Abstract
Since its first description in Drosophila by Drs Nusslein-Volhard and Wieschaus in 1980, hedgehog (Hh) signaling has been implicated in regulation of cell differentiation, proliferation, tissue polarity, stem cell maintenance, and carcinogenesis. The first link of Hh signaling to cancer was established through studies of Gorlin syndrome in 1996 by two independent teams. Later, it was shown that Hh signaling may be involved in many types of cancer, including skin, leukemia, lung, brain, and gastrointestinal cancers. In early 2012, the US Food and Drug Administration approved the clinical use of Hh inhibitor Erivedge/vismodegib for treatment of locally advanced and metastatic basal cell carcinomas. With further investigation, it is possible to see more clinical applications of Hh signaling inhibitors. In this review, we will summarize major advances in the last 3 years in our understanding of Hh signaling activation in human cancer, and recent developments in preclinical and clinical studies using Hh signaling inhibitors.
Introduction
Remarkable progress has been made since the hedgehog (Hh) mutant phenotype was first described in fruit fly in 1980.Citation1 Three vertebrate homologues of Hh and their receptors were identified in the 1990s.Citation2–Citation6 As an essential pathway during development, the Hh pathway is critical for maintaining tissue polarity and stem cell population. The first link between Hh signaling and cancer was shown in tumor-prone Gorlin syndrome in 1996.Citation7–Citation11 In early 2012, Hh signaling inhibitor GDC-0449 (Erivedge/vismodegib; Hoffmann-La Roche Ltd, Basel, Switzerland) was approved by the US Food and Drug Administration for treatment of locally advanced and metastatic basal cell carcinomas (BCCs).
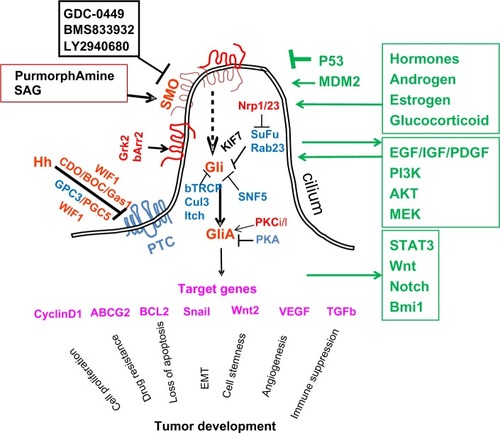
The general signaling mechanisms of the Hh pathway are conserved from flies to humans.Citation12 Mammalian Hh signaling molecules include ligands (sonic Hh, Indian Hh, and desert Hh), patched receptors (PTCH1, PTCH2), signal transducer smoothened (SMO), and transcription factors (Gli1, Gli2, Gli3) (see ). In the absence of ligands, SMO serves as the key signal transducer, whose function is inhibited by another transmembrane protein patched (PTCH1). Upon binding of an active Hh ligand, this inhibition is released, allowing SMO to signal downstream, eventually leading to activation of Gli transcription factors. Gli molecules can bind the specific consensus sequences located in the promoter region of the target genes to regulate target gene expression.Citation13,Citation14
Figure 1 A diagram of hedgehog (Hh) signaling in mammalian cells. Smoothened (SMO) is the key signal transducer of the Hh pathway. In the absence of the Hh ligands, Hh receptor patched (PTC) is thought to be localized in the cilium to inhibit SMO signaling. Coreceptors of Hh include CDO (cell adhesion molecule-related/down regulated by oncogenes), brother of CDO (BOC), Gas1, glypican 3, (GPC3), and GPC5. Wnt inhibitory factor-1 (WIF1) can also regulate Hh signaling through association with CDO, BOC, or GPC5. Gli molecules are processed with the help of suppressor of fused (SuFu)/KIF7, β-TRCP molecules into repressor forms, which turn off the Hh signaling pathway. Other negative regulators of Gli molecules include Rab23, protein kinase A (PKA), SuFu, tumor suppressor sucrose nonfermenting 5 (SNF5), Culin 3 (Cul3), and itchy E3 ubiquitin ligase (Itch) through regulation Gli protein modifications, nuclear–cytoplasm shuttling, as well as transcriptional activities. In the presence of Hh, PTC is thought to be shuttled out of cilium and is unable to inhibit SMO. The ciliary localization of SMO is thought to require β-arrestin 2 (βArr2), and G protein coupled receptor kinase 2 (GRK2). Hh reception promotes SMO conformational changes to form dimers. Gli molecules are now processed to active forms (GliA), which will activate the Hh target genes. This process can be inhibited by KIF7 and SuFu. Protein kinase C isoform ι/λ0 (PKCι/λ) is known to positively regulate Gli transcriptional activity. Positive regulators are in red, negative regulators are in blue, and target genes are in pink. KIF7 can function (in black) as a negative regulator or a positive regulator. The interacting pathways with the Hh pathway are in green. Although the role of cilium for Hh signaling during embryonic development is well established, cancer cells generally lack cilia. It has been demonstrated that lack of cilia prevents development of basal cell carcinomas in mice. It is not clear whether this is true for all other types of Hh signaling-associated cancer.
