Abstract
Background
Poor physical functioning (PF) is a common issue among critically ill patients. It was suggested that reasonable nutrition accelerates PF recovery. However, the details and types of nutritional interventions on the PF of different intensive care unit (ICU) patients at present have not been well analyzed yet. This study aimed to systematically synthesize nutritional interventions on PF in different ICU populations.
Methods
Whittemore and Knafl’s framework was employed. PubMed, EMBASE, Web of Science, CINAHL Plus with Full Text, and Cochrane Library were searched to obtain studies from January 2010 to September 2020, with a manual search of the included studies’ references. Record screening, data extraction, and quality appraisal were conducted independently by each reviewer before reaching an agreement after discussion.
Results
Twelve studies were included reporting the effects of early parenteral nutrition, early enteral nutrition, early goal-directed nutrition, early adequate nutrition, higher protein delivery, higher energy delivery, low energy delivery, energy and protein delivery, intermittent enteral feeding on PF like muscle mass, muscle strength, and function. Function was the most common outcome but showed little improvements. Muscle strength outcomes improved the most. The mechanically ventilated were the most popular target ICU population. The commenced time of the interventions is usually within 24 to 48 hours after ICU admission.
Conclusion
Research on nutritional interventions on critically ill patients’ PF is limited, but most are of a high level of evidence. Few intervention studies specified their evidence basis. Qualitative studies investigating timeframe of initiating feeding, perspectives of the patients’ perspectives and caregivers are warranted to advance research and further discuss this topic.
Introduction
Physical functioning (PF) is the physical abilities allowing functional independence and those related to movement.Citation1 Poor PF, including decreased muscle mass, muscle strength, and function, is a frequent problem in critically ill patients,Citation2–Citation4 since skeletal muscle proteolysis is enhanced due to the catabolism caused by the hypermetabolic state of acute illness,Citation5,Citation6 which is associated with adverse clinical outcomes including infections, difficult weaning from mechanical ventilation (MV), a longer length of stay, increases mortality, higher financial costs, decreased quality of life of survivors.Citation4,Citation7 Rapid muscle loss is independently correlated with increased intensive care unit (ICU) mortality and in-hospital mortality in ICU patients.Citation8 A study suggested that decreased PF was defined as the most critical outcome by ICU survivors,Citation9 who often experience permanent functional disability due to ICU-acquired weakness (ICU-AW).Citation10 These, along with skeletal muscle’s immunologic and metabolic functions, highlight the importance of preserving muscle mass and promoting PF during acute illness.Citation11,Citation12 Moreover, as Herridge pointed out, surviving critical illness is not the happy ending we imagined for our patients.Citation13
Besides, ICU patients often have difficulty eating independently and are at high risk of malnutrition and lean body mass loss,Citation14 rendering them needing nutritional support most frequently among all patients.Citation15 It was found that optimal amounts and timely provision of nutritional intake relate to faster PF recovery and reduced infectious complications, time of MV, and mortality.Citation16–Citation19 Reasonable nutrition is fundamental for ICU patients to maximize physical programs’ benefits and support recovery.Citation14 While inadequate nutritional therapy results in loss of lean body mass, lack of adequate physical activity leads to muscle weakness and inability to mobilize.Citation20 Nevertheless, it is quite challenging to plan the right nutritional intervention for ICU patients.Citation15 The best way of performing nutritional therapy remains controversial.Citation21 Even though there are several nutritional guidelines specialized for the critically ill, such as the guidelines of the European Societies for Clinical Nutrition and Metabolism (ESPEN) (2018),Citation22 the European Society of Intensive Care Medicine (ESICM) (2017),Citation23 and the Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) (2016),Citation24 adherence to these standards in clinical practice is limited. There is a significant discrepancy among nutritional interventions, hindering the interpretation of results, comparisons of trials, and the formation of strong evidence-based recommendations.Citation21,Citation25 Furthermore, as a patient-centered outcome, PF outcomes are vital in clinical trials evaluating nutritional interventions.Citation26 These speak to the significance of understanding what types of nutritional interventions have been implemented on the PF (eg, muscle mass, muscle strength, function) of different categories of ICU patients at present, to provide a reference for clinical practice and future research design and promote the functional ability of the critically ill.
The effects of certain specific types of nutritional interventions in ICU patients, such as enteral nutrition, parenteral nutrition (PN), energy, and protein delivery, etc., were explored by several systematic reviews as well as meta-analyses,Citation7,Citation27–Citation29 with some targeting PF while others the overall clinical outcomes (with or without PF). However, to our knowledge, almost none described and summarized the various types of nutritional interventions of this topic across studies, hindering better understanding and future design of the nutritional interventions on the PF for ICU patients.
This integrative review aims to identify and analyze details of different nutritional interventions on PF for critically ill patients with different characteristic (eg, with organ failure, contradictions of enteral nutrition (EN), etc.) over the past decade. So as to benefit the critically ill more instead of simply helping them survive the critical illness.
