Abstract
The inhibitors of apoptosis (IAPs) constitute a family of proteins involved in the regulation of various cellular processes, including cell death, immune and inflammatory responses, cell proliferation, cell differentiation, and cell motility. There is accumulating evidence supporting IAP-targeting in tumors: IAPs regulate various cellular processes that contribute to tumor development, such as cell death, cell proliferation, and cell migration; their expression is increased in a number of human tumor samples, and IAP overexpression has been correlated with tumor growth, and poor prognosis or low response to treatment; and IAP expression can be rapidly induced in response to chemotherapy or radiotherapy because of the presence of an internal ribosome entry site (IRES)-dependent mechanism of translation initiation, which could contribute to resistance to antitumor therapy. The development of IAP antagonists is an important challenge and was subject to intense research over the past decade. Six molecules are currently in clinical trials. This review focuses on the role of IAPs in tumors and the development of IAP-targeting molecules for anticancer therapy.
Keywords:
Introduction: IAP family of proteins
The inhibitors of apoptosis (IAPs) constitute a family of proteins highly conserved throughout evolution. IAPs were initially discovered in baculoviruses two decades ago,Citation1 as potent inhibitors of apoptosis in infected insect cells. The first human homologous neuronal apoptosis inhibitory protein (NAIP) and cellular IAP 1 and 2 (cIAP1 and cIAP2) were characterized 2 years later,Citation2,Citation3 followed by X-chromosome linked IAP (XIAP),Citation4,Citation5 survivin,Citation6 Apollon (also called BRUCE),Citation7 melanoma IAP (ML-IAP) (also called Livin),Citation8 and IAP-like protein 2 (ILP2).Citation9 The IAP family is defined by the presence of one to three conserved protein motifs named a baculoviral IAP repeat (BIR). Most of them form a surface hydrophobic groove that specifically binds a conserved tetrapeptide motif, called IAP binding motif (IBM), found in the active subunits of apoptotic protease caspase-3, -7, and -9 and in cellular IAP antagonists, such as the second mitochondria-derived activator of caspases (Smac) (also named direct IAP-binding protein with low isoelectric point (pI) [DIABLO])Citation10–Citation13 and the high temperature requirement protein A2 (HtrA2)Citation12,Citation14 (). The first BIR of XIAP and cIAPs does not bind IBM but rather, the signaling molecule transforming growth factor beta (TGFβ)-activated kinase 1-binding protein 1 (TAB1)Citation15 or the tumor necrosis factor (TNF) receptor (TNFR) associated factors (TRAFs),Citation16–Citation18 connecting XIAP and cIAPs with the TGF and TNF signaling pathways, respectively. In addition to the BIRs, cIAPs, XIAP, ML-IAP and ILP2 also possess a C-terminal RING (really interesting new gene) domain conferring an E3 ligase activity in the ubiquitination or neddylationCitation19 reactions (for review,Citation20,Citation21).
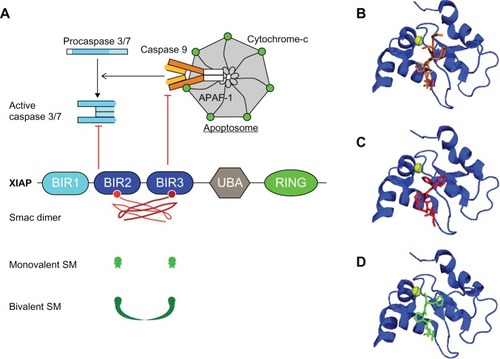
Figure 1 The inhibition of caspases by XIAP and the regulation by Smac and Smac mimetics.
Abbreviations: AVPI, Smac N-terminal tetrapeptide; BIR, baculoviral IAP repeat; HtrA2, high temperature requirement protein A2; IAPs, inhibitors of apoptosis; IBM, IAP binding motif; RING, really interesting new gene; SM, Smac mimetic; Smac, second mitochondria-derived activator of caspases; TAB1, TGFβ-activated kinase 1-binding protein 1; TGFβ, transforming growth factor beta; UBA, ubiquitin associated; XIAP, X-chromosome linked IAP; APAF-1, apoptotic peptidase activating factor.
Numerous partners of IAPs have been identified, including some caspases,Citation22–Citation24 some signaling molecules,Citation25,Citation26 some regulators of the NF-κB: nuclear factor of kappa-light polypeptide gene enhancer in B-cell activating pathways,Citation25 some regulators of the actin cytoskeleton,Citation27 and some transcriptional regulators.Citation28,Citation29 Thus, although they were initially characterized as inhibitors of apoptosis, IAPs display additional nonapoptotic functions in the regulation of cell proliferation, cell division, cell differentiation, cell motility, and in proinflammatory and immune response (for review,Citation25,Citation26), which could contribute to oncogenesis.
Expression of IAPs in tumors
The expression of IAPs or cellular IAP antagonists such as Smac,Citation11 HtrA2, or the septin-like mitochondrial protein, ARTS,Citation30,Citation31 were shown to be altered in a number of human tumor samples (). Overexpression of IAPs or downregulation of the cellular IAP antagonists have been correlated with advanced progressive disease, aggressiveness, and poor prognosis or low response to treatment (). The alterations of IAP expression can be associated or not, with gene mutations. The baculoviral IAP repeat containing protein (BIRC)2 and BIRC3 genes, encoding cIAP1 and cIAP2, respectively, are located on chromosome 11q21–22, a region found amplified in human hepatocarcinoma,Citation32 mammary carcinoma,Citation33 medulloblastoma,Citation34 and in pancreatic,Citation35 cervical,Citation36 lung,Citation37 oral squamous cell,Citation38 and esophagealCitation39 carcinomas. Some (30%) mucosa-associated lymphoid tissue (MALT) lymphoma are associated with the chromosomal translocation t(11;18) (q21;q21) generating a chimeric protein composed of the N-terminal sequences of cIAP2 fused to the C-terminal sequence of MALT1.Citation17,Citation40 Conversely, IAPs can also display antitumoral properties in lymphocytes. The BIRC2 and/or BIRC3 genes were found to be mutated in some multiple myeloma samples,Citation41,Citation42 and the BIRC4 encoding XIAP in X-linked lymphoproliferative disease.Citation43 The expression and functions of the atypical IAP survivin in tumors, and the development of specific survivin-targeted therapy were recently reviewed by Coumar et alCitation44 and won’t be discussed here.
Table 1 Expression of IAPs and IAP antagonists in human tumors
Role of IAPs in cancer
IAPs as apoptotic regulators
IAPs were first characterized as inhibitors of apoptosis because of their ability to bind caspases. Indeed, cIAPs, XIAP and ML-IAP can bind caspase-3, -7, and -9 via the BIRsCitation10,Citation11,Citation45,Citation46 and can induce their ubiquitination or neddylation via the RING domain.Citation19,Citation22–Citation24 The influence of the ubiquitination is still not very well established, triggering degradative or nondegradative consequences,Citation22–Citation24 while the neddylation of caspase-7, by XIAP, inhibits its activity.Citation19 In addition, XIAP is able to directly inhibit the enzymatic activity of caspases (). The XIAP BIR3 binds the dimer interface of caspase-9, and the linker region upstream of BIR2 binds across the substrate binding pocket of caspase-3 and -7, which hinder substrate accessibility and hide the catalytic residue of caspases.Citation47–Citation49 The capacity of XIAP to inhibit caspase activity could account for the resistance of cancer cells to antitumor therapy. Indeed, DNA-damaging treatments, such as ionizing irradiations, induce a translational upregulation of XIAP as a consequence of the presence of an internal ribosome entry site (IRES)-dependent translation mechanism, which results in the resistance of carcinoma cells to radiation-induced apoptosis.Citation50,Citation51
IAPs can also inhibit cell death at an earlier step, preventing the assembly of caspase-8- or -10-activating platforms. Caspase-8 and -10 are initiator caspases recruited by the adaptor Fas-associated death domain protein (FADD) in multiprotein complexes, which provide the proximity required for caspase homodimerization and self-activation (for review,Citation52). These molecular platforms are assembled either in response to the engagement of death receptor from the TNFR superfamily (in which case, these are referred to as death-inducing signaling complex [DISC] and complex II)Citation52,Citation53 or in response to genotoxic stress, tumor necrosis factor-like weak inducer of apoptosis (TWEAK) engagement, or toll-like receptor (TLR) 3 stimulation (in which case, they are referred to as Ripoptosome).Citation54,Citation55 Complex IICitation50,Citation51 and RipoptosomeCitation52,Citation53 share, in addition to the caspase and the adaptor FADD, the serine/threonine kinase receptor interacting protein (RIP) (). cIAPs and XIAP are potent regulators of proteins from the RIP family, catalyzing the conjugation of ubiquitin chains that control either protein degradation or signal transduction pathwaysCitation56–Citation62 (). In the absence of cIAPs, non-ubiquitinated RIP1 promotes (through its kinase activity) the assembly of the caspase-activating platforms that leads to cell deathCitation56,Citation62 (). Thus, cIAPs inhibit RIP1-containing caspase-activating platform assembly, either by promoting the ubiquitin-proteasome-mediated degradation of the components of the RipoptosomeCitation54 or by inducing a nondegradative ubiquitination of RIP1, which inhibits the cell death complex assembly and promotes survival-signaling pathway transduction.Citation56,Citation59,Citation63
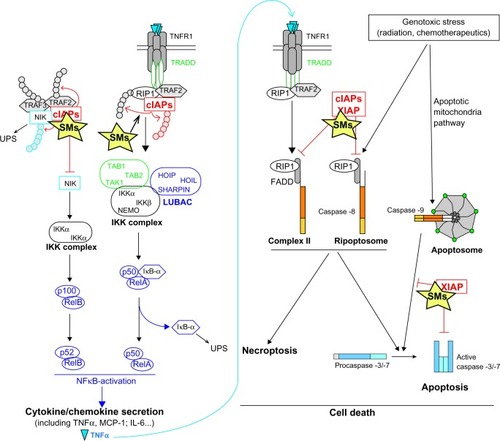
Figure 2 Mechanisms of action of Smac mimetics.