In the last 3 years, there has been significant progress regarding Hh signaling and its significance in cancer development and therapeutics. The total number of publications on Hh signaling in the last 3 years is close to 30% of all Hh-related publications, and progress has been made in the following areas: 1) better understanding of Hh signal transduction and the associated target genes, 2) more reliable mouse models linking Hh signaling to human malignancies, 3) better understanding of Hh signaling mechanisms during cancer development and metastasis, 4) an increasing number of clinical and preclinical studies on cancer treatment using Hh signaling inhibitors, and 5) emerging evidence of Hh signaling in supporting residual cancer cells and cancer stem cells.
Signal transduction of the Hh pathway
All Hh proteins are secreted molecules, functioning at short range on nearby cells or at long range to distant cells during development.Citation15–Citation17 Hh protein precursors undergo post-translational modifications (autocleavage to release the N-terminal fragment [HhN], covalently binding to a cholesterol moiety at the C-terminal end, and palmitoylation by a palmitoylacyltransferase at the N-terminus of HhN).Citation18–Citation21 Molecules involved in Hh protein transport and distribution include the transmembrane transporter-like protein dispatched (Disp),Citation22–Citation24 metalloproteinases,Citation25 the heparan sulfate proteoglycans Dally-like (Dlp) and DallyCitation26,Citation27 or their regulators,Citation28 as well as enzymes such as sulfateless and tout velu.Citation29–Citation31
shows the mammalian Hh signaling pathway with major players in the diagram. Several molecules are engaged in reception of Hh ligands, with patched (PTC, one PTC in fly, and two PTCs in vertebrates: PTCH1 and PTCH2) as the major receptor.Citation32 Studies from cultured cells indicate that PTC inhibits SMO at a substochiometric concentration.Citation33 Hh-interacting protein (HIP) can compete with PTC on Hh binding, resulting in negative regulation of Hh signaling.Citation34 On the other hand interference Hh or its vertebrate homologues cell adhesion molecule related/downregulated by oncogenes (CDO) and BOC (brother of CDO), GAS1, and glypican-3 (GPC3) serve as coreceptors of Hh.Citation35–Citation42 In contrast to the inhibitory effect of glypican-3, glypican-5 (GPC5) and other heparan sulfate proteoglycans are shown to stimulate Hh signaling by promoting binding of sonic Hh to PTCH1.Citation43,Citation44 The effect of GPC5 and interference Hh homologues requires another secreted extracellular molecule: Wnt inhibitory factor-1 (WIF1).Citation45,Citation46 It is still not entirely clear how binding of Hh proteins results in the pathway activation. It is proposed that PTC limits SMO signaling via transporting endogenous small molecules specifically targeted to SMO. Candidates of these small molecules include PI4P, lipoproteins, and provitamin D3.Citation47–Citation50 It is currently not very clear how these molecules regulate SMO signaling.