Methods
Design
This review was guided by Whittemore and Knafl’s frameworkCitation30 for integrative reviews, which is composed of five steps: (1) problem identification, (2) literature search, (3) data evaluation, (4) data analysis, and (5) presentation. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)Citation31 were applied to present the flow diagram of the identification, screening, exclusion, and inclusion of the literature. Abstracts, conference proceedings, dissertations, commentaries, non-peer-reviewed journal articles, research protocols, case reports, reviews (not including systematic reviews and meta-analyses), and researches that did not study PF were excluded. According to ICF framework,Citation32 which was introduced by the World Health Organization (WHO) to provide a unified and standard language for the description of health and health-related well-being,Citation1 PF is composed of muscle mass, muscle strength, and function.Citation33 Muscle mass is a passive nonvolitional outcome enabling quantification of muscle morphology.Citation33 Muscle strength, as a dynamic measure, provides greater detail on the patient’s level of impairment.Citation33 Function reflects activity limitation within the ICF framework.Citation33 If the intervention topic of the sub-analysis of one trial was the same as the original trial, it would be combined with the original trial and treated as one (eg, trial A + sub-analyses B, C of trial A= trial A). The details of the inclusion and exclusion criteria of this research are provided in .
Table 1 Inclusion and Exclusion Criteria
Five electronic databases were included in this integrative review: PubMed, EMBASE, Web of Science, CINAHL Plus with Full Text, and Cochrane Library, with time-limited from January 2010 to September 2020. Reference lists of the included studies were manually searched. Subject headings, key terms, and the complete search strategy can be accessed in Supplementary File 1.
Data Extraction and Quality Appraisal
The level of evidence (LOE) for each study was evaluated independently by two researchers (WZ and SR) using the Rating System for the Hierarchy of Evidence for Intervention and Treatment Questions by Melnyk and Fineout-Overholt.Citation34 The LOE of the literature is designated as follows: systematic reviews or meta-analyses of randomized controlled trials (RCT), and clinical guidelines based on systematic reviews or meta-analyses (Level 1); well-designed RCTs (Level 2); controlled trials with no randomization (Level 3); case-control or cohort study (Level 4); systematic reviews of descriptive and qualitative studies (Level 5); single descriptive or qualitative study (Level 6); expert opinions (Level 7) (not included in this review).Citation34
Data Analysis
The articles were classified into a literature matrix that included author, year, country, duration, design, commenced time, total sample size, target population, description of the intervention, PF measure, finding, and level of evidence.
Results
Article Characteristics
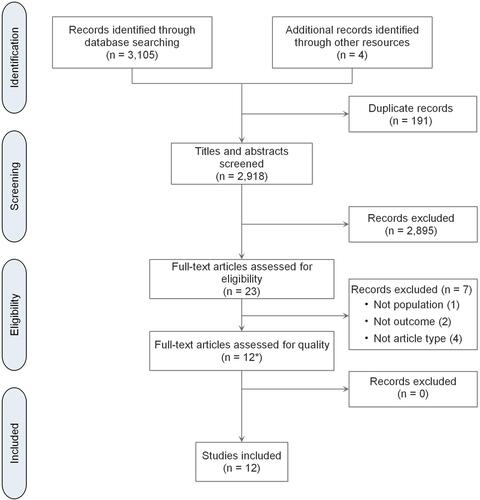
An initial search of the literature generated 3109 articles. A total of 12 articles were included in this integrative review after final screening and quality appraisal. The PRISMA checklistCitation31 was utilized to outline the retrieval process (see ).
Figure 1 PRISMA flow chart. *Original Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically Ill Patients (EPaNIC) study and four sub-analyses of it were treated as one EPaNIC study. Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.Citation31
Almost 84% (n = 10) of included studies were published from 2015 to 2020. Four (30.77%) studies were conducted in Australia, one (7.69%) was conducted in New Zealand, Belgium, Japan, United States, Denmark, United Kingdom, and China each, and two (15.38%) not applicable. The numbers of studies conducted in single-center and multi-center settings were both five (41.67%).
Article types consisted of six (50%) randomized controlled trials, three (25%) descriptive studies, two (16.67%) systematic reviews, and one (8.33%) non-randomized control trial. One hundred to two hundred (n = 4, 33.33%) was the common sample size of study followed by 0 to 100 (n = 3, 25%), above 1000 (n = 2, 16.67%), and 200 to 1000 (n = 1, 8.33%). The LOE of the articles was two (16.67%) for Level 1, six (50%) for Level 2, one (8.33%) for Level 3, and three (25%) for Level 6 (see ).
Table 2 The Characteristics of Included Studies
Participant Characteristics
The target population in the included studies were ICU patients with mechanically ventilated (n = 6, 50.00%), chronic critical illness (n = 1, 8.33%), relative contraindications to early EN (n = 1, 8.33%), and a requirement of PN (n = 1, 8.33%) (see ). Three (25.00%) articles included all types of ICU patients.
Synthesis of Interventions, Outcomes, and Results
Description of Nutritional Interventions
Altogether, nine nutritional interventions were evaluated in 12 studies. shows that the majority of nutritional intervention type is early PN (n = 2, 16.67%) and low energy delivery (n = 2, 16.67%). Other interventions like early EN, early goal-directed nutrition (EGDN), early adequate nutrition, higher protein delivery, higher energy delivery, energy and protein delivery, and intermittent enteral feeding were one (8.33%) each. Almost half of the interventions are characterized as early nutrition delivery (41.67%). Nearly half concerned with energy and protein delivery (41.67%). Details of the interventions are presented in .