Abbreviations: BIR, baculoviral IAP repeat; cIAP, cellular IAP; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; IAPs, inhibitors of apoptosis; IL, interleukin; IKK, IκB kinase complex; LUBAC, linear ubiquitin chain assembly complex; MCP-1, monocyte chemoattractant protein; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NIK, NF-κB-inducing kinase; RIP1, receptor interacting protein 1; SM, Smac mimetic; Smac, second mitochondria-derived activator of caspases; TAK, TGFβ-activated kinase; TGFβ, transforming growth factor beta; TNF, tumor necrosis factor; TNFR1, tumor necrosis factor receptor 1; TRADD, TNFR1-associated death domain; TRAF, TNFR associated factor; UBA, ubiquitin proteasome system; XIAP, X-chromosome linked IAP; TAB, TAK1-binding partners; HOIL, heme-oxidized IRP2 ligase-1; HOIP, HOIL-1L-interaction protein; NEMO, nuclear factor-κB (NF-κB) essential modulator; UPS, ubiquitin-proteasome system.
IAPs as cell-signaling regulators
The role of IAPs in the regulation of the NF-κB-activating signaling pathways is well documented (for review,Citation25,Citation26). NF-κB is a transcription factor induced by the stimulation of antigen or cytokine receptors, by the recognition of microbiological patterns by the TLRs, the nucleotide-binding oligomerization domain-containing proteins (NODs), or the NOD-like receptors (NLRs), or in response to intracellular injuries, such as DNA damage or reactive oxygen species. NF-κB contributes to the adaptive response of cells, by mediating the expression of the proinflammatory molecules that counter microbial invasion and by promoting the expression of genes involved in cell survival, cell differentiation, and cell proliferation.Citation64 The transcription factor consists of heterodimers formed by one Rel subunit (RelA [also called p65], RelB, or c-Rel) and one NF-κB subunit (the p50 subunit of NF-κB1 or the p52 subunit of NF-κB2). In resting cells, the p50/RelA dimer is sequestered into the cytoplasm by the inhibitor of κB (IκB) proteins. Upon stimulation of the cell surface or intracellular receptors, or DNA damage, p50/RelA is released as a consequence of the degradation of NF-kappa-B inhibitor alpha (IκB-α) and translocated into the nucleus to stimulate proinflammatory gene transcription (). Degradation of IκB-α requires its phosphorylation by the IκB kinase (IKK) complex, which is activated by ubiquitination by the linear ubiquitin chain assembly complex (LUBAC) and by phosphorylation by TGFβ-activated protein kinase 1 (TAK1)Citation64 (). cIAPs and XIAP promote the steric proximity of TAK1, LUBAC, and IKK complex. In the TNF-R1-signaling pathway, cIAPs are recruited along with RIP1 to the receptorCitation61 and trigger self-ubiquitination and the nondegradative polyubiquitination of RIP1Citation56,Citation57,Citation66 (), and in NOD2-mediated inflammatory signaling, XIAP and cIAPs mediate the conjugation of ubiquitin chains to RIP2.Citation67–Citation69 These ubiquitin chains serve as a scaffold for the recruitment and activation of the signaling complexes leading to IKK activationCitation56,Citation61,Citation68,Citation70 (). cIAPs can also modulate NF-κB activation by catalyzing the monoubiquitination of the IKK component NF-κB essential modulator (NEMO), which is required for IKK activation,Citation71,Citation72 and XIAP promotes the activation of TAK1 and the steric proximity of TAK1 and IKK complexCitation71 during TGFβ and myelin basic protein (MBP) receptor signaling, or in response to DNA damage.Citation15,Citation71,Citation73–Citation75 A second NF-κB-activating signaling pathway, named the noncanonical pathway, involves NF-κB-inducing kinase (NIK), which catalyzes the phosphorylation of IKKα. In turn, IKKα induces the phosphorylation of the p100 NF-κB2 precursor, leading to its proteolytic activation into active p52 NF-κB2 (). cIAPs prevent the noncanonical activation of NF-κB by mediating the ubiquitination and the proteasomal-mediated degradation of NIKCitation70,Citation76–Citation79 (). Mutations in cIAP-encoding genes leading to NIK stabilization and chronic NF-kB activation could facilitate B cell malignancy and lymphomagenesis, as observed in some multiple myelomas that harbor mutations in the cIAP1- or cIAP2-encoding genesCitation41,Citation42 and as observed in MALT lymphoma that is associated with a chromosomal translocation t(11;18)(q21;q21), generating a chimeric protein composed of the N-terminal sequence of cIAP2 fused to the C-terminal sequence of MALT1.Citation17,Citation40,Citation80
Cell proliferation and migration
cIAPs are positive regulators of cell proliferation, a function correlated with the nuclear localization of the proteins.Citation29,Citation81 Interestingly, the nuclear expression of cIAP1 has been associated with advanced disease stages and poor patient prognosis in human cervical and esophageal squamous cell carcinomas and bladder cancersCitation36,Citation82,Citation83 (). The influence of IAPs on cell proliferation can be explained by their capacity to stimulate the activity of the c-Myc and E2F1 transcription factors, which are important regulators of cell cycle progression and cell proliferation with oncogenic properties.Citation28,Citation29 IAPs have also been involved in the regulation of the invasive properties of mammalian cancer cells, as recently reviewed.Citation84
Targeting IAPs in cancer therapy
Targeting IAPs in tumors is an important challenge and several strategies have been explored, including the use of antisense oligonucleotides and antagonist molecules. A synthetic antisense oligonucleotide to XIAP, named AEG35156, was developed by Aegera Therapeutics Inc (Montreal, QC, Canada).Citation85 It demonstrated promising efficiency in the preclinical studies. It induced a decrease of XIAP expression in tumor cell lines and tumor xenograft models, and sensitized cells to various standard chemotherapeutic agents and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) receptor agonists.Citation86 AEG35156 entered into clinical trials (http://www.clinicaltrials.gov/) in 2005, and to date, ten Phase 1,2, or 1/2 clinical trials have been completed in solid tumors and in acute myeloid leukemia (AML) () (for review,Citation86,Citation87). In the trials, AEG35156 appeared to accumulate in the liver and to have efficiently downregulated XIAP messenger ribonucleic acid (mRNA) in peripheral blood mononuclear cells and hepatocytes. AEG35156 is generally well tolerated except when administered in repeated high doses. Promising results were obtained with AEG35156 used as a single agent in solid tumorsCitation88 and in combination with cytarabine and idarubicin in AMLCitation89 in the Phase 1 studies, but it failed to show any significant antitumoral activity in the randomized Phase 2 studies in pancreatic adenocarcinoma, when combined with Gemcitabine,Citation90 or in AML, when it was given in combination with cytarabine and idarubicin.Citation91
Table 2 AEG35156 XIAP antisense oligonucleotide in clinical trials (http://www.clinicaltrials.gov/)
The structural characterization of the interaction of XIAP with caspases, or with Smac, or the drosophila Smac homologs has provided very potent tools for the design of synthetic IAP antagonists aiming to inhibit the capacity of XIAP to neutralize caspases.Citation11,Citation92–Citation94 The surface hydrophobic groove of IAP BIRs binds the IBM found in the N-terminal of the active subunits of caspase-3, -7, and -9 and exposed by activating proteolytic processing.Citation10,Citation11 Cellular IAP antagonists also own an IBM.Citation10–Citation13 During the apoptotic process, the Smac IBM is exposed as a consequence of the cleavage of the mitochondria-targeting signal, and matured Smac is released from the mitochondria into the cytosol.Citation10–Citation12 The tetrapeptide Ala-Val-Pro-Ile (AVPI) IBM motif of Smac inserts into the XIAP BIR2 and BIR3-caspase interaction pocket and abrogates XIAP-mediated caspase inhibitionCitation93,Citation95,Citation96 (). The Smac N-terminal peptide was also derived to produce cell permeable peptides and was shown to mimic the activity of Smac and to sensitize human cancer cell lines to diverse chemotherapeutic agents, including etoposide, teniposide, cisplatin, paclitaxel, 7-ethyl-10-hydroxycamptothecin (SN-38), and TRAIL agonists.Citation97–Citation100 In xenograft models, a Smac-derived peptide, made permeable by linking to the shuttle peptide trans-activation of transcription (TAT) from HIV, enhanced the antitumoral effect of TRAIL in glioma,Citation99 and a polyarginine-conjugated Smac peptide was shown to sensitize non-small cell lung carcinoma cells to cisplatin,Citation98 with little toxicity to normal tissues. The pharmacological properties of such Smac-derived peptides were not good enough to merit consideration of these molecules as therapeutic agents; however, they provided the bases for the structure-based design of IAP antagonists named Smac mimetics (SMs). Several approaches were used, including the screening of peptide or peptidomimetic libraries,Citation101,Citation102 and the structure-based design of conformationally constrained SMsCitation103,Citation104 (). Considerable efforts were invested to improve the affinity of the compounds to the IAP BIR domains, to improve their ability to antagonize IAPs,Citation104–Citation107 to improve cellular delivery and activity (ie, their capacity to induce apoptosis or to sensitize to apoptotic agents), and to improve their in vivo stability and bioavailability. The preclinical assays demonstrated their capacity to inhibit tumor growth in multiple solid tumors,Citation102,Citation107,Citation108 acute lymphoblastic leukemia (ALL),Citation108 and multiple myelomaCitation109 xenograft models and to sensitize cells to TRAIL, proteasome inhibitors, B-cell lymphoma protein 2 (Bcl-2) family-targeting compounds, and more conventional therapeutic agents, such as radiation, melphalan, or cisplatin.Citation103,Citation109–Citation114 Importantly, these compounds were well tolerated by animals and did not display toxicity against normal lymphocytes and bone marrow stromal cellsCitation109 or normal mammary epithelial cells.Citation115 The analysis of binding affinity revealed that similarly to Smac,Citation93,Citation95,Citation96 SMs can bind to XIAP-BIR2, preventing XIAP-caspase-7 and -3 binding, and to XIAP-BIR3, abrogating the XIAP-mediated inhibition of caspase-9. Structural and biochemical studies of the apoptotic activity of Smac cellular protein revealed, first, that it forms a symmetric dimer;Citation93,Citation94 second, that dimerization is essential for Smac function;Citation93,Citation116 and third, that the capacity of Smac to abrogate XIAP-mediated caspase inhibition required the binding to both BIR2 and BIR3.Citation95 Overall, these observations support the conclusion that compounds targeting both BIR domains could be more efficient as XIAP antagonists and lead to the development of bivalent small molecules containing two Smac AVPI IBM motif mimetics.Citation117 As expected, these compounds appeared to be more potent than their monovalent counterparts, in antagonizing XIAP and in activating caspases.Citation104,Citation117,Citation118 Like the monovalent versions, the bivalent molecules either inhibited tumor growth or sensitized cells to both conventional and nonconventional anticancer therapies in the preclinical assays and did not display toxicity to normal human primary cells;Citation104,Citation117–Citation121 however, unlike the monovalent molecules, the bivalent SMs are not orally bioavailable. To date, more than 50 applications for patents related to IAP antagonists have been filed (for review,Citation122), and six SMs have entered human clinical trials (http://www.clinicaltrials.gov/) for the treatment of cancer (described in ).