It is now known that glucocorticoid molecules can modulate SMO signaling through regulating its ciliary localization.Citation51 Several recent reports support SMO to G protein coupling,Citation52–Citation55 but the physiological relevance of the G protein coupling of SMO in carcinogenesis has not been convincingly demonstrated. Gα can also regulate Gli proteins independent of SMO.Citation56 It is quite clear that two important events occur during SMO signaling in mammalian cells. First, SMO protein undergoes conformational change to favor SMO signaling,Citation57 although the regulatory mechanism underlying this conformational change is not clear. Second ciliary translocation of mammalian SMO protein is critical for Hh signaling.Citation58–Citation63 Several reports now link neuropilin 1/2 (Nrp1/2) to SMO signaling.Citation64–Citation67
Several molecules are identified to be genetically downstream of SMO in Drosophila, including COS2, suppressor of fused (SuFu), and fused. A COS2 homologue, kinesin like-protein KIF7, functions in the Hh pathway but not directly associated with SMO,Citation68–Citation72 suggesting that KIF7 does not contain all COS2 functions in vertebrates. In contrast, the phenotype of fused−/− mice is very different from Shh null mice,Citation73–Citation75 indicating that fused is not critical for Hh signaling during early embryonic development in mice.
In addition to the Drosophila homologues, mammalian cells have several novel cytoplasmic regulators of Hh signaling, including Rab23Citation76 and tectonic.Citation77 Rab23 and tectonic are all negative regulators downstream of SMO. We have shown that Rab23 is involved in Gli–SuFu interactionCitation78 (see ). Unlike many Rab proteins, we found that Rab23 is localized both in the nucleus and in cytoplasm,Citation79 suggesting that Rab23 may have other unrevealed functions apart from membrane trafficking.
The ultimate effect of Hh signaling is activation of downstream Gli transcription factors, which regulate target genes by directly binding a consensus binding site (5′-tgggtggtc-3′) in the promoter.Citation13,Citation14,Citation80,Citation81 The activity of Gli transcription factors can be regulated at several levels. First, nuclear–cytoplasmic shuttling of Gli molecules is tightly regulated.Citation82–Citation85 Protein kinase A can retain Gli1 protein in the cytoplasm via a protein kinase A site in the nuclear localization signal domain,Citation83 whereas activated Ras signaling induces Gli nuclear localization.Citation85 Second ubiquitination, acetylation, and protein degradation of Gli molecules are regulated by several distinct mechanisms, including β-TRCP-, cul3/BTB-, and numb/itch-mediated Gli ubiquitination, sumoylation, and acetylation.Citation86–Citation93 In addition, Gli3 (Gli2 to a lesser extent) can be processed into transcriptional repressors, which may be mediated by the β-TRCP E3 ligase.Citation88,Citation94 SuFu not only prevents nuclear translocation of Gli molecules but also inhibits Gli1-mediated transcriptional activity.Citation95–Citation97 Other mechanisms to modify Gli functions include interaction with a negative regulator sucrose nonfermenting 5 (SNF5)Citation98 and a positive regulator protein kinase C isoform ι/λ.Citation99
Several feedback regulatory loops exist in this pathway to maintain a certain level of Hh signaling in a given cell. PTC, HIP, GAS1, neuropilins, and Gli1 are components, as well as the target genes of this pathway. PTC and HIP provide negative feedback regulation, whereas Gli1 and Nrp1/2 form positive regulatory loops. On the other hand GAS1 is downregulated by the Hh pathway but is a positive regulator for Hh signaling.Citation100 Alterations of these loops would lead to abnormal signaling of this pathway, such as inactivation of PTCH1 in BCCs.
