published in English that included 87,000 patients with acute vivax malaria reported that ~20% of the patients experienced malaria relapse, with a relapse rate ranging from 0% to 100%.Citation19 Studies have also identified that the events of vivax relapse are associated with patient and parasite factors.Citation20,Citation21
Malaria interventions
Global malaria incidences reduced from 262 million in 2000 to 214 million in 2015. The World Health Organization has also planned to eliminate malaria in more than 20 countries by 2025 through implementation of integrated malaria control programs that use interventions such as vector control, effective diagnosis, and use of artemisinin-based combinations therapies.Citation1,Citation3,Citation22,Citation23 Moreover, RTS, S, a recombinant protein-based malaria vaccine, which has been approved by the European Medicines Agency, with a protective efficacy of ~26% in young infants and 36% in children,Citation24 may also be integrated with other tools in malaria control.
Vector control
In sub-Saharan Africa, ~50% of the population used either insecticide-treated mosquito nets or indoor residual spraying to prevent malaria in 2013.Citation3,Citation25 Despite emergence of pyrethroid-resistant Anopheles mosquitoes (mainly Anopheles gambiae and Anopheles funestus) worldwide, countries such as Ethiopia, Zambia, and Rwanda have reduced malaria incidence by more than two-thirds compared to what was projected for the countries between 2000 and 2011. Similarly, 4% of global and 7% of African populations were protected from malaria by indoor residual spraying in 2013.Citation2,Citation26–Citation30
Malaria diagnosis
Accurate diagnosis and prompt treatment of all patients with proper antimalarial drugs is one of the essential components of malaria control and elimination strategies. Early diagnosis and treatment of acute cases is the primary approach to reduce mortality and morbidity.Citation31,Citation32 Diagnosis of malaria is done based on a defined set of criteria. Even though any patient with acute febrile illness should be a malaria suspect, the criteria, particularly the parasite density, may vary with the level of malaria transmission intensity. The World Health Organization also recommends that parasitological confirmation of malaria cases should be done with either microscopy or rapid diagnostic test in order to improve care of parasite-positive patients.Citation33–Citation35
Current approaches applied for controlling P. falciparum malaria may not be effective against P. vivax. Control program against P. vivax is proven to be a formidable task due to lack of suitable diagnostic tools to detect low vivax parasitic loads during blood stage, presence of hypnozoites, and lack of effective whole stage drugs against vivax.Citation2,Citation7,Citation11,Citation19,Citation36
Treatment of uncomplicated vivax
Chloroquine (CQ) is still the first line of treatment for malaria due to P. vivax infectionCitation37 although CQ-resistant P. vivax has been identified in various countries.Citation38 A review of ten nonrandomized trials showed that the therapeutic success of CQ at 28 days of follow-up ranges from 49.2% to 96.2%.Citation39 In addition to CQ, P. vivax and P. ovale infections require treatment with primaquine (PQ) for radical cure because of the presence of hypnozoites in their life cycles. In areas with CQ-resistant P. vivax, artemisinin-based combinations therapies containing piperaquine, mefloquine, or lumefantrine are the recommended treatments.Citation40,Citation41 Artemisinin-based combinations therapies are also effectiveCitation37,Citation42–Citation44 for treatment of malaria due to coinfection with multiple species.Citation43–Citation45 Radical treatment with PQ is needed for P. vivax or P. ovale coinfections.Citation37
Treatment of complicated vivax
P. vivax is considered relatively benign disease,Citation46 even though manifestations such as severe anemia and acute respiratory distress syndrome are encountered occasionally.Citation47 A recent systematic review highlights a marked increase of reported cases of severe vivax in certain P. vivax-endemic regions of the world.Citation47 Patients with severe vivax malaria are treated aggressively with either parenteral cinchona alkaloids, ie, quinine or quinidine, or artemisinin derivatives, preferably intravenous artesunate.Citation48
P. vivax infections that recur after drug treatment may be a recrudescent of asexual blood-stage parasites that survived drug treatment, reinfection from new mosquito inoculation, or relapse due to hypnozoites.Citation41
Patients with vivax malaria may benefit from the major blood schizonticidal agents only for clinical cure. Similarly, chemoprophylaxis with blood schizonticidal agents provides only suppressive (clinical) prophylaxis.Citation49 Hence, a drug targeting the hepatic stages of primary schizonts and secondary schizonts (hypnozoites) would be critical for causal prophylaxis, terminal prophylaxis/presumptive anti-relapse therapy (PART), and radical cure of infection caused by vivax malaria.Citation50 PQ, a synthetic derivative of quinine,Citation51 has been instrumental in these aspectsCitation52 ().
Malaria prophylaxis
Causal prophylaxis
So far, eight clinical studies with different research designs, including experimental challenge studies, controlled trials, and prospective observational studies, have been conducted to evaluate the efficacy of PQ in preventing malaria. The results from these studies showed PQ’s protective efficacy against vivax malaria at 30 mg/d to be >85%.Citation50 In these studies, PQ was started 1 day before travel to an endemic area and continued throughout the stay in the area as well as for 7 days after return. However, PQ is not being used as a causal prophylactic agent in many countries up until now.Citation53
Terminal prophylaxis
Terminal prophylaxis is alternatively called PART. PQ is used for PART with a regimen of 15 mg/d orally for 14 days immediately after the individuals have traveled out of vivax-endemic areas.Citation54 Terminal prophylaxis is considered for persons who have resided for prolonged periods (eg, ≥6 months) in high-risk vivax-endemic areas or who experience intense exposure to P. vivax.Citation50
Radical cure
A standard PQ therapy of 15 mg/d orally for 14 days has been reported to fail in preventing relapse in different geographic locations such as the Solomon Islands, Southeast Asia, Brazil, Colombia, Guyana, Guatemala, Somalia, Ethiopia, Afghanistan, and elsewhere.Citation50,Citation55 These reports may not represent actual failures of the 15 mg daily regimen since the extent of adherence to the recommended regimen and quality of the medications were not confirmed in these studies.