Figure 3 Structure of the Smac N-terminal tetrapeptide (AVPI) and SMs used in clinical trials.
Abbreviations: CAS RN, CAS Registry Number®; SM, Smac mimetic; Smac, second mitochondria-derived activator of caspases.
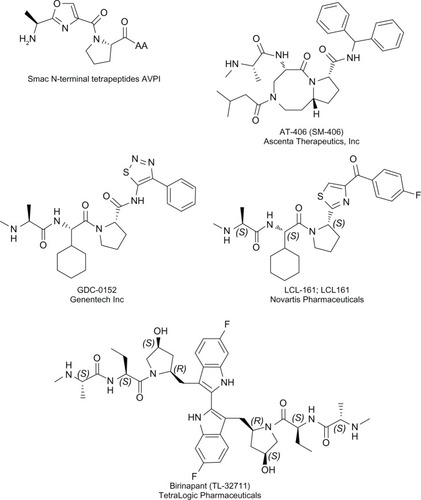
Table 3 SMs in clinical trials
Mechanisms of action of SMs
As expected, SMs abrogate XIAP-mediated caspase inhibition and therefore increase caspase-3 and -7 activities (). However, in addition to binding XIAP BIRs, SMs also bind the BIR domains of ML-IAP, cIAP1 and cIAP2.Citation56,Citation104,Citation105,Citation115,Citation118 SMs stimulate the E3-ubiquitine ligase activity of cIAPs, which results in the ubiquitination of RIP1, leading in turn, to canonical NF-κB activation and the rapid autoubiquitination and subsequent proteasome-mediated degradation of cIAPsCitation123–Citation126 (). Depletion of cIAPs abolishes the cIAP-mediated ubiquitination and degradation of NIK and induces canonical activation of NF-κB. In turn, NF-κB induces the expression of proinflammatory cytokines and chemokines, including TNFα, which can trigger cell death by an autocrine pathway.Citation66,Citation125–Citation127 Furthermore, depletion of cIAPs favors the assembly of the RIP1-containing cytoplasmic cell death complexes, such as complex II and Ripoptosome, resulting in cell death in some sensitive cancer cells, or in the sensitization to TNFα or DNA-damaging chemotherapeutic agentsCitation54,Citation55 (). SMs exert their activity through XIAP and cIAPs and both effects are required for their maximal antitumoral activity.Citation128–Citation130 Indeed, IAP antagonists displaying a high and selective affinity for cIAPs over XIAP appeared less potent than pan-IAP antagonists in promoting cancer cell deathCitation129 and in sensitizing cancer cells to TRAIL.Citation131
As a consequence of cIAP degradation and NF-κB activation, the administration of SMs such as LCL161, GDC-0152, and HGS1029, resulted in the upregulation of cytokines and chemokines,Citation132–Citation134 including TNFα, monocyte chemoattractant protein (MCP)-1, interleukin (IL)-7, IL-6, and interferon (IFN)γ.Citation134 MCP-1 was used as a clinical biomarker for SMs efficiency in clinical programs.Citation133,Citation135 The analysis of the proinflammatory characteristics of cellular Smac-induced cell death suggests that the proinflammatory response elicited by SMs could activate the adaptive antitumor immune response in cancers.Citation136 In dogs, intravenous (IV) administration of GDC-0152 induced an acute systemic inflammatory response with lung and hepatic injury, which are consistent with TNF-α mediated toxicity;Citation134 however, a similar TNF-α-driven inflammatory response was not observed in humans.Citation133 Although the first clinical trials did not reveal extensive toxicity of SMs when orally or intravenously administered, additional analysis of the consequences of cytokine and chemokine secretion are required. Because osteoclast differentiation and function are stimulated by activation of the noncanonical NF-κB pathway and because osteoclasts are susceptible to TNF-mediated death, Yang et al analyzed the influence of SMs on bone metastasis and demonstrated that SMs stimulated osteoporosis and specifically enhanced metastasis in bone.Citation137
Conclusion
SMs are a very promising new class of anticancer therapeutics. Results from preclinical studies have demonstrated an acceptable safety profile and some signs of antitumoral activity, in their use as a single agent or in combination with conventional or nonconventional therapies, such as dead receptor agonists, Bcl-2, or kinase-targeting therapies. The first clinical trials demonstrated a good tolerance and target inhibition. Ongoing and future clinical trials will determine the safety, appropriate indications, and drugs combinations. It will be important to determine the level and the site of production of TNFα and other cytokines and the consequences of cytokine production for tumoral and non-tumoral cells. Since IAPs are involved in the regulation of various cellular functions, it will be interesting to target specific IAP functions in order to limit possible adverse impacts. The consequences of SMs on the immune system in vivo and the use of cIAPs as potential therapeutic targets for inflammatory or immune disorders are still important questions that need to be addressed.
Acknowledgments
Our work is supported by grants from the “Comité de Côte d’Or de la Ligue contre le Cancer,” from the “Association pour la recherche sur le Cancer (ARC),” and from the “Conseil Régional de Bourgogne.” JB received a fellowship from the “Ministère de l’Enseignement Supérieur et de la Recherche” of France, and VG received a fellowship from the “Ligue Nationale contre le Cancer.”
Disclosure
The authors report no conflicts of interest in this work.