Activation of the Hh pathway in human cancer
The initial link between Hh signaling and human cancers was made from the discovery that mutations of human PTCH1 are associated with a rare and hereditary form of BCC, basal cell nevus syndrome (BCNS) (also Gorlin syndrome).Citation101–Citation103 Gorlin syndrome is a rare autosomal genetic disease with two distinct sets of phenotypes: an increased risk of developing cancers such as BCCs, medulloblastomas, rhabdomyosarcomas, and meningiomas, as well as developmental defects such as bifid ribs and ectopic calcification.Citation104
Almost all BCCs and about 30% of medulloblastomas have activated Hh signaling via gene mutations in PTCH1, SMO, or other Hh pathway molecules.Citation105–Citation109 In addition, cancers associated with Gorlin syndrome, including rhabdomyosarcomaCitation110,Citation111 and meningiomas,Citation112–Citation114 are reported to have gene mutations in the Hh signaling pathway or elevated Hh target gene expression. Activated Hh signaling has been detected in a variety of human cancer types, either in the tumor or in the stroma.Citation100,Citation115–Citation117
Genetically engineered mice with Ptch1 and Smo genes have generated more convincing evidence for the critical role of Hh signaling in cancer. In addition to BCCs and medulloblastomas, rhabdomyosarcomas develop in mice with expression of oncogenic SmoM2 or knockout of Ptch1.Citation118–Citation121 One surprising finding from tissue-specific Ptch1 knockout is the development of gastrointestinal stromal-like tumors (GIST),Citation122 suggestive of a role of Hh signaling in GIST. Even in the situation of a small cell lung cancer (SCLC) mouse model, expression of oncogenic SmoM2 increases the tumor number, whereas Smo knockout reduces the tumor number.Citation123 Recent study of Barrett’s esophagus indicates that sonic Hh expression in the epithelium of the esophagus can lead to stromal expression of Hh signaling target genes, which is similar to the human situation.Citation124,Citation125 In contrast, tissue-specific expression of oncogenic Smo molecule SmoM2 has no effects on K-Ras-induced pancreatic cancerCitation126 or on prostate cancer.Citation127 The negative data, however, do not rule out the promoting effects of Hh signaling for tumor metastasis, a major factor for cancer mortality. Currently, there are only a limited number of mouse models for cancer metastasis. Even for the available mouse models for cancer metastasis, several variable factors make cancer metastasis models less robust, and these factors include mouse genetic backgrounds, low incidence, and long duration to observe metastasis in mice.
Hh signaling in tumor initiation, promotion, and metastases
Hh signaling plays different roles in different types of cancer.Citation100 Based on the published data, we attempt to divide the functions of Hh signaling during cancer development into three types: the tumor driver, the tumor promoter, or the regulator for residual cancer cells after therapy. For example, activated Hh signaling can drive development of BCCs, medulloblastomas, rhabdomyosarcoma, GIST, and Barrett’s esophagus,Citation118,Citation119,Citation122,Citation124,Citation128,Citation129 and Hh signaling in these lesions serves as the tumor driver, at least in the mouse models. For SCLC, Hh signaling can promote cancer development but is not sufficient to drive tumor formation, and thus serves as a tumor promoter.Citation123 In pancreatic cancer, inhibition of Hh signaling does not affect tumor formation but can promote tumor metastasis.Citation130–Citation137 For other cancer types, Hh signaling may regulate the number of cancer stem cells or the tumor microenvironment, such as leukemia and liver cancer.Citation138,Citation139 As more in vivo data are available, we predict more revelation of the tumor promoting role of Hh signaling. Tumor recurrence after therapy is a major issue in clinical care of cancer patients, such as chemotherapy or radiotherapy resistance, and will be discussed in “Hh signaling, cancer stem cell, and residual cancer cells.” For some cancer types, Hh signaling may not have any roles to play.
Activation of Hh signaling does not work in isolation but rather crosstalks with other signaling pathways during cancer development and metastasis. Earlier studies indicated that Ptch1+/− mice with P53 knock out all developed medulloblastomas, whereas <30% of Ptch1+/− mice (with wild-type P53) had this type of tumor.Citation140 We have shown that skin-specific knockout of Stat3 or its upstream activator Il11ra significantly reduced Hh signaling-mediated BCC formation.Citation141 Increasing data have indicated close collaboration between Hh signaling and growth factor signaling pathways. Our earlier work indicated that platelet-derived growth factor α (PDGFRα) is regulated by Hh signaling and is responsible for cell proliferation in BCCs.Citation142 Now more links are reported between Hh and other pathways, including epidermal growth factor, insulin growth factor, transforming growth factor β (TGFβ), mTOR/S6K1, RACK1, notch, and protein kinase C.Citation100,Citation143–Citation151 Although some of these molecules are involved in regulation of tumor microenvironment, such as TGFβ, others are known to regulate cancer stem cells, such as PDGFRα and notch. We will have more discussion on cancer stem cells in “Hh signaling, cancer stem cell, and residual cancer cells.”