A meta-analysis evaluated the efficacy of PQ in preventing vivax malaria recurrence in 59,735 patients.Citation55 A marked heterogeneity was noted in the study design and particularly on PQ dosing. Despite differences in design, it was possible to draw a conclusion from 87 clinical trials selected for the systematic review. Three dose ranges of PQ, expressed as total doses per kilogram, were considered. The dose ranges included very low (<2.5 mg/kg body weight), low (>2.5 mg/kg–<5.0 mg/kg body weight), and high (≥5.0 mg/kg body weight). The median rate of recurrence following very low dose of PQ in 44 studies was 25% (range, 0%–90%) at 4–6 months, whereas in 82 studies following low dose of PQ, the recurrence was 6.7% (range, 0%–59%) at 4–6 months. High-dose PQ regimens assessed in 28 treatment arms were associated with a median recurrence rate of 0% (range, 0%–15%) at 1 month. Some strains of P. vivax may also show inherent resistance to 15 mg/d regimen without any previous exposure to PQ. Taking all these into consideration, higher doses of PQ (30 mg base for 14 days; 420 mg total dose) are administered to prevent relapse in some regions.Citation50,Citation56
Why PQ use is not at its maximum?
Even though PQ was licensed for use in 1952 for the prevention and cure of malaria, its use is not maximized to the need of the society.Citation57 The major factor that limits the wide usage of PQ is the high risk of hemolysis in patients with gluocse-6-phosphate dehydrogenase (G6PD) deficiency.Citation52
Hemolysis related to G6PD deficiency
Polymorphisms of the G6PD gene are numerous, with G6PD deficiency due to single-point mutations, deletions, insertions, and rarely, splicing variants.Citation58 Approximately 400 variant alleles have been described.Citation59 The overall prevalence of G6PD deficiency allele across malaria-endemic countries is estimated to be 8.0% (interquartile range, 7.4–8.8). Using the 2010 population data, this corresponds to 220 million males (interquartile range, 203–241) and an estimated 133 million females (interquartile range, 122–148), including 17 million homozygous females.Citation60
Treatment of 22 G6PD-deficient patients in Thailand with a 3-day course of CQ followed by PQ, 15 mg/d for 14 days, resulted in a significant reduction in hematocrit. The treatment did not result in blood transfusion.Citation61 Another report from Brazil revealed the risks of hemolysis in 18 patients who were referred to a tertiary care unit after therapy with PQ (0.5 mg/kg/day for 7 days) for radical cure. All patients had jaundice, 14 patients had dark urine, and one patient presented with low urinary output. Blood transfusion was required in 12 out of the 18 patients included in the study due to PQ-induced hemolysis. A patient who developed severe renal failure required hemodialysis. The study confirmed that all the abovementioned adverse phenomena were associated with the patients’ G6PD deficiency.Citation62 The association between the extent of PQ-induced hemolysis and the dose of PQ as well as degree of G6PD deficiency is well established.Citation40 Hence, it is recommended that patients are tested for G6PD deficiency before treatment with PQ and many countries restrict the use of PQ, including single-dose treatment, for the gametocidal purpose.Citation63
Other adverse effects associated with PQ
Though not a major reason for abandoning the use of PQ, individuals taking PQ can develop additional adverse effects such as gastrointestinal discomfort and methemoglobinemia.Citation54 Administration of 22.5–30 mg of PQ per day resulted in mild-to-moderate abdominal cramps in 10%–12% of patients.Citation52 Standard PQ therapy elevates methemoglobin levels slightlyCitation54 and could be treated with intravenous methylene blue. However, in the presence of G6PD deficiency, patients may not respond to treatment.Citation64
Need for high dose for a better outcome
Evidence for failure of the standard PQ regimen, which is 15 mg/d orally for 14 days, in preventing relapse emerged from experimental challenge with the Chesson strain of P. vivax. This strain was isolated from an American soldier infected in New Guinea in 1944.Citation65 Another trial in Thailand demonstrated reduced efficacy of a 15 mg PQ regimen in preventing relapse.Citation66 A study in Germany also demonstrated that infection acquired on the islands of New Guinea had a high risk of relapse after PQ therapy.Citation67 The higher dose required to tackle PQ-resistant vivax in radical cure or terminal prophylaxis would increase dose-dependent adverse effects from the drug.
Tafenoquine: the next generation 8-aminoquinoline
Currently available antimalarial drugs are stage specific. Drugs such as PQ have activity mainly on hypnozoites and hence help in preventing relapse, while drugs such as CQ have effect on blood stages (asexual stages), which makes it important for clinical cure of patients with malaria. This fact should attract researchers to work further in discovering and developing products that target multiple stages of the Plasmodium. Efforts are also being exerted to come up with a better alternative drug that can be used in treatment failures due to resistance and that shorten treatment duration. As an example, PQ’s liabilities include prolonged treatment duration; increasing trend of failing treatments, particularly at 15 mg/d regimen; and the risk of hemolytic anemia in G6PD-deficient patients. These identified gaps in PQ treatment should put a pressure on the scientific community to search for alternative interventions that can fill these gaps.