Supplementary material
Table S1 Role of IAPs in cancer
References
- TammIRichterSScholzFXIAP expression correlates with monocytic differentiation in adult de novo AML: impact on prognosisHematol J20045648949515570290
- TammIKornblauSMSegallHExpression and prognostic significance of IAP-family genes in human cancers and myeloid leukemiasClin Cancer Res2000651796180310815900
- Grzybowska-IzydorczykOCebulaBRobakTSmolewskiPExpression and prognostic significance of the inhibitor of apoptosis protein (IAP) family and its antagonists in chronic lymphocytic leukaemiaEur J Cancer201046480081020045309
- HussainARUddinSAhmedMPrognostic significance of XIAP expression in DLBCL and effect of its inhibition on AKT signallingJ Pathol2011222218019020632385
- LiMSongTYinZFNaYQXIAP as a prognostic marker of early recurrence of nonmuscular invasive bladder cancerChin Med J (Engl)2007120646947317439739
- ZhangYZhuJTangYX-linked inhibitor of apoptosis positive nuclear labeling: a new independent prognostic biomarker of breast invasive ductal carcinomaDiagn Pathol201164921645409
- LiuSSTsangBKCheungANAnti-apoptotic proteins, apoptotic and proliferative parameters and their prognostic significance in cervical carcinomaEur J Cancer20013791104111011378340
- XiangGWenXWangHChenKLiuHExpression of X-linked inhibitor of apoptosis protein in human colorectal cancer and its correlation with prognosisJ Surg Oncol2009100870871219777490
- MoussataDAmaraSSiddeekBXIAP as a radioresistance factor and prognostic marker for radiotherapy in human rectal adenocarcinomaAm J Pathol201218141271127822867709
- AugelloCCarusoLMaggioniMInhibitors of apoptosis proteins (IAPs) expression and their prognostic significance in hepatocellular carcinomaBMC Cancer2009912519397802
- ShiYHDingWXZhouJExpression of X-linked inhibitor-of-apoptosis protein in hepatocellular carcinoma promotes metastasis and tumor recurrenceHepatology200848249750718666224
- HiscuttELHillDSMartinSTargeting X-linked inhibitor of apoptosis protein to increase the efficacy of endoplasmic reticulum stress-induced apoptosis for melanoma therapyJ Invest Dermatol201013092250225820520630
- FerreiraCGvan der ValkPSpanSWExpression of X-linked inhibitor of apoptosis as a novel prognostic marker in radically resected non-small cell lung cancer patientsClin Cancer Res2001782468247411489828
- FerreiraCGvan der ValkPSpanSWAssessment of IAP (inhibitor of apoptosis) proteins as predictors of response to chemotherapy in advanced non-small-cell lung cancer patientsAnn Oncol200112679980511484955
- BrunckhorstMKLernerDWangSYuQAT-406, an orally active antagonist of multiple inhibitor of apoptosis proteins, inhibits progression of human ovarian cancerCancer Biol Ther201213980481122669575
- SeligsonDBHongoFHuerta-YepezSExpression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrenceClin Cancer Res200713206056606317947468
- KrajewskaMKrajewskiSBanaresSElevated expression of inhibitor of apoptosis proteins in prostate cancerClin Cancer Res20039134914492514581366
- RampUKriegTCaliskanEXIAP expression is an independent prognostic marker in clear-cell renal carcinomasHum Pathol20043581022102815297970
- YanYMahotkaCHeikausSDisturbed balance of expression between XIAP and Smac/DIABLO during tumour progression in renal cell carcinomasBr J Cancer20049171349135715328523
- MizutaniYNakanishiHLiYNOverexpression of XIAP expression in renal cell carcinoma predicts a worse prognosisInt J Oncol200730491992517332931
- GuLQLiFYZhaoLBRAFV600E mutation and X-linked inhibitor of apoptosis expression in papillary thyroid carcinomaThyroid200919434735419355825
- ImotoITsudaHHirasawaAExpression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapyCancer Res200262174860486612208731
- ImotoIYangZQPimkhaokhamAIdentification of cIAP1 as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomasCancer Res200161186629663411559525
- ZenderLSpectorMSXueWIdentification and validation of oncogenes in liver cancer using an integrative oncogenomic approachCell200612571253126716814713
- ChengLZhouZFlesken-NikitinARb inactivation accelerates neoplastic growth and substitutes for recurrent amplification of cIAP1, cIAP2 and Yap1 in sporadic mammary carcinoma associated with p53 deficiencyOncogene201029425700571120676140
- ReardonDAMichalkiewiczEBoyettJMExtensive genomic abnormalities in childhood medulloblastoma by comparative genomic hybridizationCancer Res19975718404240479307291
- DaiZZhuWGMorrisonCDA comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenesHum Mol Genet200312779180112651874
- BashyamMDBairRKimYHArray-based comparative genomic hybridization identifies localized DNA amplifications and homozygous deletions in pancreatic cancerNeoplasia20057655656216036106
- EspositoIKleeffJAbiatariIOverexpression of cellular inhibitor of apoptosis protein 2 is an early event in the progression of pancreatic cancerJ Clin Pathol200760888589516775116
- VallatLMagdelénatHMerle-BéralHThe resistance of B-CLL cells to DNA damage-induced apoptosis defined by DNA microarraysBlood2003101114598460612586635
- SilvaKLVasconcellosDVCastroEDCoelhoAMLindenRMaiaRCApoptotic effect of fludarabine is independent of expression of IAPs in B-cell chronic lymphocytic leukemiaApoptosis200611227728516502265
- CheXYangDZongHNuclear cIAP1 overexpression is a tumor stage- and grade-independent predictor of poor prognosis in human bladder cancer patientsUrol Oncol201230445045621795072
- PonnelleTChapusotCMartinLSubcellular expression of c-IAP1 and c-IAP2 in colorectal cancers: relationships with clinicopathological features and prognosisPathol Res Pract20031991172373114708638
- TanimotoTTsudaHImazekiNNuclear expression of cIAP-1, an apoptosis inhibiting protein, predicts lymph node metastasis and poor patient prognosis in head and neck squamous cell carcinomasCancer Lett2005224114115115911110
- QiSMogiSTsudaHExpression of cIAP-1 correlates with nodal metastasis in squamous cell carcinoma of the tongueInt J Oral Maxillofac Surg200837111047105318621506
- KeatsJJFonsecaRChesiMPromiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myelomaCancer Cell200712213114417692805
- AnnunziataCMDavisREDemchenkoYFrequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myelomaCancer Cell200712211513017692804
- StanculescuABembinsterLABorgenKBergamaschiAWileyEFrasorJEstrogen promotes breast cancer cell survival in an inhibitor of apoptosis (IAP)-dependent mannerHorm Cancer20101312713521152357
- AkagiTMotegiMTamuraAA novel gene, MALT1 at 18q21, is involved in t(11;18) (q21;q21) found in low-grade B-cell lymphoma of mucosa-associated lymphoid tissueOncogene199918425785579410523859
- DierlammJBaensMWlodarskaIThe apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomasBlood199993113601360910339464
- El-MesallamyHOHegabHMKamalAMExpression of inhibitor of apoptosis protein (IAP) livin/BIRC7 in acute leukemia in adults: correlation with prognostic factors and outcomeLeuk Res201135121616162221700335
- ChoiJHwangYKSungKWExpression of Livin, an antiapoptotic protein, is an independent favorable prognostic factor in childhood acute lymphoblastic leukemiaBlood2007109247147716990595
- GazzanigaPGradiloneAGiulianiLExpression and prognostic significance of LIVIN, SURVIVIN and other apoptosis-related genes in the progression of superficial bladder cancerAnn Oncol2003141859012488298
- WangXXuJJuSNiHZhuJWangHLivin gene plays a role in drug resistance of colon cancer cellsClin Biochem2010437–865566020171199
- WangTSDingQQGuoRHExpression of livin in gastric cancer and induction of apoptosis in SGC-7901 cells by shRNA-mediated silencing of livin geneBiomed Pharmacother201064533333819914791
- NachmiasBAshhabYBucholtzVCaspase-mediated cleavage converts Livin from an antiapoptotic to a proapoptotic factor: implications for drug-resistant melanomaCancer Res200363196340634914559822
- KimDKAlvaradoCSAbramowskyCRExpression of inhibitor-of-apoptosis protein (IAP) livin by neuroblastoma cells: correlation with prognostic factors and outcomePediatr Dev Pathol20058662162916328668
- NedelcuTKubistaBKollerALivin and Bcl-2 expression in high-grade osteosarcomaJ Cancer Res Clin Oncol2008134223724417632732
- KempkensteffenCHinzSChristophFExpression of the apoptosis inhibitor livin in renal cell carcinomas: correlations with pathology and outcomeTumour Biol200728313213817519534
- HaferkampABedkeJVetterCHigh nuclear Livin expression is a favourable prognostic indicator in renal cell carcinomaBJU Int2008102111700170618990137
- KempkensteffenCHinzSKrauseHExpression of splicing variants of the inhibitor of apoptosis livin in testicular germ cell tumorsTumour Biol2008292768218515985
- PlutaAWrzesien-KusACebula-ObrzutBInfluence of high expression of Smac/DIABLO protein on the clinical outcome in acute myeloid leukemia patientsLeuk Res201034101308131320061022
- MizutaniYKatsuokaYBonavidaBLow circulating serum levels of second mitochondria-derived activator of caspase (Smac/DIABLO) in patients with bladder cancerInt J Oncol20124041246125022218530