Table 3 Summary of Included Studies
Among the seven intervention trials identified, the energy targets of six were individualized, namely using equations (the Harris-Benedict equation,Citation35 the Quark RMR Indirect Calorimeter,Citation36 and the modified Penn State EquationCitation37 in three trial respectively), indirect calorimetry (around 25 kcal/kg/d (24 kcal/kg/d for the higher protein group, 26 kcal/kg/d for the lower protein group),Citation38 25–30 kcal/kg/d (25 kcal/kg/d initially)Citation39 and 1.5 kcal/kg/hCitation40) in three trials respectively, etc. to dynamically measure individuals’ energy targets. Only one trialCitation41 of the seven ones had its energy targets fixed in specific amounts (400 kcal on day 1, 800 kcal on day 2, and 2880 kcal/d after day 2).
Four out of the seven intervention trials had individualized protein targets (0.8 g/kg or 1.2 g/kg,Citation38 1.2–2.0 g/kg/d (1.2 g/kg/d initially),Citation39 at least 1.2 g/kg,Citation37 and at least 1.5 g/kgCitation36), which were set based on the patients’ weight only. One of the rests of the seven trials had a fixed protein target (0.056 g/mL).Citation40 The others did not specify their protein delivery targets.
Most of the seven trials (five) were carried out by multidisciplinary teams, of which clinicians and nursing staff were the most common members. Two teamsCitation37,Citation38 included dietitians, and oneCitation37 of them had a physiotherapist to assess the PF. The above five studies all clarified each team member’s corresponding tasks, of which one study by McNelly et alCitation37 demonstrated most clearly by showing a chart of this information. TwoCitation39,Citation40 of the seven trials specified neither the professions of the research members nor their responsibilities.
Among the seven intervention studies, threeCitation36,Citation38,Citation41 were designed based on well-accepted evidence. The one by Casaer et alCitation41 was based on the guidelines for early initiation of parenteral nutrition, but without mentioning which specific guidelines were utilized. The protein delivery of the trial conducted by Ferrie et alCitation38 was based on the 2006 ESPEN guidelines,Citation42 the 2009 ESPEN guidelines,Citation43 and the 2009 ASPEN guidelines.Citation44 The energy expenditure measurement of the one by Allingstrup et alCitation36 was based on the 2009 ESPEN guidelines,Citation45 the 2016 SCCM, and the ASPEN guidelines.Citation46
The most common commenced time for included articles was within 24 hours and within 48 hours after ICU admission (n = 3, 23.08%, both). Within 72 hours after ICU admission was the least (n = 2, 15.38%), and many were unspecified (n = 3, 23.08%).
Description of Physical Functioning Related Outcome Measures
Function (n = 12, 54.55%), including walk, sitting and daily living tasks, was the primary PF measure, followed by muscle strength (n = 6, 27.27%, consisting of muscle weakness, handgrip, fatigue, etc.) and muscle mass (n = 4, 18.18%, consisting of muscle thickness and muscle loss, etc.).
Description of Physical Functioning Related Results
Function
Among the nine studies that studied the influence of nutritional interventions on function, only one multi-centered observational study by Yatabe et alCitation47 found that low caloric intake (less than 10 kcal/kg/day) until day three was associated with good physical status in mechanically ventilated (for at least 24 hours) patients, which was defined as more than end sitting.
Five studiesCitation35–Citation37,Citation40,Citation41 found no significant effect on function from nutritional interventions. One prospective observational studyCitation48 found that cumulative energy deficit was associated with decreased function scores in the mechanically ventilated (for at least 48 hours).
One systematic reviewCitation21 found that nutritional interventions deteriorated ICU patients’ function. Similarly, one retrospective studyCitation49 found that adequate evidence-based ICU nutrition worsened chronic critical illness (CCI) patients’ function.
Muscle Strength
Three studies showed that muscle strength benefited from nutritional interventions, including early PN in patients with relative contraindications to early EN,Citation35 higher protein/amino acid provision in those requiring PN,Citation38 early EN in mechanically ventilated patients with sepsis.Citation39 One prospective observational studyCitation48 found that cumulative energy deficit was associated with greater ICU-AW odds in mechanically ventilated patients (for at least 48 hours).
However, the RCT by Casaer et alCitation41 demonstrated that an early PN intervention was detrimental to muscle strength. One systematic reviewCitation21 found that nutritional interventions worsened muscle strength.
Muscle Mass
Only the one by Ferrie et alCitation38 found nutritional interventions beneficial to muscle mass (sum of 3 muscle sites (forearm, biceps, and thigh), forearm muscle thickness, thigh muscle thickness), but with no significant effect on biceps muscle thickness.
The one by Casaer et alCitation41 found that early EN either worsened muscle mass or have no significant effect on it. One systematic reviewCitation7 found no significant association between energy and protein delivery and changes in skeletal muscle mass. One RCTCitation37 found that intermittent enteral feeding did not significantly affect muscle mass in mechanically ventilated patients with multiorgan failure.