The 8-aminoquinoline tafenoquine (TQ; WR 238605) was discovered by scientists at the Walter Reed Army Institute of Research in 1978. It is currently being developed in a collaborative approach between GlaxoSmithKline and Medicines for Malaria Venture.Citation68
Pharmacokinetics of TQ
Bioavailability and absorption
First-time-in-humans safety and pharmacokinetics study showed that the time to peak concentration (tmax) of TQ is 13.8 hours. This study speculated that the prolonged absorption from the gut could be due to absorption at distal gastrointestinal tract combined with the drug’s slow clearance.Citation69 In a Phase I trial involving 156 individuals taking different doses of TQ, it was demonstrated that TQ is slowly absorbed. The median tmax values for TQ were 15 hours for the 300 mg dose and 12 hours for the 600 mg and 1,200 mg doses. Area under the curve and maximum concentration observed (Cmax) exhibited moderate intersubject variability.Citation70
Bioavailability of TQ increases when the drug is taken with high-fat meal.Citation71 From a population kinetics study of TQ in Thai soldiers, it was demonstrated that food affects the amount of TQ absorbed, rather than the rate of absorption.Citation72
Distribution
TQ has a large volume of distribution (~2,560 L) and a low clearance (~6 L/h).Citation69 Though body weight affects the plasma concentration in general, females tended to achieve higher drug concentrations compared to males of equivalent weight.Citation73 The concentration of TQ in whole blood is approximately twofold higher than the corresponding concentration in plasma. In individuals with a normal hematocrit of 45%, the drug concentration in the erythrocytes is estimated to be threefold higher than that in plasma. However, there is no change in accumulation of the drug in RBCs over time.Citation69 Little is known about the distribution of TQ to extra vascular tissues in human beings, but animal study showed that the drug is highly distributed to the liver. Area under the curve up to the last measurable concentration (AUClast) in the liver after intravenous administration is ~80 times more than that in the plasma.Citation74
Metabolism and excretion
Like PQ,Citation75 the activation of TQ needs metabolism by cytochrome P450 2D6 (CYP2D6) liver microsomal enzyme.Citation76,Citation77 This was demonstrated by the lack of the anti-malarial activity of TQ in CYP2D6 knockout mice when given at a dose of 3 mg/kg and the partial restoration of its antimalarial activity in humanized CYP2D6 knockin mice.Citation76
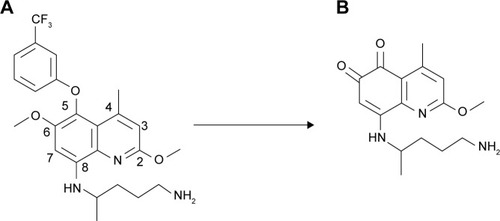
A metabolite of TQ, 5,6 ortho-quinone TQ, has been identified from animals taking the drug (). The level of 5,6 ortho-quinone TQ is high after administration of TQ in wild-type extensive metabolizer phenotype. This report from laboratory animals shows the association between CYP2D6 metabolism and TQ pharmacokinetics. The findings in the laboratory animals could suggest that TQ is metabolized by CYP2D6 in human beings in whom the isoenzyme shows polymorphism.Citation77
Figure 3 Metabolism of TQ (A) to 5,6 ortho-quinone TQ (B) in the presence of 2D6 isoenzyme.Citation77
Pharmacodynamics of TQ
Plasmodium stages affected by TQ
An in vitro test evaluating the activity of 8-aminoquinolines showed that TQ has an average 50% inhibitory concentration (IC50) of 0.436 μM (range, 0.059–1.47 μM) against blood stages of seven P. falciparum clones and strains (NIG59, NIG9171, D6, W2, TM91C235, WR75-235, and TM91C40).Citation78
Another study evaluated an in vitro activity of TQ and PQ in wild isolates of P. falciparum from Djibouti, Gabon, and Senegal, where the isolates are CQ resistant. The IC50 values for TQ were in the range of 0.9–9.7 μM for the Djiboutian isolates, 0.6–33.1 μM for the Gabonese isolates, and 0.5–20.7 μM for the Senegalese isolates.Citation79 PQ’s activities were inferior to those of TQ.
A study from Thailand investigated the transmission blocking potential of TQ. The study evaluated the efficacy of TQ against the sporogonic stage of vivax parasite after letting mosquitoes feed on gametocytemic blood containing TQ. TQ reduced the transmission of the parasite to the mosquito at doses of ≥25 mg/kg.Citation80
Mechanism of action
TQ is a prodrug that needs activation to quinone TQ metabolite through metabolism by CYP2D6 ().Citation76 The mechanism of action of TQ is not yet precisely known. Research Councils UKCitation81 demonstrated the metabolites of 8-aminoquinolines to be redox cycled by P. falciparum ferredoxin-NADP+ reductase and diflavin reductase enzymes, which are upregulated in gametocytes and liver stages. The spontaneous oxidation of metabolites also generates hydrogen peroxide and hydroxyl radicals. It is hypothesized that the reactive oxygen species generated through P. falciparum ferredoxin-NADP+ reductase and diflavin reductase enzymes leads to parasite kill.Citation81 The upregulation of these enzymes in TQ-sensitive stages of the parasite supports the hypothesis.