- PlutaPCebula-ObrzutBEhemannVCorrelation of Smac/DIABLO protein expression with the clinico-pathological features of breast cancer patientsNeoplasma201158543043521744997
- Arellano-LlamasAGarciaFJPerezDHigh Smac/DIABLO expression is associated with early local recurrence of cervical cancerBMC Cancer2006625617067390
- EndoKKohnoeSWatanabeAClinical significance of Smac/DIA-BLO expression in colorectal cancerOncol Rep200921235135519148507
- DobrzyckaBTerlikowskiSJBernaczykPSPrognostic significance of smac/DIABLO in endometrioid endometrial cancerFolia Histochem Cytobiol201148467868121478115
- XuYZhouLHuangJRole of Smac in determining the chemotherapeutic response of esophageal squamous cell carcinomaClin Cancer Res201117165412542221676925
- SekimuraAKonishiAMizunoKExpression of Smac/DIABLO is a novel prognostic marker in lung cancerOncol Rep200411479780215010875
- MizutaniYKatsuokaYBonavidaBPrognostic significance of second mitochondria-derived activator of caspase (Smac/DIABLO) expression in bladder cancer and target for therapyInt J Oncol201037250350820596678
- ShibataTMahotkaCWethkampNHeikausSGabbertHERampUDisturbed expression of the apoptosis regulators XIAP, XAF1, and Smac/DIABLO in gastric adenocarcinomasDiagn Mol Pathol20071611817471152
- KempkensteffenCHinzSChristophFExpression levels of the mitochondrial IAP antagonists Smac/DIABLO and Omi/HtrA2 in clear-cell renal cell carcinomas and their prognostic valueJ Cancer Res Clin Oncol2008134554355017922292
- OuyangYQzur HausenAOrlowska-VolkMExpression levels of hnRNP G and hTra2-beta1 correlate with opposite outcomes in endometrial cancer biologyInt J Cancer201112892010201920607830
- YangXXingHGaoQRegulation of HtrA2/Omi by X-linked inhibitor of apoptosis protein in chemoresistance in human ovarian cancer cellsGynecol Oncol200597241342115863139
- HuXYXuYMChenXCPingHChenZHZengFQImmunohistochemical analysis of Omi/HtrA2 expression in prostate cancer and benign prostatic hyperplasiaAPMIS20061141289389817207090
- HuXChenXPingHChenZZengFLuGImmunohistochemical analysis of Omi/HtrA2 expression in prostate cancer and benign prostatic hyperplasiaJ Huazhong Univ Sci Technolog Med Sci200525667167316696322
- LeeSHLeeJWKimHSImmunohistochemical analysis of Omi/HtrA2 expression in stomach cancerAPMIS2003111558659012887511
- Zurawa-JanickaDKobielaJGalczynskaNChanges in expression of human serine protease HtrA1, HtrA2 and HtrA3 genes in benign and malignant thyroid tumorsOncol Rep20122851838184422923201
- NarkiewiczJLapinska-SzumczykSZurawa-JanickaDSkorko-GlonekJEmerichJLipinskaBExpression of human HtrA1, HtrA2, HtrA3 and TGF-beta1 genes in primary endometrial cancerOncol Rep20092161529153719424634
- NarkiewiczJKlasa-MazurkiewiczDZurawa-JanickaDSkorko-GlonekJEmerichJLipinskaBChanges in mRNA and protein levels of human HtrA1, HtrA2 and HtrA3 in ovarian cancerClin Biochem2008417–856156918241672
- GottfriedYVoldavskyEYodkoLSaboEBen-ItzhakOLarischSExpression of the pro-apoptotic protein ARTS in astrocytic tumors: correlation with malignancy grade and survival rateCancer2004101112614262115517578
- ElhasidRSaharDMerlingAMitochondrial pro-apoptotic ARTS protein is lost in the majority of acute lymphoblastic leukemia patientsOncogene200423325468547515122323
References
- CrookNEClemRJMillerLKAn apoptosis-inhibiting baculovirus gene with a zinc finger-like motifJ Virol1993674216821748445726
- RotheMPanMGHenzelWJAyresTMGoeddelDVThe TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteinsCell1995837124312528548810
- RoyNMahadevanMSMcLeanMThe gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophyCell19958011671787813013
- DuckettCSNavaVEGedrichRWA conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitorsEMBO J19961511268526948654366
- UrenAGPakuschMHawkinsCJPulsKLVauxDLCloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factorsProc Natl Acad Sci U S A19969310497449788643514
- AmbrosiniGAdidaCAltieriDCA novel anti-apoptosis gene, survivin, expressed in cancer and lymphomaNat Med1997389179219256286
- ChenZNaitoMHoriSMashimaTYamoriTTsuruoTA human IAP-family gene, apollon, expressed in human brain cancer cellsBiochem Biophys Res Commun1999264384785410544019
- VucicDStennickeHRPisabarroMTSalvesenGSDixitVMML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomasCurr Biol200010211359136611084335
- RichterBWMirSSEibenLJMolecular cloning of ILP-2, a novel member of the inhibitor of apoptosis protein familyMol Cell Biol200121134292430111390657
- SrinivasulaSMHegdeRSalehAA conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosisNature2001410682411211611242052
- LiuZSunCOlejniczakETStructural basis for binding of Smac/DIABLO to the XIAP BIR3 domainNature200040868151004100811140637
- HegdeRSrinivasulaSMZhangZIdentification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interactionJ Biol Chem2002277143243811606597
- ShiYA conserved tetrapeptide motif: potentiating apoptosis through IAP-bindingCell Death Differ200292939511840157
- VerhagenAMSilkeJEkertPGHtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteinsJ Biol Chem2002277144545411604410
- LuMLinSCHuangYXIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerizationMol Cell200726568970217560374
- SamuelTWelshKLoberTTogoSHZapataJMReedJCDistinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspasesJ Biol Chem200628121080109016282325
- VarfolomeevEWaysonSMDixitVMFairbrotherWJVucicDThe inhibitor of apoptosis protein fusion c-IAP2.MALT1stimulates NF-kappaB activation independently of TRAF1 AND TRAF2J Biol Chem200628139290222902916891304
- MacePDSmitsCVauxDLSilkeJDayCLAsymmetric recruitment of cIAPs by TRAF2J Mol Biol2010400181520447407
- BroemerMTenevTRigboltKTSystematic in vivo RNAi analysis identifies IAPs as NEDD8-E3 ligasesMol Cell201040581082221145488
- VucicDDixitVMWertzIEUbiquitylation in apoptosis: a post-translational modification at the edge of life and deathNat Rev Mol Cell Biol201112743945221697901
- BroemerMMeierPUbiquitin-mediated regulation of apoptosisTrends Cell Biol200919313014019217783
- HuangHKJoazeiroCABonfocoEKamadaSLeversonJDHunterTThe inhibitor of apoptosis, cIAP2, functions as a ubiquitin-protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7J Biol Chem200027535266612666410862606
- SuzukiYNakabayashiYTakahashiRUbiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell deathProc Natl Acad Sci U S A200198158662866711447297
- ChoiYEButterworthMMalladiSDuckettCSCohenGMBrattonSBThe E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and -7 via unique mechanisms at distinct steps in their processingJ Biol Chem200928419127721278219258326
- Gyrd-HansenMMeierPIAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancerNat Rev Cancer201010856157420651737
- BeugSTCheungHHLaCasseECKornelukRGModulation of immune signalling by inhibitors of apoptosisTrends Immunol2012331153554522836014
- OberoiTKDoganTHockingJCIAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradationEMBO J2011311142822117219
- XuLZhuJHuXc-IAP1 cooperates with Myc by acting as a ubiquitin ligase for Mad1Mol Cell200728591492218082613
- CartierJBertheletJMarivinACellular inhibitor of apoptosis protein-1 (cIAP1) can regulate E2F1 transcription factor-mediated control of cyclin transcriptionJ Biol Chem201128630264062641721653699
- GottfriedYRotemALotanRStellerHLarischSThe mitochondrial ARTS protein promotes apoptosis through targeting XIAPEMBO J20042371627163515029247
- García-FernándezMKisselHBrownSSept4/ARTS is required for stem cell apoptosis and tumor suppressionGenes Dev201024202282229320952537
- ZenderLSpectorMSXueWIdentification and validation of oncogenes in liver cancer using an integrative oncogenomic approachCell200612571253126716814713
- ChengLZhouZFlesken-NikitinARb inactivation accelerates neoplastic growth and substitutes for recurrent amplification of cIAP1, cIAP2 and Yap1 in sporadic mammary carcinoma associated with p53 deficiencyOncogene201029425700571120676140
- ReardonDAMichalkiewiczEBoyettJMExtensive genomic abnormalities in childhood medulloblastoma by comparative genomic hybridizationCancer Res19975718404240479307291
- BashyamMDBairRKimYHArray-based comparative genomic hybridization identifies localized DNA amplifications and homozygous deletions in pancreatic cancerNeoplasia20057655656216036106
- ImotoITsudaHHirasawaAExpression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapyCancer Res200262174860486612208731
- DaiZZhuWGMorrisonCDA comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenesHum Mol Genet200312779180112651874
- SnijdersAMSchmidtBLFridlyandJRare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinomaOncogene200524264232424215824737
- ImotoIYangZQPimkhaokhamAIdentification of cIAP1 as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomasCancer Res200161186629663411559525
- GarrisonJBSamuelTReedJCTRAF2-binding BIR1 domain of c-IAP2/MALT1 fusion protein is essential for activation of NF-kappaBOncogene200928131584159319234489
- KeatsJJFonsecaRChesiMPromiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myelomaCancer Cell200712213114417692805