Increasing evidence indicates that Hh signaling plays an important role during tumor metastasis in several types of cancer, such as pancreatic and breast cancers.Citation135,Citation152 Studies from many groups indicate activation of Hh signaling in the stromal as well as tumor compartments in metastatic pancreatic cancer.Citation130,Citation133–Citation137,Citation153 In fact, Hh signaling inhibitors are effective in suppressing tumor metastases of pancreatic cancer.Citation135 Hh signaling also regulates bone homeostasis as well as bone metastasis in breast cancer independent of the Hh ligands.Citation143 During tumor metastasis, Hh signaling activation is observed both in the tumor compartment and in the stroma.Citation135 The molecules mediating Hh’s metastatic functions remain largely untested, but there are reports to indicate the following molecules: snail, TGFβ, Wnt, HGF, and muc5 AC.Citation135,Citation154–Citation157 Further studies will be needed to understand the molecular basis by which Hh signaling mediates cancer metastases.
Hh signaling inhibitors: preclinical and clinical studies
More than 200 compounds have been disclosed to have inhibitory effects on Hh signaling. Of these, eight have been used for clinical trials (see for the list). There are three major targeting sites for Hh signaling inhibitors identified so far: Hh molecules (Shh neutralizing antibodies, small molecule Robotnikinin), SMO protein (cyclopamine and its derivatives IPI-926 and CycT, and synthetic compounds GDC-0449, XL-139/BMS833923, LDE-225, PF04449913, and LY2940680), and Gli inhibitors (HPI-1, HPI-2, GANT-56, and GANT-61).Citation100 The major advances include successful clinical trials using GDC-0449 and US Food and Drug Administration approval of GDC-0449 for treatment of locally advanced and metastatic BCCs. However, combination of Hh signaling inhibitors with gemcitabine or Hh signaling inhibitors alone did not show any improvements in the outcomes of pancreatic cancer patients. We summarize these data below.
Table 1 A list of hedgehog signaling inhibitors in clinical trials (from http://clinicaltrials.gov)Table Footnotea
shows the list of Hh signaling inhibitors in clinical trials, with all eight small molecules targeting SMO. Clinical trials with GDC-0449 in BCCs are the most successful. The successful Phase II clinical trials were preceded with a remarkable Phase I clinical trial in patients with metastatic BCCs.Citation158 This drug is well tolerated by patients.Citation159–Citation161 Two independent groups used GDC-0449 to treat BCNS patients and sporadic BCCs, respectively, via oral administration. Although the overall outcomes were very encouraging, the responses of two groups of patients were quite different. Although BCNS patients had virtually a 100% response rate, sporadic BCCs had only a 33% response rate. Previous studies in mouse models indicate that tumors acquire somatic mutations in Smo or other signaling pathways following GDC-0449 administration,Citation162 which may explain why not all sporadic BCCs responded well. A more rational way to treat sporadic BCCs is topical application. Two groups (one from Novartis AG and one from Hoffmann-La Roche Ltd/Genentech) indeed tested that possibility with BCNS patients and obtained impressive responses.Citation163,Citation164 Mechanisms to Smo antagonist resistance include mutations in the target SMO gene or alterations in the PI3K pathway.Citation165,Citation166 Several ways have been explored to mitigate drug resistance to SMO antagonists, such as itraconazole and arsenic trioxide, polymeric nanoparticle-encapsulated Hh signaling inhibitors, or vitamin D3.Citation167–Citation170 Hopefully, some of these combined treatments will provide benefits to BCC patients.
Studies in animal models demonstrated significant inhibition of Hh signaling inhibitors on medulloblastoma development. For example, oral administration of IPI-926 or PF-5274857 can reduce tumor development, leading to a longer lifespan in mouse medulloblastoma models.Citation171,Citation172 However, an early clinical trial on a medulloblastoma patient using GDC-0449 yielded only a transient therapeutic effect, due to an SMO mutation occurring soon after treatment.Citation173 The outcome data of current medulloblastoma clinical trials are not available, but there is still a high expectation.