Similar to the blood schizonticide CQ, TQ inhibits heme polymerase in blood stage of the parasites.Citation78 This may explain the reason why TQ has activity against asexual blood stage of parasites, unlike PQ that does not inhibit the polymerization of hematin.
Clinical development of TQ
TQ, an investigational 8-aminoquinoline derivative for the treatment and relapse prevention of P. vivax malaria, has been granted a breakthrough therapy designation by the US Food and Drug Administration.Citation82 Breakthrough therapy designation is the US Food and Drug Administration’s program aimed at accelerating the development and review times of drugs for serious or life-threatening conditions. The designation was granted since preliminary clinical evidence indicated that TQ has substantial improvement over the existing therapy.Citation83
Efficacy of TQ in radical cure
There were six controlled trials published, out of which three were randomized controlled dose selection trials. Subsequently, out of the three randomized trials, only one trial was a double-blind study.Citation84–Citation87 High-quality evidence on the efficacy of TQ for radical cure is obtained from this double-blind study, which was also a multicenter, randomized, placebo-controlled Phase IIb study (the Dose and Efficacy Trial Evaluating Chloroquine and Tafenoquine In Vivax Elimination [DETECTIVE] trial). The trial evaluated efficacy of TQ in a range of doses in preventing relapse within 6 months. Single dose of TQ at 600 mg, following the standard CQ therapy for clinical cure, prevented relapse of vivax malaria by 91.9%, whereas 15 mg PQ administered for 14 days prevented relapse only by 77.3%.Citation85 The same study reevaluated the efficacy of TQ in homologous vivax strain. The recurrence rate was 3.5% and 1.8% at 300 mg and 600 mg single doses of TQ, respectively. At a dose of 300 mg TQ, there was a ninefold reduction in homologous recurrence compared with CQ alone. At the same dose of 300 mg, heterologous recurrences also reduced by threefold.Citation84 The DETECTIVE trial concluded that 50 mg and 100 mg of TQ do not provide a satisfactory prevention of relapse ().
Table 1 Efficacy of TQ in radical cure
Two open-label controlled trials showed that a single dose of 500 mg or 600 mg of TQ added to the standard dose of CQ reduced the recurrence rate of vivax infection by 75% and 91.1%, respectively, compared with the control arm treated with CQ alone.Citation86,Citation87 Although these studies involved few study participants, the findings were somewhat in agreement with those of the DETECTIVE trial ().
Trials investigating the effect of repeated doses of TQ to prevent relapse of vivax malaria were also conducted. A 3-day course of TQ was found to be noninferior to a 14-day course of PQ treatment with a relapse rate of 3.48% and 3.3%, respectively.Citation88 Two randomized open-label controlled trials from Thailand also revealed that a 7-day course of TQ after the standard dose of CQ prevented relapse completely ().Citation86,Citation87
Chemoprophylactic efficacy of TQ
In a human challenge study, administration of TQ (600 mg single dose) 1 day prior to mosquito challenge prevented falciparum infection in three of the four volunteers included in the study. In this study, the volunteer who got infected had a low plasma TQ concentration.Citation89 A study on protective efficacy of TQ against falciparum malaria in Ghana revealed that the drug’s efficacy ranges from 84.4% to 87.2% using a regimen of 50–200 mg/d for 3 days as loading followed by weekly maintenance doses. Shanks et al support the idea of adding weekly maintenance dose of TQ in the regimen in order to achieve a better efficacy.Citation90,Citation91 Another study in Gabon confirms the prophylactic efficacy of TQ at 250 mg for 3 days to be 100% during a 2-month study period.Citation92 Another study in East Timor comparing the chemoprophylactic efficacy of TQ and mefloquine against vivax infection showed that TQ’s prophylactic efficacy of 99.1% was not inferior to that of mefloquine’s, which was 99.3% as shown in .Citation93
Table 2 Chemoprophylactic efficacy of TQ
Efficacy of TQ for terminal prophylaxis
Randomized controlled trials evaluated the terminal prophylactic efficacy of TQ in Australian defense force personnel returning from Bougainville and East Timor.Citation94 The relapse rate was higher in study participants returning from East Timor than in those returning from Bougainville even though the treatment did not differ in the two locations. This study revealed TQ to be a good drug for PART. TQ administered with a twice daily (bid) dose of 200 mg for 3 days had a similar efficacy to 400 mg of the drug administered once daily for 3 days (). The pooled relapse rate in treatment arms getting the divided dose of 200 mg TQ bid (3.1%; 85% confidence interval [CI]: 1–7.1) was not significantly different from that getting the single dose of 400 mg daily (7.9%; 95% CI: 4.8–12).Citation94
Table 3 Efficacy of TQ in terminal prophylaxis
Another trial compared the terminal prophylactic efficacy of 400 mg TQ for 3 days (divided and undivided dose) with PQ (7.5 mg tid for 14 days) in study participants returning from Bougainville.Citation95 The relapse rate in the TQ arm was 1.9% (95% CI: 0.5–3.3), whereas that in the PQ arm was 2.8% (95% CI: 0.6–5; ).