Discussion
To our knowledge, this is the first integrative review to focus exclusively on studies concerned with nutritional interventions on PF for ICU patients. It shows that the related studies over the past decade have been mostly quantitative, of which are primarily multicentered RCTs and have ample sample sizes, allowing them to show the actual effect and generalizability of their interventions and demonstrating a relatively high level of evidence of the studies of this topic. One included systematic review was conducted in 2016 but was published two years later, with the inclusion of only six studies, of which merely two were RCTs, while others were observational ones, which might render the evidence found in this included systematic review inconclusive. We found that most included studies were published in recent five years, suggesting that this realm is a relatively new research hotspot. It is an inevitable trend to explore more about the nutritional interventions on PF in the future to contribute to the form of evidence to deliver nutrition in a way that prevents the deterioration of the PF of critically ill patients. It thus adds to the possibility of a better quality of life after a critical illness.Citation50
Given the rapid onset of muscle wasting within hours of MV,Citation50 it is imperative to pay close attention to the PF-related changes of mechanically ventilated ICU patients, which is corresponding with what we found in our review that the majority of the target ICU population is the mechanically ventilated. Nevertheless, as pointed out by Chapple et al,Citation51 the number of patients who are either never mechanically ventilated or only ventilated for a short time during their ICU stay is growing, while current nutrition guidelines provide limited recommendations specific for this population. Therefore, the nutritional needs of this population are to be ascertained in future trail designs. As recommended by the ESPEN guideline (2018),Citation22 which is one of the most updated nutritional guidelines for the critically ill to date, the nutrition delivery should be commenced within 48h of ICU admission, and almost half of the included studies in this review set their commenced time accordingly and have focal considerations of early administration of nutrition. However, one limitation of the guidelines to date is that they concern little to the effects of nutrition on ICU patients’ PF. So there is great possibility that the most suitable time to start feeding benefiting PF lies elsewhere (like 17 h for instance). Therefore, though with studies of high quality in the field in terms of the feeding commenced time (eg, early/late PN/EN), the best commenced time of nutritional intervention on PF still requires further exploration. More large-scale observational studies concerning the starting timeframe are warranted, before the design of experimental studies, to find the possible suitable commenced time in different populations, either the distinct types of ICU population (eg, disease type, mechanically ventilated or not) or the various races of population (eg, country, northern or the southern part of one country). In order to get a knowing of what timeframe is possibly better to start what kind of nutrition for the sake of the patient’s PF through observation in the first place, as well as which category of patients might benefit more from certain interventions. Then plan and carry out experimental studies to further pin down the suitable time, nutrition therapy, and the population afterwards.
More than half of the reviewed studies’ assessed PF are function, which showed little improvement. This finding, both on the proportion and the result of function, is similar to what has been found in a systematic review by Taverny et al.Citation21 One possible reason why almost all function outcomes failed to improve is that this PF outcome is relatively hard to measure in ICU patients than in general wards. For one thing, the needed treatments to the patient’s critical illness (eg, sedation, continuous renal replacement therapy (CRRT)) along with the caregivers’ fear of causing adverse events (eg, fall, tube peeling) impedes the patient to perform the movements (eg, walking, transfer from bed to chair, using the toilet) needed for measuring his or her function.Citation50,Citation52 For another, the critical illness itself, which commonly needs a relatively long time to recover and usually will not be fully healed even when the patient is going to be discharged from ICU, render the muscle strength and activity tolerance of the patient too limited to do the required movements even when it is the time that he or she is ready to get out of ICU.Citation53 Besides, even after the critical illness is cured, the function impaired during the disease still requires some time to recover from fulfilling higher movement goals,Citation54 of which the degree of difficulty is much greater than their last achieved ones, take, for example, the Barthel Index which is common in function assessment of ICU patients,Citation55,Citation56 from lying in bed incapable of walking to propelling a wheelchair independently at least 50 yards.Citation55
Among the six muscle strength outcomes measured, half were improved by nutritional interventions, and most muscle strength outcomes were muscle weakness primarily assessed by the Medical Research Council sum score (MRC-SS), which is commonly used for the detection of ICU-AW.Citation57 This satisfying improvement is probably due to the relative easiness for the critically ill patient to perform the movements (eg, wrist extension, elbow flexion, knee extension) required for assessing muscle strength in contrast to the assessment of function since the patient do not have to get out of bed to do these movements.Citation58 Furthermore, achieving a higher goal in terms of muscle strength (eg, from resisting partial force to resisting full force when extending the wrist) is less demanding than that of function.
In terms of the measurement of energy target/energy expenditure (EE), around half of the included interventional trials applied predictive equations, and about half adopted indirect calorimetry. Indirect calorimetry is preferred over predictive equations in most updated critical nutrition guidelines (A.S.P.E.N. (2016)Citation24 and ESPEN (2018)Citation22), due to the significant inaccuracy (up to 60%) of predictive equations as a result of the difficulty of assessing body weight accurately.Citation22,Citation59 In the absence of indirect calorimetry, oxygen consumption (VO2) from pulmonary arterial catheter or carbon dioxide production (VCO2) from the ventilator can be more accurate on the evaluation of EE than predictive equations, though less than indirect calorimetry.Citation22 If indirect calorimetry, VO 2 or VCO 2 measurements are all unavailable, simple weight-based equations (eg, 20–25 kcal/kg/d) may be preferred.Citation22
Two out of seven intervention studies did not specify their protein delivery when their intervention is mainly on energy delivery. Two out of seven intervention studies did not specify what discipline the persons made up of their research team and what responsibility each member took. Despite the relatively small proportion these studies account for, it is worth noting that researchers should provide a detailed report of the provision of energy and protein, as well as the details of the constituent disciplines of the team members and their respective responsibilities in their research since transparency on these details, will promote the uptake of research evidence into practice.Citation60–Citation62 Otherwise, questions such as “How much protein/energy should we deliver to our patients?” and “Which discipline of personnel should we include in our research team and what kind of task he or she should shoulder?” will be challenging to answer and therefore hamper both the design and the replication of the intervention.