There is evidence to support that rhabdomyosarcoma is very responsive to Hh signaling inhibitors. First, gene expression analyses revealed elevated Hh target gene expression in embryonic rhabdomyosarcomas.Citation111 Second, preliminary studies used forskolin or SMO inhibitor to shrink tumors in mouse models.Citation174 In addition, evidence for Hh signaling in meningiomas and SCLC is quite clear, and Hh signaling inhibitors should be effective in these tumor types as well.
Hh signaling, cancer stem cell, and residual cancer cells
Increasing evidence indicates that Hh signaling is critical for cancer stem cell maintenance and function.Citation138,Citation175,Citation176 For example, leukemia stem cell maintenance and expansion are dependent on Hh signaling.Citation138,Citation175 The effect of Hh signaling on a normal hematopoietic stem cell population, however, is still quite controversial, with some showing effects but others with no effects.Citation138,Citation177–Citation180 Based on cancer stem cell theory, it is anticipated that Hh signaling activation exerts resistance to cancer chemotherapy and radiotherapy.Citation181 Several studies have indeed shown that Hh signaling activation is associated with chemotherapy or radiotherapy resistance.Citation182–Citation183 Hh signaling inhibitor IPI-926 enhances delivery of the chemotherapeutical drug gemcitabine in a mouse model of pancreatic cancer. Relevance to the cancer stem cell theory is the link between Hh signaling activation and cancer relapse from drug resistance.
Based on the published data, we propose that Hh signaling may help maintain the stemness of cancer stem cells, which are generally insensitive to chemotherapy and radiotherapy. There is evidence to indicate that Hh signaling regulates expression of cancer stem cell-related markers, such as aldehyde dehydrogenase, Bmi1, snail, Wnt2, PDGFRα, jagged-1, CD44, and c-MET.Citation135,Citation155,Citation184–Citation188 The level of Hh expression is often higher in the cancer stem cell population in several cancer types.Citation189–Citation193 Thus, we have reasons to believe that inhibition of Hh signaling may be effective in reducing the number of cancer stem cells, which may play an important role in chemotherapy and radiotherapy resistance.
Chemotherapy and radiotherapy play an important role in the clinical care of cancer patients, but resistance to these treatments remains a major obstacle in cancer patient care. Recent studies revealed a few examples for the role of Hh signaling in chemotherapy and radiotherapy resistance. Resistance to docetaxel is a major clinical challenge for prostate cancer patients. A recent study revealed an important role of Hh signaling on docetaxel resistance in prostate cancer.Citation194 Combination of notch and Hh signaling inhibitors was able to reverse docetaxel resistance both in cultured cells and in xenografts. Activation of Hh signaling via PI3 K is also reported in tamoxifen-resistant breast cancer,Citation195 and a combination of Hh signaling inhibitor GDC-0449 with tamoxifen significantly reduced cell colony formation and tumor development in xenografts. In addition, activated Hh signaling is shown to be responsible for drug resistance in ovarian cancer, cervical cancer, and myeloid leukemic cells.Citation196–Citation198 A recent study also suggests that Hh signaling may be associated with antiepidermal growth factor receptor therapy (targeted therapy) resistance observed in head and neck cancer.Citation144 The exact mechanisms by which Hh signaling activation confers drug resistance are not entirely clear, but it is reported that Hh signaling can regulate expression of several drug resistance-related genes such as ABCG2 and MDR.Citation198,Citation199 The cancer stem cell theory can also explain some of the mechanisms.