Curative efficacy of TQ
In vitro experiments showed that TQ has antimalarial effect on the blood stage of P. falciparum isolates. The IC50 of TQ against falciparum isolates ranges from 59 nM to 9.7 μM from different studies.Citation78,Citation79 Evidences support the effect of TQ to be related with the inhibition of heme polymerase.Citation78 The reported IC50 of TQ against falciparum isolates were higher than the artesunate IC50 (range, 1.85–2.42 nM) and lower than the CQ IC50 (range, 104–334 nM). This is explained by the fact that the isolates used for test were resistant to CQ.Citation79
An exploratory study investigated the curative efficacy of TQ (400 mg single dose followed by 200 mg bid for 2 days) in two vivax malaria patients after their return from Papua New Guinea. The patients had no positive blood smears during the 2-year follow-up period. The investigators observed that malaria parasites were cleared rapidly. These findings, however, need to be supported by randomized controlled trial(s) before TQ is used for curative purpose.Citation96
Safety of TQ
A study investigating the safety of 200 mg weekly dose of TQ for 6 months in 492 participants showed that 13% of the participants encountered at least one adverse event. The most frequent complaint was gastrointestinal abnormalities (nausea and abdominal pain). Treatment-related mild vortex keratopathy, corneal deposits, was also detected in 93% (69 of 74) of subjects taking TQ. The vortex keratopathy was not associated with any effect on visual acuity and was fully resolved in all subjects by 1 year.Citation93
Another study assessed possible adverse effects of TQ in 369 participants with a dose range of 25 mg to 200 mg in a weekly prophylactic therapy. Gastrointestinal abnormalities (diarrhea, dysentery, and abdominal pain) were the most common reported adverse events with a frequency ranging from 13% to 18%. The study also confirmed that there is no evidence of a relationship between TQ dosage and reports of physical complaints or the occurrence of abnormal laboratory parameters. The laboratory parameters assessed in the study were alanine aminotransferase, hemoglobin, white blood cell counts, platelet counts, and bilirubin levels.Citation90
Study participants included in the clinical trial of TQ are screened for normal level of G6PD activity. A single study reported hemolytic anemia in study participants with G6PD deficiency who were wrongly recruited in a TQ trial.Citation91
Prolongation of the QT interval is also one of the concerns in patients treated with antimalarial drugs. In the DETECTIVE trial, QT prolongation occurred in 2% of patients taking TQ, while the phenomenon was observed in 8% and 4% of patients taking PQ and CQ, respectively.Citation85 In another study,Citation70 TQ did not cause prolongation of QT interval even at a dose of 1,200 mg. Hence, TQ could be taken as a safer drug in terms of QT prolongation compared with other quinoline antimalarial drugs probably due to short duration of treatment with TQ.Citation70
Conclusion
The conclusions of this review are as follows:
TQ is an efficacious drug for radical cure, terminal prophylaxis, and chemoprophylaxis of vivax malaria. TQ has at least similar efficacy to PQ for radical cure and terminal prophylaxis with the additional benefit of short treatment duration, which can significantly improve patient adherence. A weekly administration of TQ also demonstrated equivalent chemoprophylactic efficacy with that of mefloquine.
Most of TQ efficacy studies are conducted in individuals with normal G6PD activity. However, one clinical study reported severe hemolytic anemia in G6PD-deficient individuals who were wrongly included in the study. Data on the relative safety of TQ over PQ in patients with G6PD deficiency are lacking.
Though TQ has a better activity against clinical isolates of blood stage P. vivax parasite in vitro compared to PQ and CQ, there is not enough evidence supporting its use for clinical cure.
The authors recommend further multicenter clinical studies on TQ with appropriate sample size and considering special populations before labeling TQ as an alternative medication for radical cure, terminal prophylaxis, and chemoprophylaxis of vivax malaria. Additional studies might be also required to investigate possible drug interactions with TQ.