However, what is uncertain is if the researchers reflect that their interventions are founded on principles of specific guidelines. Only 28.57% of the intervention studies stated their theoretical base clearly, which limits understanding of nutritional interventions on PF in ICU patients within a broader context, including the significant transition of interventions into practice.Citation63,Citation64
Implications for Future Research
Future nutritional interventions on PF in ICU patients are especially encouraged to incorporate the following details. Firstly, the intervention should be based on well-accepted evidence, which should be specified in the report to guarantee the research’s rationality and provide further guidance in the implementation process. Moreover, every delivered nutrition ingredient’s details are to be explicated even if only one component is the primary intervention to promote the intervention’s generalization. Also, the disciplines of the research members and their respective tasks should be stated clearly. The addition of muscle strength-related measures (ICU-AW especially) can also be considered since this PF is more possible to be performed within the ICU setting.
As mentioned above, more large-scale observational studies concerning the starting timeframe are required to find the possible suitable commenced time of feedings in different populations, facilitating the further design of experimental studies.
Besides, it is pointed out by a research that the optimal nutritional management among ICU patients with lower body mass index (BMI) remains unclear,Citation47 calling for more researches in terms of this population since it will benefit most Asian population and lean Western and Oceanian population. Additionally, the nutritional management of ICU patients who are not mechanically ventilated or only ventilated for a short period requires further exploration.Citation51
Most included studies adopted quantitative approaches in investigating nutritional interventions on PF in ICU patients, but almost all failed to take the feeling and experience of the patients and/or their caregivers into account, so were the qualitive ones, not in line with the wholistic health concept advocated by the World Health Organization (WHO).Citation65 As suggested in a study, patients might be burdened by the nutritional treatment both during hospitalization and after discharge.Citation66 Considering that qualitative research could contribute to practical intervention delivery insights, this review highlights the need for more qualitative studies investigating the perspectives of the patients and/or their caregivers, in an effort to treat the person as a whole. Current qualitative studies have investigated the association between nutrition and the PF of ICU patients in either a nation (ie, Japan)Citation47 or certain types of ICU patients (ie, chronic critical illness patients).Citation49 However, depth interviews are needed to provide more insight into the patients’ own experience of having certain types of nutrition delivery and especially on their PF in or out of ICU, as well as their caregivers’ (medical staffs, family members) perspectives and experience when taking care of them, which is practically ignored in the current studies. Nutritional interventions on PF in the critically ill could then be optimized and improve the patients’ outcomes, and meanwhile, taking into account the patients’ and the caregivers’ feeling and experience rather than focus merely on the symptoms. Moreover, it is suggested that the incorporation of co-design including both clinicians/researchers and patients should be adopted in the trial design of nutritional interventions on the critically ill,Citation51,Citation67 which is likely to benefit from the investigation of patients’/caregivers’ insights. Theories about wholistic health could be applied in various types of research studies concerning this topic.
Apart from nutritional interventions solely, it is recommended that bundled or synergistic therapies should be taken into consideration,Citation51 such as combined nutritional and physical interventions, as this enhanced synergistic treatment may increase muscle mass, and improve activities of daily living within a short period after discharge for ICU patients.Citation66
Furthermore, our results can be used as a guideline for design of the research and practice related to nutritional interventions on the critically ill’s PF, such as the energy/protein target, patient populations, PF, etc.
Limitations
First of all, it is essential to highlight that only a minimal number of sources were identified for inclusion, a challenging factor for conducting a systematic review or meta-analysis and drawing clear conclusions from the evidence. However, the aim of this review is to summarize the details of the researches concerning the nutritional intervention on functioning of critically ill patients, promoting a more comprehensive understanding of this topic, in order to provide references for future studies, instead of paying more attention to the level of evidence of the current researches and pinning down the effects of this type of intervention like in the case of systematic reviews.Citation68,Citation69 Moreover, compared to scoping reviews, which aim to identify and mapping certain characteristics or concepts of studies as well as analyze the knowledge gaps,Citation70,Citation71 the integrative review design, focusing on using diverse data sources to develop holistic understanding of the topic of interest,Citation72 suits our aim best. In addition, even though muscle mass, muscle strength, and function, constituting the PF analyzed in this review, share a common background, mechanisms leading to their impairments are not similar. So we analyzed these outcomes separately to make up for this shortcoming. Besides, protocols and pilot studies were excluded in this review, considering the former’s lack of results and the latter’s flimsy evidence. However, this review included both primary and secondary studies with a range of objectives, as well as various research types (quantitative studies, qualitative studies and systematic reviews/meta-analyses), and thus it offers broad coverage of literature in this area.