Overcoming recurrence to radiotherapy is also very challenging, but recent studies suggest that inhibiting Hh signaling may help mitigate radiotherapy resistance in pancreatic and head/neck cancer. For pancreatic cancer, we found that a combination of Hh signaling inhibitor BMS833932 (see for details) and radiation could significantly reduce the number of lymph node metastasis.Citation135 Similarly, high expression of Gli1 is reported to be associated with lymph node metastases and tumor progression after radiotherapy in squamous cell carcinomas of the head/neck.Citation200
Summary and future perspectives
In summary, the link of Hh signaling activation to a variety of human cancer implies the relevance of studying Hh signaling to human health. Rapid advancement in the discovery of novel Hh signaling inhibitors has provided many opportunities for developing novel cancer therapeutic strategies. It is not surprising to learn that several major challenges still exist to prevent the use of Hh signaling inhibitors in clinics. These challenges include a lack of basic understanding of the molecular mechanisms by which Hh signaling mediates carcinogenesis; no clear criteria to identify the right tumors for therapeutic application; only a few reliable, physiologically relevant, and reproducible mouse models for cancer metastases to test and optimize drug dosages in order to minimize side effects; and a lack of clear strategies to mitigate drug resistance. Over the last 3 years, research in this area has greatly improved, as indicated in this review. It is anticipated that additional novel therapeutic strategies will be developed for cancer clinical trials using Hh signaling inhibitors in the next few years.
Acknowledgments
Current research in the authors’ laboratory is supported by grants from the National Cancer Institute (CA94160, CA155086), Riley Children’s Foundation, and Wells Center for Pediatric Research. Due to space limit, we could not include many important findings in this review but want to take this opportunity to thank all the investigators in this field for their work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Nusslein-VolhardCWieschausEMutations affecting segment number and polarity in DrosophilaNature198028757857958016776413
- KraussSConcordetJPInghamPWA functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryosCell1993757143114448269519
- EchelardYEpsteinDJSt-JacquesBSonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarityCell1993757141714307916661
- RiddleRDJohnsonRLLauferETabinCSonic hedgehog mediates the polarizing activity of the ZPACell1993757140114168269518
- ChangDTLópezAvon KesslerDPProducts, genetic linkage and limb patterning activity of a murine hedgehog geneDevelopment199412011333933537720571
- RoelinkHAugsburgerAHeemskerkJFloor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochordCell19947647617758124714
- EpsteinEHBasal cell carcinomas: attack of the hedgehogNat Rev Cancer200881074375418813320
- XieJHedgehog signaling in prostate cancerFuture Oncol20051333133816556007
- XieJHedgehog signaling pathway: development of antagonists for cancer therapyCurr Oncol Rep200810210711318377823
- XieJMolecular biology of basal and squamous cell carcinomasAdv Exp Med Biol200862424125118348461
- JiangJHuiCCHedgehog signaling in development and cancerDev Cell200815680181219081070
- InghamPWPlaczekMOrchestrating ontogenesis: variations on a theme by sonic hedgehogNat Rev Genet200671184185017047684
- SasakiHHuiCNakafukuMKondohHA binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitroDevelopment19971247131313229118802
- KinzlerKWVogelsteinBThe Gli gene encodes a nuclear protein which binds specific sequences in the human genomeMol Cell Biol19901026346422105456
- McMahonAPInghamPWTabinCJDevelopmental roles and clinical significance of hedgehog signalingCurr Top Dev Biol200353111412509125
- InghamPWMcMahonAPHedgehog signaling in animal development: paradigms and principlesGenes Dev200115233059308711731473
- TaipaleJBeachyPAThe hedgehog and Wnt signalling pathways in cancerNature2001411683534935411357142
- LeeJJEkkerSCvon KesslerDPPorterJASunBIBeachyPAAutoproteolysis in hedgehog protein biogenesisScience19942665190152815377985023
- PorterJAYoungKEBeachyPACholesterol modification of hedgehog signaling proteins in animal developmentScience199627452852552598824192