Authors contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Addis Ababa University for providing articles. The authors declare that there is no financial support for the current review.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationWorld Malaria Report 2015GenevaWorld Health Organization2015
- World Health OrganizationWorld Malaria Report 201455GenevaWorld Health Organization2014
- World Health OrganizationGlobal Technical Strategy for Malaria 2016–2030GenevaWorld Health Organization2015
- BattleKEGethingPWElyazarIRFThe Global Public Health Significance of Plasmodium Vivax80AmsterdamElsevier2012
- NaingCWhittakerMANyunt WaiVMakJWIs Plasmodium vivax malaria a severe malaria?: a systematic review and meta-analysisPLoS Negl Trop Dis201488e307125121491
- CarltonJMAdamsJHSilvaJCComparative genomics of the neglected human malaria parasite Plasmodium vivaxNature2008455721475776318843361
- BairdJKResistance to therapies for infection by Plasmodium vivaxClin Microbiol Rev200922350853419597012
- PopoviciJMénardDChallenges in antimalarial drug treatment for vivax malaria controlTrends Mol Med2015211277678826611336
- PriceRNTjitraEGuerraCAUKPMC Funders GroupVivax malaria: neglected and not benignAm J Trop Med Hyg2007776798718165478
- BhardwajMBharadwajLTrigunayatKTrigunayatMMThermal characterization of Plasmodium falciparum species specific proteins in Indian geographical areaNat Preced2010 Available from: http://precedings.nature.com/documents/4565/version/1Accessed July 28, 2016
- LacerdaMVMourãoMPAlexandreMAUnderstanding the clinical spectrum of complicated Plasmodium vivax malaria: a systematic review on the contributions of the Brazilian literatureMalar J2012111222230294
- GuerinPJOlliaroPNostenFMalaria: current status of control, diagnosis, treatment, and a proposed agenda for research and developmentLancet Infect Dis20022956457312206972
- FlanneryELChatterjeeAKWinzelerEAAntimalarial drug discovery – approaches and progress towards new medicinesNat Rev Microbiol2013111284986224217412
- KhengSMuthSTaylorWRTolerability and safety of weekly primaquine against relapse of Plasmodium vivax in Cambodians with glucose-6-phosphate dehydrogenase deficiencyBMC Med201513111025563062
- ValFFSampaioVSCasseraMBPlasmodium vivax malaria elimination: should innovative ideas from the past be revisited?Mem Inst Oswaldo Cruz2014109552252425184997
- CollinsWEJefferyGMPrimaquine resistance in Plasmodium vivaxAm J Trop Med Hyg19965532432498842108
- CowmanAFBerryDBaumJThe cellular and molecular basis for malaria parasite invasion of the human red blood cellJ Cell Biol2012198696197122986493
- WhiteMTKarlSBattleKEHaySIMuellerIGhaniACModelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmissionElife20143e04692
- WhiteNJDeterminants of relapse periodicity in Plasmodium vivax malariaMalar J201110129721989376
- VelhoDPAlves-jrERRibatski-silvaDGomesLTFactors associated with recurrent Plasmodium vivax malaria in Porto Velho, Rondônia State, BrazilCad Saude Publica20143071403141725166938
- ChenNAuliffARieckmannKGattonMChengQRelapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervalsJ Infect Dis2007195793494117330782
- PindoliaDKGarciaAJHuangZThe demographics of human and malaria movement and migration patterns in East AfricaMalar J20131239724191976
- World Health OrganizationControl and Elimination of Plasmodium vivax Malaria: A Technical Brief17GenevaWorld Health Organization2015
- RTS S Clinical Trials PartnershipEfficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomized, controlled trialLancet20153869988314525913272
- World Health OrganizationReversing the Incidence of Malaria 2000–2015GenevaWorld Health Organization2015
- United NationsThe Millennium Development Goals Report 2014New YorkUnited Nations2014
- AregawiMLynchMBekeleWTime series analysis of trends in malaria cases and deaths at hospitals and the effect of antimalarial interventions, 2001–2011, EthiopiaPLoS One2014911e10635925406083
- Roll Back Malaria PartnershipWorld Malaria Day – Asia, April 2014GenevaRoll Back Malaria Partnership2015
- KaremaCAregawiMWRukundoATrends in malaria cases, hospital admissions and deaths following scale-up of anti-malarial interventions, 2000–2010, RwandaMalar J20121123622823945
- MasaningaFChandaEChanda-kapataPReview of the malaria epidemiology and trends in ZambiaAsian Pac J Trop Biomed201332899423593585
- World Health OrganizationMalaria: Global Fund Proposal DevelopmentGenevaWorld Health Organization2011120
- UkpeISMoonasarDRamanJBarnesKIBakerLBlumbergLCase management of malaria: treatment and chemoprophylaxisS Afr Med J201410310793798
- WilsonMLLaboratory diagnosis of malaria: conventional and rapid diagnostic methodsArch Pathol Lab Med2013137680581123721276
- World Health OrganizationParasitological Confirmation of Malaria DiagnosisGenevaWorld Health Organization2009
- World Health OrganizationUniversal Access to Malaria Diagnostic Testing: An Operational Manual12GenevaWorld Health Organization2013
- BattleKEKarhunenMSBhattSGeographical variation in Plasmodium vivax relapseMalar J201413114424731298
- World Health OrganizationGuidelines for the Treatment of Malaria2nd edGenevaWorld Health Organization2010
- EibachDCeronNKrishnalallKTherapeutic efficacy of artemether-lumefantrine for Plasmodium vivax infections in a prospective study in GuyanaMalar J20121134723083017