Conclusions
This review demonstrates that research on nutritional interventions on critically ill patients’ PF is limited in number, but most of these are large-scale RCTs of a high level of evidence. However, though of high quality, there are still gaps to be filled in this field. Firstly, few intervention studies specified their evidence basis. Qualitative studies are warranted to further investigate the timeframe of feeding commencement in the best interest of PF. Additionally, the management of ICU patients with lower BMI lacks evidence of this topic. Moreover, the existing research highlights a need for more studies to the wholistic health of the related patients, especially qualitative studies, looking into the perspectives of the patients themselves and their medical caregivers as well as their families during or after the provision of certain types of nutrition to the patients, to advance research and to trigger a further discussion on this topic.
Disclosure
The authors report no conflicts of interest for this work.
Additional information
Funding
References
- González-Seguel F, Corner EJ, Merino-Osorio C. International classification of functioning, disability, and health domains of 60 physical functioning measurement instruments used during the adult intensive care unit stay: a scoping review. Phys Ther. 2019;99(5):627–640. doi:10.1093/ptj/pzy158
- Witteveen E, Wieske L, Sommers J, et al. Early prediction of intensive care unit–acquired weakness: a Multicenter External Validation Study. J Intensive Care Med. 2020;35(6):595–605. doi:10.1177/0885066618771001
- Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46(4):637–653. doi:10.1007/s00134-020-05944-4
- Zhang L, Hu W, Cai Z, et al. Early mobilization of critically ill patients in the intensive care unit: a systematic review and meta-analysis. PLoS One. 2019;14(10):e0223185. doi:10.1371/journal.pone.0223185
- Ndahimana D, Kim E. Energy requirements in critically ill patients. Clin Nutr Res. 2018;7(2):81–90. doi:10.7762/cnr.2018.7.2.81
- Puthucheary Z, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi:10.1001/jama.2013.278481
- Lambell KJ, King SJ, Forsyth AK, et al. Association of energy and protein delivery on skeletal muscle mass changes in critically ill adults: a systematic review. J Parenter Enteral Nutr. 2018;42(7):1112–1122. doi:10.1002/jpen.1151
- Ju S, Choi SM, Park YS, et al. Rapid muscle loss negatively impacts survival in critically ill patients with cirrhosis. J Intensive Care Med. 2020;35(7):663–671. doi:10.1177/0885066618775706
- Nedergaard H, Haberlandt T, Reichmann P, et al. Patients’ opinions on outcomes following critical illness. Acta Anaesthesiol Scand. 2018;62(4):531–539. doi:10.1111/aas.13058
- Dos Santos C, Hussain SNA, Mathur S, et al. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. Am J Respir Crit Care Med. 2016;194(7):821–830. doi:10.1164/rccm.201512-2344OC
- Pedersen B, Febbraio M. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi:10.1038/nrendo.2012.49
- Sherwood L. Human Physiology: From Cells to Systems. Pacific Grove, CA: Brooks/Cole; 2001:240.
- Herridge M. Textbook of Post-ICU Medicine: The Legacy of Critical Care. Introduction: Life After the ICU. Oxford University Press; 2014.
- Cherry-Bukowiec JR. Optimizing nutrition therapy to enhance mobility in critically ill patients. Crit Care Nurs Q. 2013;36(1):28–36. doi:10.1097/CNQ.0b013e31827507d7
- Gostyńska A, Stawny M, Dettlaff K, et al. Clinical nutrition of critically ill patients in the context of the latest ESPEN guidelines. Medicina. 2019;55(12):12. doi:10.3390/medicina55120770
- Wei X, Day AG, Ouellette-Kuntz H, et al. The association between nutritional adequacy and long-term outcomes in critically ill patients requiring prolonged mechanical ventilation: a Multicenter Cohort Study. Crit Care Med. 2015;43(8):1569–1579. doi:10.1097/CCM.0000000000001000
- Heyland D, Stephens K, Day A, et al. The success of enteral nutrition and ICU-acquired infections: a multicenter observational study. Clin Nutr. 2011;30(2):148–155. doi:10.1016/j.clnu.2010.09.011
- Heyland D, Cahill N, Day A. Optimal amount of calories for critically ill patients: depends on how you slice the cake! Crit Care Med. 2011;39(12):2619–2626. doi:10.1097/CCM.0b013e318226641d
- Alberda C, Gramlich L, Jones N, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35(10):1728–1737. doi:10.1007/s00134-009-1567-4
- Mooi NM, Ncama BP. Evidence on nutritional therapy practice guidelines and implementation in adult critically ill patients: a scoping review protocol. Syst Rev. 2019;8(1):291. doi:10.1186/s13643-019-1194-2
- Taverny G, Lescot T, Pardo E, et al. Outcomes used in randomised controlled trials of nutrition in the critically ill: a systematic review. Crit Care. 2019;23(1):12. doi:10.1186/s13054-018-2303-7
- Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79.
- Reintam blaser A, Starkopf J, Alhazzani W, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43(3):380–398. doi:10.1007/s00134-016-4665-0
- Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med. 2016;44(2):390–438.