- AnnunziataCMDavisREDemchenkoYFrequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myelomaCancer Cell200712211513017692804
- FilipovichAHZhangKSnowALMarshRAX-linked lymphoproliferative syndromes: brothers or distant cousins?Blood2010116183398340820660790
- CoumarMSTsaiFYKanwarJRSarvagallaSCheungCHTreat cancers by targeting survivin: just a dream or future reality?Cancer Treat Rev [Epub February 28, 2013]
- EckelmanBPDragMSnipasSJSalvesenGSThe mechanism of peptide-binding specificity of IAP BIR domainsCell Death Differ200815592092818239672
- TenevTZachariouAWilsonRDitzelMMeierPIAPs are functionally non-equivalent and regulate effector caspases through distinct mechanismsNat Cell Biol200571707715580265
- ScottFLDenaultJBRiedlSJShinHRenatusMSalvesenGSXIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPsEMBO J200524364565515650747
- ShiozakiENChaiJRigottiDJMechanism of XIAP-mediated inhibition of caspase-9Mol Cell200311251952712620238
- RiedlSJRenatusMSchwarzenbacherRStructural basis for the inhibition of caspase-3 by XIAPCell2001104579180011257232
- HolcikMYehCKornelukRGChowTTranslational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell deathOncogene200019364174417710962579
- GuLZhuNZhangHDurdenDLFengYZhouMRegulation of XIAP translation and induction by MDM2 following irradiationCancer Cell200915536337519411066
- MacePDRiedlSJMolecular cell death platforms and assembliesCurr Opin Cell Biol201022682883620817427
- MicheauOTschoppJInduction of TNF receptor I-mediated apoptosis via two sequential signaling complexesCell2003114218119012887920
- TenevTBianchiKDardingMThe Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPsMol Cell201143343244821737329
- FeoktistovaMGeserickPKellertBcIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoformsMol Cell201143344946321737330
- BertrandMJMilutinovicSDicksonKMcIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitinationMol Cell200830668970018570872
- DynekJNGoncharovTDueberECc-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signallingEMBO J201029244198420921113135
- ParkSMYoonJBLeeTHReceptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitroFEBS Lett20045661–315115615147886
- GeserickPHupeMMoulinMCellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitmentJ Cell Biol200918771037105420038679
- VinceJEWongWWGentleIInhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activationImmunity201236221522722365665
- HaasTLEmmerichCHGerlachBRecruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene inductionMol Cell200936583184420005846
- O’DonnellMALegarda-AddisonDSkountzosPYehWCTingATUbiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signalingCurr Biol200717541842417306544
- VanlangenakkerNVanden BergheTBogaertPcIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species productionCell Death Differ201118465666521052097
- HaydenMSGhoshSNF-kappaB, the first quarter-century: remarkable progress and outstanding questionsGenes Dev201226320323422302935
- ShihVFTsuiRCaldwellAHoffmannAA single NFκB system for both canonical and non-canonical signalingCell Res20112118610221102550
- VarfolomeevEGoncharovTFedorovaAVc-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activationJ Biol Chem200828336242952429918621737
- BertrandMJDoironKLabbéKKornelukRGBarkerPASalehMCellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2Immunity200930678980119464198
- DamgaardRBNachburUYabalMThe ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunityMol Cell201246674675822607974
- KriegACorreaRGGarrisonJBXIAP mediates NOD signaling via interaction with RIP2Proc Natl Acad Sci U S A200910634145241452919667203
- VarfolomeevEGoncharovTMaeckerHCellular inhibitors of apoptosis are global regulators of NF-κB and MAPK activation by members of the TNF family of receptorsSci Signal20125216ra2222434933
- JinHSLeeDHKimDHChungJHLeeSJLeeTHcIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activationCancer Res20096951782179119223549
- HinzMStilmannMArslanSÇKhannaKKDittmarGScheidereitCA cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-κB activationMol Cell2010401637420932475
- Hofer-WarbinekRSchmidJAStehlikCBinderBRLippJde MartinRActivation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1J Biol Chem200027529220642206810807933
- Birkey ReffeySWurthnerJUParksWTRobertsABDuckettCSX-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-beta signalingJ Biol Chem200127628265422654911356828
- SannaMGda Silva CorreiaJDucreyOIAP suppression of apoptosis involves distinct mechanisms: the TAK1/JNK1 signaling cascade and caspase inhibitionMol Cell Biol20022261754176611865055
- ZarnegarBJWangYMahoneyDJNoncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIKNat Immunol20089121371137818997794
- VallabhapurapuSMatsuzawaAZhangWNonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signalingNat Immunol20089121364137018997792
- FelthamRMoulinMVinceJETumor necrosis factor (TNF) signaling, but not TWEAK (TNF-like weak inducer of apoptosis)-triggered cIAP1 (cellular inhibitor of apoptosis protein 1) degradation, requires cIAP1 RING dimerization and E2 bindingJ Biol Chem201028523175251753620356846
- MaoAPLiSZhongBVirus-triggered ubiquitination of TRAF3/6 by cIAP1/2 is essential for induction of interferon-beta (IFN-beta) and cellular antiviral responseJ Biol Chem2010285139470947620097753
- RosebeckSMaddenLJinXCleavage of NIK by the API2-MALT1 fusion oncoprotein leads to noncanonical NF-kappaB activationScience2011331601646847221273489
- SamuelTOkadaKHyerMWelshKZapataJMReedJCcIAP1 Localizes to the nuclear compartment and modulates the cell cycleCancer Res200565121021815665297
- TanimotoTTsudaHImazekiNNuclear expression of cIAP-1, an apoptosis inhibiting protein, predicts lymph node metastasis and poor patient prognosis in head and neck squamous cell carcinomasCancer Lett2005224114115115911110
- CheXYangDZongHNuclear cIAP1 overexpression is a tumor stage- and grade-independent predictor of poor prognosis in human bladder cancer patientsUrol Oncol201230445045621795072
- FuldaSRegulation of cell migration, invasion and metastasis by IAP proteins and their antagonistsOncogene [Epub March 11, 2013]
- McManusDCLefebvreCACherton-HorvatGLoss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeuticsOncogene200423498105811715378029
- LacasseECPulling the plug on a cancer cell by eliminating XIAP with AEG35156Cancer Lett2013332221522422776562
- KatragaddaLCarterBZBorthakurGXIAP antisense therapy with AEG 35156 in acute myeloid leukemiaExpert Opin Investig Drugs2013225663670
- DeanEJodrellDConnollyKPhase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancerJ Clin Oncol200927101660166619237630
- SchimmerADEsteyEHBorthakurGPhase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemiaJ Clin Oncol200927284741474619652057
- MahadevanDChalasaniPRensvoldDPhase I trial of AEG35156 an antisense oligonucleotide to XIAP plus gemcitabine in patients with metastatic pancreatic ductal adenocarcinomaAm J Clin Oncol201336323924322441342
- SchimmerADHerrWHanelMAddition of AEG35156 XIAP antisense oligonucleotide in reinduction chemotherapy does not improve remission rates in patients with primary refractory acute myeloid leukemia in a randomized phase II studyClin Lymphoma Myeloma Leuk201111543343821729686
- ChaiJYanNHuhJRMolecular mechanism of Reaper-Grim-Hid-mediated suppression of DIAP1-dependent Dronc ubiquitinationNat Struct Biol2003101189289814517550
- ChaiJDuCWuJWKyinSWangXShiYStructural and biochemical basis of apoptotic activation by Smac/DIABLONature2000406679885586210972280
- WuGChaiJSuberTLStructural basis of IAP recognition by Smac/DIABLONature200040868151008101211140638
- HuangYRichRLMyszkaDGWuHRequirement of both the second and third BIR domains for the relief of X-linked inhibitor of apoptosis protein (XIAP)-mediated caspase inhibition by SmacJ Biol Chem200327849495174952214512414
- DuCFangMLiYLiLWangXSmac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibitionCell20001021334210929711
- ArntCRChioreanMVHeldebrantMPGoresGJKaufmannSHSynthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situJ Biol Chem200227746442364424312218061
- YangLMashimaTSatoSPredominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptideCancer Res200363483183712591734
- FuldaSWickWWellerMDebatinKMSmac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivoNat Med20028880881512118245
- GuoFNimmanapalliRParanawithanaSEctopic overexpression of second mitochondria-derived activator of caspases (Smac/DIABLO) or cotreatment with N-terminus of Smac/DIABLO peptide potentiates epothilone B derivative-(BMS 247550) and Apo-2L/TRAIL-induced apoptosisBlood20029993419342611964312
- FranklinMCKadkhodayanSAckerlyHStructure and function analysis of peptide antagonists of melanoma inhibitor of apoptosis (ML-IAP)Biochemistry200342278223823112846571
- OostTKSunCArmstrongRCDiscovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancerJ Med Chem200447184417442615317454
- SunHNikolovska-ColeskaZYangCYStructure-based design of potent, conformationally constrained Smac mimeticsJ Am Chem Soc200412651166861668715612682