- NaingCAungKWinDKWahMJEfficacy and safety of chloroquine for treatment in patients with uncomplicated Plasmodium vivax infections in endemic countriesTrans R Soc Trop Med Hyg20101041169570520850161
- World Health OrganizationGuidelines for the Treatment of Malaria3rd edGenevaWorld Health Organization2015
- ImwongMSnounouGPukrittayakameeSRelapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoitesJ Infect Dis2007195792793317330781
- DouglasNMAnsteyNMAngusBJNostenFPriceRNEurope PMC Funders Group artemisinin combination therapy for vivax malaria?Lancet Infect Dis201210640541620510281
- MekonnenSKAseffaAMedhinGBerheNVelavanTPRe-evaluation of microscopy confirmed Plasmodium falciparum and Plasmodium vivax malaria by nested PCR detection in southern EthiopiaMalar J20141314824502664
- SennHAlattasNBoggildAKMorrisSKMixed-species Plasmodium falciparum and Plasmodium ovale malaria in a paediatric returned travellerMalar J20141317824593188
- ZakeriSKakarQGhasemiFDetection of mixed Plasmodium falciparum & Plasmodium vivax infections by nested-PCR in Pakistan, Iran & AfghanistanIndian J Med Res2010132313520693586
- NicholasDMJohnGKvon SeidleinLNicholasAMRicPNChemotherapeutic Strategies for Reducing Transmission of Plasmodium vivax Malaria80AmsterdamElsevier2012
- RahimiBAThakkinstianAWhiteNJSirivichayakulCDondorpAMChokejindachaiWSevere vivax malaria : a systematic review and meta-analysis of clinical studies since 1900Malar J201413111024383426
- PasvolGThe treatment of complicated and severe malariaBr Med Bull2006757612947
- BairdJKSchwartzEHoffmanSLPrevention and treatment of vivax malariaCurr Infect Dis Rep200791394617254503
- HillDRBairdJKPariseMELewisLSRyanETMagillAJPrimaquine: report from CDC expert meeting on malaria chemoprophylaxis IAm J Trop Med Hyg200675340241516968913
- HobbsCDuffyPDrugs for malaria: something old, something new, something borrowedF1000 Biol Rep201132422076126
- ValeNMoreiraRGomesPPrimaquine revisited six decades after its discoveryEur J Med Chem200944393795318930565
- BairdJKSuppressive chemoprophylaxis invites avoidable risk of serious illness caused by Plasmodium vivax malariaTravel Med Infect Dis2013111606523454204
- BairdJKFryauffDJHoffmanSLPrimaquine for prevention of malaria in travelersClin Infect Dis200337121659166714689349
- JohnGKDouglasNMvon SeidleinLPrimaquine radical cure of Plasmodium vivax: a critical review of the literatureMalar J201211128022900786
- CampoBVandalOWescheDLBurrowsJNKilling the hypnozoite – drug discovery approaches to prevent relapse in Plasmodium vivaxPathog Glob Health2015109310712225891812
- FernandoDRodrigoCRajapakseSPrimaquine in vivax malaria: an update and review on management issuesMalar J201110135122152065
- BeutlerEDuparcSG6PD Deficiency Working GroupGlucose-6-phosphate dehydrogenase deficiency and antimalarial drug developmentAm J Trop Med Hyg200777477978917978087
- CarterNPambaADuparcSWaitumbiJNFrequency of glucose-6-phosphate dehydrogenase deficiency in malaria patients from six African countries enrolled in two randomized anti-malarial clinical trialsMalar J201110124121849081
- HowesREPielFBPatilAPG6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based mapPLoS Med2012911e100133923152723
- BuchachartKKrudsoodSSinghasivanonPEffect of primaquine standard dose (15 mg/day for 14 days) in the treatment of vivax malaria patients in ThailandSoutheast Asian J Trop Med Public Health200132472072612041544
- Ramos JúniorWMSardinhaJFCostaMRSantanaMSAlecrimMGLacerdaMVClinical aspects of hemolysis in patients with Plasmodium vivax malaria treated with primaquine, in the Brazilian AmazonBraz J Infect Dis201014441041220963329
- World Health OrganizationThe Safety and Effectiveness of Single Dose Primaquine as a Plasmodium falciparum GametocytocideGenevaWorld Health Organization2012119
- ColemanMColemanNDrug-induced methaemoglobinaemia. Treatment issuesDrug Saf19961463944058828017
- BairdJKHoffmanSLPrimaquine therapy for malariaClin Infect Dis20043991336134515494911
- BunnagDKarbwangJThanavibulAHigh dose of primaquine in primaquine resistant vivax malariaTrans R Soc Trop Med Hyg19948822182198036680
- JelinekTNothdurftHDVon SonnenburgFLoscherTLong-term efficacy of primaquine in the treatment of vivax malaria in nonimmune travelersAm J Trop Med Hyg19955243223247741169
- GlaxoSmithKline [webpage on the Internet]GSK and MMV Announce Start of Phase III Programme of Tafenoquine for Plasmodium vivax Malaria201413 Available from: https://us.gsk.com/en-us/media/press-releases/2014/gsk-and-mmv-announce-start-of-phase-iii-programme-of-tafenoquine-for-plasmodium-vivax-malaria/Accessed July 28, 2016
- BruecknerRPLasseterKCLinETSchusterBGFirst-time-in-humans safety and pharmacokinetics of WR 238605, a new antimalarialAm J Trop Med Hyg19985856456499598455
- GreenJAPatelAKPatelBRTafenoquine at therapeutic concentrations does not prolong fridericia-corrected QT interval in healthy subjectsJ Clin Pharmacol2014549995100524700490
- EdsteinMDKociskoDAWalshDSEamsilaCCharlesBGRieckmannKHPlasma concentrations of tafenoquine, a new long-acting antimalarial agent, in Thai soldiers receiving monthly prophylaxisClin Infect Dis200337121654165814689348
- EdsteinMDKociskoDABrewerTGWalshDSEamsilaCCharlesBGPopulation pharmacokinetics of the new antimalarial agent tafenoquine in Thai soldiersBr J Clin Pharmacol200152666367011736877