- van Lieshout R, Tick LW, de Laat D, et al. Adherence to guidelines on nutrition support during intensive treatment of acute myeloid leukemia patients: a nationwide comparison. Clin Nutr ESPEN. 2020;39:242–250. doi:10.1016/j.clnesp.2020.05.003
- Arabi YM, Casaer MP, Chapman M, et al. The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med. 2017;43(9):1239–1256. doi:10.1007/s00134-017-4711-6
- Machado LDS, Rizzi P, Silva FM. Administration of enteral nutrition in the prone position, gastric residual volume and other clinical outcomes in critically ill patients: a systematic review. Rev Bras Ter Intensiva. 2020;32(1):133–142. doi:10.5935/0103-507X.20200019
- Luo Y, Qian Y. Effect of combined parenteral and enteral nutrition for patients with a critical illness: a meta-analysis of randomized controlled trials. Medicine. 2020;99(3):e18778. doi:10.1097/MD.0000000000018778
- Asrani VM, Brown A, Bissett I, et al. Impact of intravenous fluids and enteral nutrition on the severity of gastrointestinal dysfunction: a systematic review and meta-analysis. J Crit Care Med. 2020;6(1):5–24. doi:10.2478/jccm-2020-0009
- Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs. 2005;52(5):546–553. doi:10.1111/j.1365-2648.2005.03621.x
- Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
- World Health O. International Classification of Functioning, Disability and Health: ICF. Geneva: World Health Organization; 2001.
- Parry SM, Granger CL, Berney S, et al. Assessment of impairment and activity limitations in the critically ill: a systematic review of measurement instruments and their clinimetric properties. Intensive Care Med. 2015;41(5):744–762. doi:10.1007/s00134-015-3672-x
- Melnyk B, Fineout-Overholt E. Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2015.
- Doig GS, Simpson F, Sweetman EA, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013;309(20):2130–2138. doi:10.1001/jama.2013.5124
- Allingstrup MJ, Kondrup J, Wiis J, et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017;43(11):1637–1647. doi:10.1007/s00134-017-4880-3
- McNelly AS, Bear DE, Connolly BA, et al. Effect of intermittent or continuous feed on muscle wasting in critical illness: a Phase II clinical trial. Chest. 2020;158(1):183–194. doi:10.1016/j.chest.2020.03.045
- Ferrie S, Allman-Farinelli M, Daley M, et al. Protein requirements in the critically ill: a randomized controlled trial using parenteral nutrition. J Parenter Enteral Nutr. 2016;40(6):795–805. doi:10.1177/0148607115618449
- Liu Y, Zhao W, Chen W, et al. Effects of early enteral nutrition on immune function and prognosis of patients with sepsis on mechanical ventilation. J Intensive Care Med. 2018;35(10):1053–1061. doi:10.1177/0885066618809893
- Reid DB, Chapple LS, O’Connor SN, et al. The effect of augmenting early nutritional energy delivery on quality of life and employment status one year after ICU admission. Anaesth Intensive Care. 2016;44(3):406–412. doi:10.1177/0310057X1604400309
- Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506–517. doi:10.1056/NEJMoa1102662
- Volkert D, Berner YN, Berry E, et al. ESPEN guidelines on enteral nutrition: geriatrics. Clin Nutr. 2006;25(2):330–360. doi:10.1016/j.clnu.2006.01.012
- Bozzetti F, Forbes A. The ESPEN clinical practice guidelines on parenteral nutrition: present status and perspectives for future research. Clin Nutr. 2009;28(4):359–364. doi:10.1016/j.clnu.2009.05.010
- McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J Parenter Enteral Nutr. 2009;33(3):277–316. doi:10.1177/0148607109335234
- Singer P, Berger MM, Van den Berghe G, et al. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009;28(4):387–400. doi:10.1016/j.clnu.2009.04.024
- McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J Parenter Enteral Nutr. 2016;40(2):159–211. doi:10.1177/0148607115621863
- Yatabe T, Egi M, Sakaguchi M, et al. Influence of nutritional management and rehabilitation on physical outcome in Japanese intensive care unit patients: a multicenter observational study. Ann Nutr Metab. 2019;74(1):35–43. doi:10.1159/000495213
- Fetterplace K, Beach LJ, MacIsaac C, et al. Associations between nutritional energy delivery, bioimpedance spectroscopy and functional outcomes in survivors of critical illness. J Hum Nutr Diet. 2019;32(6):702–712. doi:10.1111/jhn.12659
- Rosenthal MD, Bala T, Wang ZK, et al. Chronic critical illness patients fail to respond to current evidence-based intensive care nutrition secondarily to persistent inflammation, immunosuppression, and catabolic syndrome. J Parenter Enteral Nutr. 2020;44(7):1237–1249. doi:10.1002/jpen.1794
- Clarissa C, Salisbury L, Rodgers S, et al. Early mobilisation in mechanically ventilated patients: a systematic integrative review of definitions and activities. J Intensive Care. 2019;7(1):3. doi:10.1186/s40560-018-0355-z
- Chapple L-AS, Ridley EJ, Chapman MJ. Trial design in critical care nutrition: the past, present and future. Nutrients. 2020;12(12):12. doi:10.3390/nu12123694
- Brock C, Marzano V, Green M, et al. Defining new barriers to mobilisation in a highly active intensive care unit - have we found the ceiling? An observational study. Heart Lung. 2018;47(4):380–385. doi:10.1016/j.hrtlng.2018.04.004
- Zhou W, Shi B, Fan Y, et al. Effect of early activity combined with early nutrition on acquired weakness in ICU patients. Medicine. 2020;99(29):e21282. doi:10.1097/MD.0000000000021282
- Meyer-Frießem CH, Malewicz NM, Rath S, et al. Incidence, time course and influence on quality of life of intensive care unit-acquired weakness symptoms in long-term intensive care survivors. J Intensive Care Med. 2020:885066620949178. doi:10.1177/0885066620949178
- Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61–65.