- LiLThomasRMSuzukiHDe BrabanderJKWangXHarranPGA small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell deathScience200430556891471147415353805
- ZobelKWangLVarfolomeevEDesign, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPsACS Chem Biol20061852553317168540
- SharmaSKStraubCZawelLDevelopment of peptidomimetics targeting IAPsInt J Pept Res Ther2006121213219617919
- SunHNikolovska-ColeskaZLuJDesign, synthesis, and evaluation of a potent, cell-permeable, conformationally constrained second mitochondria derived activator of caspase (Smac) mimeticJ Med Chem200649267916792017181177
- HoughtonPJKangMHReynoldsCPInitial testing (stage 1) of LCL161, a SMAC mimetic, by the Pediatric Preclinical Testing ProgramPediatr Blood Cancer201258463663921681929
- ChauhanDNeriPVelankarMTargeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM)Blood200710931220122717032924
- ChenKFLinJPShiauCWInhibition of Bcl-2 improves effect of LCL161, a SMAC mimetic, in hepatocellular carcinoma cellsBiochem Parmacol2012843268277
- YuanZSyrkinGAdemABlockade of inhibitors of apoptosis (IAPs) in combination with tumor-targeted delivery of tumor necrosis factor-α leads to synergistic antitumor activityCancer Gene Ther2013201465623154431
- XuYZhouLHuangJRole of Smac in determining the chemotherapeutic response of esophageal squamous cell carcinomaClin Cancer Res201117165412542221676925
- ZieglerDSKeatingJKesariSA small-molecule IAP inhibitor overcomes resistance to cytotoxic therapies in malignant gliomas in vitro and in vivoNeuro Oncol201113882082921724651
- KeatingJTsoliMHallahanARIngramWJHaberMZieglerDSTargeting the inhibitor of apoptosis proteins as a novel therapeutic strategy in medulloblastomaMol Cancer Ther201211122654266323012247
- FlygareJABeresiniMBudhaNDiscovery of a potent small-molecule antagonist of inhibitor of apoptosis (IAP) proteins and clinical candidate for the treatment of cancer (GDC-0152)J Med Chem20125594101411322413863
- FlanaganLSebastiaJDelgadoMELennonJCRehmMDimerization of Smac is crucial for its mitochondrial retention by XIAP subsequent to mitochondrial outer membrane permeabilizationBiochim Biophys Acta20111813581982621354220
- SunHNikolovska-ColeskaZLuJDesign, synthesis, and characterization of a potent, nonpeptide, cell-permeable, bivalent Smac mimetic that concurrently targets both the BIR2 and BIR3 domains in XIAPJ Am Chem Soc200712949152791529417999504
- LuJBaiLSunHSM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAPCancer Res200868229384939319010913
- BrunckhorstMKLernerDWangSYuQAT-406, an orally active antagonist of multiple inhibitor of apoptosis proteins, inhibits progression of human ovarian cancerCancer Biol Ther201213980481122669575
- CaiQSunHPengYA Potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatmentJ Med Chem20115482714272621443232
- FuldaSInhibitor of apoptosis (IAP) proteins as therapeutic targets for radiosensitization of human cancersCancer Treat Rev201238676076622342104
- FlygareJAFairbrotherWJSmall-molecule pan-IAP antagonists: a patent reviewExpert Opin Ther Pat201020225126720100005
- FelthamRBettjemanBBudhidarmoRSmac mimetics activate the E3 ligase activity of cIAP1 protein by promoting RING domain dimerizationJ Biol Chem201128619170151702821393245
- DueberECSchoefflerAJLingelAAntagonists induce a conformational change in cIAP1 that promotes autoubiquitinationScience2011334605437638022021857
- VarfolomeevEBlankenshipJWWaysonSMIAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosisCell2007131466968118022362
- VinceJEWongWWKhanNIAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosisCell2007131468269318022363
- PetersenSLWangLYalcin-ChinAAutocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosisCancer Cell200712544545617996648
- CohenFAlickeBElliottLOOrally bioavailable antagonists of inhibitor of apoptosis proteins based on an azabicyclooctane scaffoldJ Med Chem20095261723173019228017
- NdubakuCVarfolomeevEWangLAntagonism of c-IAP and XIAP proteins is required for efficient induction of cell death by small-molecule IAP antagonistsACS Chem Biol20094755756619492850
- GalbánSHwangCRumbleJMCytoprotective effects of IAPs revealed by a small molecule antagonistBiochem J2009417376577118851715
- AllensworthJLSauerSJLyerlyHKMorseMADeviGRSmac mimetic Birinapant induces apoptosis and enhances TRAIL potency in inflammatory breast cancer cells in an IAP-dependent and TNF-alpha-independent mechanismBreast Cancer Res Treat2013137235937123225169
- KnightsAJFucikovaJPasamAKoernigSCebonJInhibitor of apoptosis protein (IAP) antagonists demonstrate divergent immunomodulatory properties in human immune subsets with implications for combination therapyCancer Immunol Immunother201362232133522923192
- WongHBudhaNRWestKDogs are more sensitive to antagonists of inhibitor of apoptosis proteins than rats and humans: a translational toxicokinetic/toxicodynamic analysisToxicol Sci2012130120521322843607
- EricksonRITarrantJCainGToxicity profile of small-molecule IAP antagonist GDC-0152 is linked to TNF-α pharmacologyToxicol Sci2013131124725822956632
- InfanteJRPorterDSenSKA phase I study of LCL161, an oral IAP inhibitor, in patients with advanced cancerPaper presented at: 101st Annual Meeting of the American Association for Cancer ResearchApril 17–21, 2010Washington, DC. Philadelphia, PAAACR2010 Abstract nr 2775
- EmeagiPUVan LintSGoyvaertsCProinflammatory characteristics of SMAC/DIABLO-induced cell death in antitumor therapyCancer Res20127261342135222379024
- YangCDavisJLZengRAntagonism of inhibitor of apoptosis proteins increases bone metastasis via unexpected osteoclast activationCancer Discov20133221222323269702
- TammIRichterSScholzFXIAP expression correlates with monocytic differentiation in adult de novo AML: impact on prognosisHematol J20045648949515570290
- Grzybowska-IzydorczykOCebulaBRobakTSmolewskiPExpression and prognostic significance of the inhibitor of apoptosis protein (IAP) family and its antagonists in chronic lymphocytic leukaemiaEur J Cancer201046480081020045309
- HussainARUddinSAhmedMPrognostic significance of XIAP expression in DLBCL and effect of its inhibition on AKT signallingJ Pathol2010222218019020632385
- LiMSongTYinZFNaYQXIAP as a prognostic marker of early recurrence of nonmuscular invasive bladder cancerChin Med J (Engl)2007120646947317439739
- ZhangYZhuJTangYX-linked inhibitor of apoptosis positive nuclear labeling: a new independent prognostic biomarker of breast invasive ductal carcinomaDiagn Pathol201164921645409
- LiuSSTsangBKCheungANAnti-apoptotic proteins, apoptotic and proliferative parameters and their prognostic significance in cervical carcinomaEur J Cancer20013791104111011378340
- XiangGWenXWangHChenKLiuHExpression of X-linked inhibitor of apoptosis protein in human colorectal cancer and its correlation with prognosisJ Surg Oncol2009100870871219777490
- MoussataDAmaraSSiddeekBXIAP as a radioresistance factor and prognostic marker for radiotherapy in human rectal adenocarcinomaAm J Pathol201218141271127822867709
- ShiYHDingWXZhouJExpression of X-linked inhibitor-of-apoptosis protein in hepatocellular carcinoma promotes metastasis and tumor recurrenceHepatology200848249750718666224
- AugelloCCarusoLMaggioniMInhibitors of apoptosis proteins (IAPs) expression and their prognostic significance in hepatocellular carcinomaBMC Cancer2009912519397802
- HiscuttELHillDSMartinSTargeting X-linked inhibitor of apoptosis protein to increase the efficacy of endoplasmic reticulum stress-induced apoptosis for melanoma therapyJ Invest Dermatol201013092250225820520630
- FerreiraCGvan der ValkPSpanSWExpression of X-linked inhibitor of apoptosis as a novel prognostic marker in radically resected non-small cell lung cancer patientsClin Cancer Res2001782468247411489828
- FerreiraCGvan der ValkPSpanSWAssessment of IAP (inhibitor of apoptosis) proteins as predictors of response to chemotherapy in advanced non-small-cell lung cancer patientsAnn Oncol200112679980511484955
- SeligsonDBHongoFHuerta-YepezSExpression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrenceClin Cancer Res200713206056606317947468
- KrajewskaMKrajewskiSBanaresSElevated expression of inhibitor of apoptosis proteins in prostate cancerClin Cancer Res20039134914492514581366
- RampUKriegTCaliskanEXIAP expression is an independent prognostic marker in clear-cell renal carcinomasHum Pathol20043581022102815297970
- YanYMahotkaCHeikausSDisturbed balance of expression between XIAP and Smac/DIABLO during tumour progression in renal cell carcinomasBr J Cancer20049171349135715328523
- MizutaniYNakanishiHLiYNOverexpression of XIAP expression in renal cell carcinoma predicts a worse prognosisInt J Oncol200730491992517332931
- GuLQLiFYZhaoLBRAFV600E mutation and X-linked inhibitor of apoptosis expression in papillary thyroid carcinomaThyroid200919434735419355825
- EspositoIKleeffJAbiatariIOverexpression of cellular inhibitor of apoptosis protein 2 is an early event in the progression of pancreatic cancerJ Clin Pathol200760888589516775116
- VallatLMagdelénatHMerle-BéralHThe resistance of B-CLL cells to DNA damage-induced apoptosis defined by DNA microarraysBlood2003101114598460612586635