- EdsteinMDNasveldPEKociskoDAKitchenerSJGattonMLRieckmannKHGender differences in gastrointestinal disturbances and plasma concentrations of tafenoquine in healthy volunteers after tafenoquine administration for post-exposure vivax malaria prophylaxisTrans R Soc Trop Med Hyg2007101322623016814823
- LiQO’NeilMXieLAssessment of the prophylactic activity and pharmacokinetic profile of oral tafenoquine compared to primaquine for inhibition of liver stage malaria infectionsMalar J201413114124731238
- PybusBSMarcsisinSRJinXThe metabolism of primaquine to its active metabolite is dependent on CYP 2D6Malar J201312121223782898
- MarcsisinSRSousaJCReichardGATafenoquine and NPC-1161B require CYP 2D metabolism for anti-malarial activity: implications for the 8-aminoquinoline class of anti-malarial compoundsMalar J2014131224386891
- VuongCXieLHPotterBBDifferential CYP 2D metabolism alters tafenoquine pharmacokineticsAntimicrob Agents Chemother20155973864386925870069
- VennerstromJLNuzumEOMillerRE8-Aminoquinolines active against blood stage Plasmodium falciparum in vitro inhibit hematin polymerizationAntimicrob Agents Chemother199943359860210049273
- PradinesBMamfoumbiMMTallAIn vitro activity of tafenoquine against the asexual blood stages of Plasmodium falciparum isolates from Gabon, Senegal, and DjiboutiAntimicrob Agents Chemother20065093225322616940138
- PonsaNSattabongkotJKittayapongPEikaratNColemanRETransmission-blocking activity of tafenoquine (WR-238605) and artelinic acid against naturally circulating strains of Plasmodium vivax in ThailandAm J Trop Med Hyg200369554254714695093
- Research Councils UK [webpage on the Internet]Defining the Mechanism of Action of the 8-Aminoquinolines: A Pre-Requisite to Rationally Designed Safe Antimalarials for the Elimination Era Available from: http://gtr.rcuk.ac.uk/projects?ref=MR/L000644/1Accessed April 13, 2016
- Malaria Nexus [webpage on the Internet]Tafenoquine Gets a U.S. Food and Drug Administration (FDA) Breaththrough Therapy Designation2014 Available from: http://www.malarianexus.com/news/tafenoquine-gets-u-s-food-and-drug-administration-fda-breakthrough/Accessed May 23, 2016
- Center for Health Policy [webpage on the Internet]Breakthrough Therapy Designation: Exploring the Qualifying Criteria2015 Available from: http://www.brookings.edu/events/2015/04/24-fda-breakthrough-therapy-criteriaAccessed May 23, 2016
- BeckHPWampflerRCarterNEstimation of the antirelapse efficacy of tafenoquine, using Plasmodium vivax genotypingJ Infect Dis2016213579479926500351
- Llanos-CuentasALacerdaMVRueangweerayutRTafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection studyLancet201438399221049105824360369
- WalshDSLooareesuwanSWilairatanaPRandomized dose-ranging study of the safety and efficacy of WR 238605 (tafenoquine) in the prevention of relapse of Plasmodium vivax malaria in ThailandJ Infect Dis199918041282128710479159
- WalshDSWilairatanaPTangDBRandomized trial of 3-dose regimens of tafenoquine (WR238605) versus low-dose primaquine for preventing Plasmodium vivax malaria relapseClin Infect Dis20043981095110315486831
- EdsteinMWalshDEamsilaCMalaria prophylaxis/radical cure: recent experiences of the Australian Defense ForceMed Trop200161112
- BruecknerRPCosterTWescheDLShmuklarskyMSchusterBGProphylaxis of Plasmodium falciparum infection in a human challenge model with WR 238605, a new 8-aminoquinoline antimalarialAntimicrob Agents Chemother1998425129312949593172
- HaleBROwusu-AgyeiSFryauffDJA randomized, double-blind, placebo-controlled, dose-ranging trial of tafenoquine for weekly prophylaxis against Plasmodium falciparumClin Infect Dis200336554154912594633
- ShanksGDOlooAJAlemanGMA new primaquine analogue, tafenoquine (WR 238605), for prophylaxis against Plasmodium falciparum malariaClin Infect Dis200133121968197411700577
- LellBFaucherJFMissinouMAMalaria chemoprophylaxis with tafenoquine: a randomised studyLancet200035592202041204510885356
- NasveldPEEdsteinMDReidMRandomized, double-blind study of the safety, tolerability, and efficacy of tafenoquine versus mefloquine for malaria prophylaxis in nonimmune subjectsAntimicrob Agents Chemother201054279279819995933
- ElmesNJNasveldPEKitchenerSJKociskoDAEdsteinMDThe efficacy and tolerability of three different regimens of tafenoquine versus primaquine for post-exposure prophylaxis of Plasmodium vivax malaria in the Southwest PacificTrans R Soc Trop Med Hyg2008102111095110118541280
- NasveldPKitchenerSEdsteinMRieckmannKComparison of tafenoquine (WR238605) and primaquine in the post-exposure (terminal) prophylaxis of vivax malaria in Australian Defense Force personnelTrans R Soc Trop Med Hyg200296668368412625150
- NasveldPKitchenerSTreatment of acute vivax malaria with tafenoquineTrans R Soc Trop Med Hyg20059912515550254
- KitchenerSNasveldPEdsteinMDShort report: tafenoquine for the treatment of recurrent Plasmodium vivax malariaAm J Trop Med Hyg200776349449617360873
- WalshDEamsilaCSasipraphaTEfficacy of monthly tafenoquine for prophylaxis of Plasmodium vivax and multidrug-resistant Plasmodium falciparum malariaJ Infect Dis200419081456146315378438
- PubChem [webpage on the Internet]Primaquine: Compound Summary for CID 49082005 Available from: https://pubchem.ncbi.nlm.nih.gov/compound/primaquineAccessed May 23, 2016