- Yi Y, Ding L, Wen H, et al. Is barthel index suitable for assessing activities of daily living in patients with dementia? Front Psychiatry. 2020;11:282. doi:10.3389/fpsyt.2020.00282
- Mitobe Y, Morishita S, Ohashi K, et al. Skeletal muscle index at intensive care unit admission is a predictor of intensive care unit-acquired weakness in patients with sepsis. J Clin Med Res. 2019;11(12):834–841. doi:10.14740/jocmr4027
- Hermans G, Clerckx B, Vanhullebusch T, et al. Interobserver agreement of medical research council sum-score and handgrip strength in the intensive care unit. Muscle Nerve. 2012;45(1):18–25. doi:10.1002/mus.22219
- Zusman O, Kagan I, Bendavid I, et al. Predictive equations versus measured energy expenditure by indirect calorimetry: a retrospective validation. Clin Nutr. 2019;38(3):1206–1210. doi:10.1016/j.clnu.2018.04.020
- Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical research council guidance. Int J Nurs Stud. 2013;50(5):587–592. doi:10.1016/j.ijnurstu.2012.09.010
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. doi:10.1177/1747493018778713
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi:10.1016/j.ijsu.2014.07.014
- Davidoff F, Dixon-Woods M, Leviton L, et al. Demystifying theory and its use in improvement. BMJ Qual Saf. 2015;24(3):228–238. doi:10.1136/bmjqs-2014-003627
- Bradbury-Jones C, Taylor J, Herber O. How theory is used and articulated in qualitative research: development of a new typology. Soc Sci Med. 2014;120:135–141. doi:10.1016/j.socscimed.2014.09.014
- Larson JS. The World Health Organization’s definition of health: social versus spiritual health. Soc Indic Res. 1996;38(2):181–192. doi:10.1007/BF00300458
- Kou K, Momosaki R, Miyazaki S, et al. Impact of nutrition therapy and rehabilitation on acute and critical illness: a systematic review. J UOEH. 2019;41(3):303–315. doi:10.7888/juoeh.41.303
- Haines KJ, Holdsworth C, Cranwell K, et al. Development of a peer support model using experience-based co-design to improve critical care recovery. Crit Care Explor. 2019;1(3):e0006. doi:10.1097/CCE.0000000000000006
- Greenhalgh T. Papers that summarise other papers (systematic reviews and meta-analyses). BMJ. 1997;315(7109):672–675. doi:10.1136/bmj.315.7109.672
- Kirkevold M. Integrative nursing research--an important strategy to further the development of nursing science and nursing practice. J Adv Nurs. 1997;25(5):977–984. doi:10.1046/j.1365-2648.1997.1997025977.x
- Colquhoun HL, Levac D, O’Brien KK, et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. 2014;67(12):1291–1294. doi:10.1016/j.jclinepi.2014.03.013
- Munn Z, Peters MDJ, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. doi:10.1186/s12874-018-0611-x
- Da Silva RN, Brandão MAG, Ferreira MA. Integrative review as a method to generate or to test nursing theory. Nurs Sci Q. 2020;33(3):258–263. doi:10.1177/0894318420920602
- Heyland DK, Stapleton RD, Mourtzakis M, et al. Combining nutrition and exercise to optimize survival and recovery from critical illness: conceptual and methodological issues. Clin Nutr. 2016;35(5):1196–1206. doi:10.1016/j.clnu.2015.07.003
- Hermans G, De Jonghe B, Bruyninckx F, et al. Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst Rev. 2014;2014(1):Cd006832.
- Casaer MP, Langouche L, Coudyzer W, et al. Impact of early parenteral nutrition on muscle and adipose tissue compartments during critical illness. Crit Care Med. 2013;41(10):2298–2309. doi:10.1097/CCM.0b013e31828cef02
- Hermans G, Clerckx B, Vanhullebusch T, et al. Withholding parenteral nutrition for 1 week reduces ICU-acquired weakness. Crit Care. 2019;55(S2):S94–S5. doi:10.1186/cc12190
- Hermans G, Casaer MP, Clerckx B, et al. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med. 2013;1(8):621–629. doi:10.1016/S2213-2600(13)70183-8
- Vanhorebeek I, Casaer MP, Guiza F, et al. Impact of early versus late parenteral nutrition on morphological and molecular markers of atrophy and autophagy in skeletal muscle of critically ill patients. Crit Care. 2013;17(S2):S96. doi:10.1186/cc12194