- SilvaKLVasconcellosDVCastroEDCoelhoAMLindenRMaiaRCApoptotic effect of fludarabine is independent of expression of IAPs in B-cell chronic lymphocytic leukemiaApoptosis200611227728516502265
- PonnelleTChapusotCMartinLSubcellular expression of c-IAP1 and c-IAP2 in colorectal cancers: relationships with clinicopathological features and prognosisPathol Res Pract20031991172373114708638
- QiSMogiSTsudaHExpression of cIAP-1 correlates with nodal metastasis in squamous cell carcinoma of the tongueInt J Oral Maxillofac Surg200837111047105318621506
- StanculescuABembinsterLABorgenKBergamaschiAWileyEFrasorJEstrogen promotes breast cancer cell survival in an inhibitor of apoptosis (IAP)-dependent mannerHorm Cancer20101312713521152357
- AkagiTMotegiMTamuraAA novel gene, MALT1 at 18q21, is involved in t(11;18) (q21;q21) found in low-grade B-cell lymphoma of mucosa-associated lymphoid tissueOncogene199918425785579410523859
- DierlammJBaensMWlodarskaIThe apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomasBlood199993113601360910339464
- AnnunziataCMDavisREDemchenkoYFrequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myelomaCancer Cell200712211513017692804
- El-MesallamyHOHegabHMKamalAMExpression of inhibitor of apoptosis protein (IAP) livin/BIRC7 in acute leukemia in adults: correlation with prognostic factors and outcomeLeuk Res201135121616162221700335
- ChoiJHwangYKSungKWExpression of Livin, an antiapoptotic protein, is an independent favorable prognostic factor in childhood acute lymphoblastic leukemiaBlood2007109247147716990595
- GazzanigaPGradiloneAGiulianiLExpression and prognostic significance of LIVIN, SURVIVIN and other apoptosis-related genes in the progression of superficial bladder cancerAnn Oncol2003141859012488298
- WangXXuJJuSNiHZhuJWangHLivin gene plays a role in drug resistance of colon cancer cellsClin Biochem2010437–865566020171199
- WangTSDingQQGuoRHExpression of livin in gastric cancer and induction of apoptosis in SGC-7901 cells by shRNA-mediated silencing of livin geneBiomed Pharmacother201064533333819914791
- NachmiasBAshhabYBucholtzVCaspase-mediated cleavage converts Livin from an antiapoptotic to a proapoptotic factor: implications for drug-resistant melanomaCancer Res200363196340634914559822
- KimDKAlvaradoCSAbramowskyCRExpression of inhibitor-of-apoptosis protein (IAP) livin by neuroblastoma cells: correlation with prognostic factors and outcomePediatr Dev Pathol20058662162916328668
- NedelcuTKubistaBKollerALivin and Bcl-2 expression in highgrade osteosarcomaJ Cancer Res Clin Oncol2008134223724417632732
- KempkensteffenCHinzSChristophFExpression of the apoptosis inhibitor livin in renal cell carcinomas: correlations with pathology and outcomeTumour Biol200728313213817519534
- HaferkampABedkeJVetterCHigh nuclear Livin expression is a favourable prognostic indicator in renal cell carcinomaBJU Int2008102111700170618990137
- KempkensteffenCHinzSKrauseHExpression of splicing variants of the inhibitor of apoptosis livin in testicular germ cell tumorsTumour Biol2008292768218515985
- PlutaAWrzesien-KusACebula-ObrzutBInfluence of high expression of Smac/DIABLO protein on the clinical outcome in acute myeloid leukemia patientsLeuk Res201034101308131320061022
- MizutaniYKatsuokaYBonavidaBLow circulating serum levels of second mitochondria-derived activator of caspase (Smac/DIABLO) in patients with bladder cancerInt J Oncol20124041246125022218530
- PlutaPCebula-ObrzutBEhemannVCorrelation of Smac/DIABLO protein expression with the clinico-pathological features of breast cancer patientsNeoplasma201158543043521744997
- Arellano-LlamasAGarciaFJPerezDHigh Smac/DIABLO expression is associated with early local recurrence of cervical cancerBMC Cancer2006625617067390
- EndoKKohnoeSWatanabeAClinical significance of Smac/DIABLO expression in colorectal cancerOncol Rep200921235135519148507
- DobrzyckaBTerlikowskiSJBernaczykPSPrognostic significance of smac/DIABLO in endometrioid endometrial cancerFolia Histochem Cytobiol201148467868121478115
- SekimuraAKonishiAMizunoKExpression of Smac/DIABLO is a novel prognostic marker in lung cancerOncol Rep200411479780215010875
- MizutaniYKatsuokaYBonavidaBPrognostic significance of second mitochondria-derived activator of caspase (Smac/DIABLO) expression in bladder cancer and target for therapyInt J Oncol201037250350820596678
- ShibataTMahotkaCWethkampNHeikausSGabbertHERampUDisturbed expression of the apoptosis regulators XIAP, XAF1, and Smac/DIABLO in gastric adenocarcinomasDiagn Mol Pathol20071611817471152
- KempkensteffenCHinzSChristophFExpression levels of the mitochondrial IAP antagonists Smac/DIABLO and Omi/HtrA2 in clear-cell renal cell carcinomas and their prognostic valueJ Cancer Res Clin Oncol2008134554355017922292
- OuyangYQzur HausenAOrlowska-VolkMExpression levels of hnRNP G and hTra2-beta1 correlate with opposite outcomes in endometrial cancer biologyInt J Cancer201112892010201920607830
- YangXXingHGaoQRegulation of HtrA2/Omi by X-linked inhibitor of apoptosis protein in chemoresistance in human ovarian cancer cellsGynecol Oncol200597241342115863139
- HuXYXuYMChenXCPingHChenZHZengFQImmunohistochemical analysis of Omi/HtrA2 expression in prostate cancer and benign prostatic hyperplasiaAPMIS20061141289389817207090
- HuXChenXPingHChenZZengFLuGImmunohistochemical analysis of Omi/HtrA2 expression in prostate cancer and benign prostatic hyperplasiaJ Huazhong Univ Sci Technolog Med Sci200525667167316696322
- LeeSHLeeJWKimHSImmunohistochemical analysis of Omi/HtrA2 expression in stomach cancerAPMIS2003111558659012887511
- Zurawa-JanickaDKobielaJGalczynskaNChanges in expression of human serine protease HtrA1, HtrA2 and HtrA3 genes in benign and malignant thyroid tumorsOncol Rep20122851838184422923201
- NarkiewiczJLapinska-SzumczykSZurawa-JanickaDSkorko-GlonekJEmerichJLipinskaBExpression of human HtrA1, HtrA2, HtrA3 and TGF-beta1 genes in primary endometrial cancerOncol Rep20092161529153719424634
- NarkiewiczJKlasa-MazurkiewiczDZurawa-JanickaDSkorko-GlonekJEmerichJLipinskaBChanges in mRNA and protein levels of human HtrA1, HtrA2 and HtrA3 in ovarian cancerClin Biochem2008417–856156918241672
- GottfriedYVoldavskyEYodkoLSaboEBen-ItzhakOLarischSExpression of the pro-apoptotic protein ARTS in astrocytic tumors: correlation with malignancy grade and survival rateCancer2004101112614262115517578
- ElhasidRSaharDMerlingAMitochondrial pro-apoptotic ARTS protein is lost in the majority of acute lymphoblastic leukemia patientsOncogene200423325468547515122323
- CarterBZMakDHMorrisSJXIAP antisense oligonucleotide (AEG35156) achieves target knockdown and induces apoptosis preferentially in CD34+38– cells in a phase 1/2 study of patients with relapsed/refractory AMLApoptosis2011161677420938744
- ViertlDPerillo-AdamerFRigottiSThe SMAC-mimetic Debio 1143 efficiently enhanced chemo and radiotherapy in head and neck squamous cell carcinoma modelsPaper presented at: 140th Annual Meeting of the American Association for Cancer ResearchApril 6–10, 2013Washington, DC. Philadelphia, PAAACR2013 Abstract nr 2055
- MaWWZhangHHylanderBTL32711, a novel Smac mimetic, exerts significant antitumor efficacy in primary pancreatic adenocarcinoma modelPaper presented at: 103rd Annual Meeting of the American Association for Cancer ResearchMarch 31–April 4, 2012Chicago, IL. Philadelphia, PAAACR2012 Abstract nr 1939
- SmithMACarolHEvansKBirinapant (TL32711), a small molecule Smac mimetic, induces regressions in childhood acute lymphoblastic leukemia (ALL) xenografts that express TNFα and synergizes with TNFα in vitro – a report from the Pediatric Preclinical Testing Program (PPTP)Blood20121203565
- CernaJDDonnaCNaokoTNormanCMAMStephenYRadiosensitization of GBM by a novel peptidomimetic of the second mitochondria-derived activator of caspases (SMAC)Paper presented at: 104th Annual Meeting of the American Association for Cancer ResearchApril 6–10, 2013Washington, DC. Philadelphia, PAAACR2013 Abstract nr 1599
- BenetatosCABurnsJMBordenECThe Smac mimetic Birinapant synergistically induces apoptosis in combination with type i interferons and GM-CSFPaper presented at: 104th Annual Meeting of the American Association for Cancer ResearchApril 6–10, 2013Washington, DC. Philadelphia, PAAACR2013 Abstract 3336
- AmaravadiRKSchilderRJDyGKPhase 1study of the Smac mimetic TL32711 in adult subjects with advanced solid tumors and lymphoma to evaluate safety, pharmacokinetics, pharmacodynamics, and antitumor activityPaper presented at: 102nd Annual Meeting of the American Association for Cancer ResearchApril 2–6, 2011Orlando, FL. Philadelphia, PAAACR2010 Abstract nr LB-406
- FlygareJABeresiniMBudhaNDiscovery of a potent small-molecule antagonist of inhibitor of apoptosis (IAP) proteins and clinical candidate for the treatment of cancer (GDC-0152)J Med Chem20125594101411322413863
- EricksonRITarrantJCainGToxicity profile of small-molecule IAP antagonist GDC-0152 is linked to TNF-α pharmacologyToxicol Sci2013131124725822956632