- PorterJAvon KesslerDPEkkerSCThe product of hedgehog autoproteolytic cleavage active in local and long-range signallingNature199537465203633667885476
- BuglinoJAReshMDHhat is a palmitoylacyltransferase with specificity for N-palmitoylation of sonic hedgehogJ Biol Chem200828332220762208818534984
- KawakamiTKawcakTLiYJZhangWHuYChuangPTMouse dispatched mutants fail to distribute hedgehog proteins and are defective in hedgehog signalingDevelopment2002129245753576512421714
- MaYErknerAGongRHedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatchedCell20021111637512372301
- CasparyTGarcía-GarcíaMJHuangfuDMouse dispatched homolog1 is required for long-range, but not juxtacrine, Hh signalingCurr Biol200212181628163212372258
- DierkerTDreierRPetersenABordychCGrobeKHeparan sulfate-modulated, metalloprotease-mediated sonic hedgehog release from producing cellsJ Biol Chem2009284128013802219176481
- BeckettKFranch-MarroXVincentJPGlypican-mediated endocytosis of hedgehog has opposite effects in filies and miceTrends Cell Biol200818836036318603427
- LumLYaoSMozerBIdentification of hedgehog pathway components by RNAi in Drosophila cultured cellsScience200329956152039204512663920
- Baena-LopezLARodriguezIBaonzaAThe tumor suppressor genes dachsous and fat modulate different signalling pathways by regulating dally and dally-likeProc Natl Acad Sci U S A2008105289645965018621676
- ToyodaHKinoshita-ToyodaAFoxBSelleckSBStructural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu. Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymesJ Biol Chem200027529218562186110806213
- BellaicheYTheIPerrimonNTout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusionNature1998394668885889665133
- KozielLKunathMKellyOGVortkampAExt1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossificationDev Cell20046680181315177029
- StoneDMHynesMArmaniniMThe tumour-suppressor gene patched encodes a candidate receptor for sonic hedgehogNature199638466051291348906787
- TaipaleJCooperMKMaitiTBeachyPAPatched acts catalytically to suppress the activity of smoothenedNature2002418690089289712192414
- ChuangPTMcMahonAPVertebrate hedgehog signalling modulated by induction of a hedgehog-binding proteinNature1999397672061762110050855
- MartinelliDCFanCMGas1 extends the range of hedgehog action by facilitating its signalingGenes Dev200721101231124317504940
- SeppalaMDepewMJMartinelliDCFanCMSharpePTCobourneMTGas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehogJ Clin Invest200711761575158417525797
- AllenBLTenzenTMcMahonAPThe hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse developmentGenes Dev200721101244125717504941
- OkadaACharronFMorinSBoc is a receptor for sonic hedgehog in the guidance of commissural axonsNature2006444711736937317086203
- TenzenTAllenBLColeFKangJSKraussRSMcMahonAPThe cell surface membrane proteins Cdo and Boc are components and targets of the hedgehog signaling pathway and feedback network in miceDev Cell200610564765616647304
- ZhangWKangJSColeFYiMJKraussRSCdo functions at multiple points in the sonic hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephalyDev Cell200610565766516647303
- YaoSLumLBeachyPThe ihog cell-surface proteins bind hedgehog and mediate pathway activationCell2006125234335716630821
- CapurroMIXuPShiWLiFJiaAFilmusJGlypican-3 inhibits hedgehog signaling during development by competing with patched for hedgehog bindingDev Cell200814570071118477453
- LiFShiWCapurroMFilmusJGlypican-5 stimulates rhabdomyosarcoma cell proliferation by activating hedgehog signalingJ Cell Biol2011192469170421339334
- WittRMHechtMLPazyra-MurphyMFHeparan sulfate proteoglycans containing a glypican 5 core and 2-O-sulfo-iduronic acid function as sonic hedgehog co-receptors to promote proliferationJ Biol Chem201328836262752628823867465
- Sanchez-HernandezDSierraJOrtigao-FariasJRGuerreroIThe WIF domain of the human and Drosophila Wif-1 secreted factors confers specificity for Wnt or hedgehogDevelopment2012139203849385822951645
- AvanesovAHoneyagerSMMalickiJBlairSSThe role of glypicans in Wnt inhibitory factor-1 activity and the structural basis of Wif1’s effects on Wnt and hedgehog signalingPLoS Genetics201282e100250322383891
- YavariANagarajROwusu-AnsahERole of lipid metabolism in smoothened derepression in hedgehog signalingDev Cell2010191546520643350
- KhaliullinaHPanakovaDEugsterCRiedelFCarvalhoMEatonSPatched regulates smoothened trafficking using lipoprotein-derived lipidsDevelopment2009136244111412119906846
- CallejoACuliJGuerreroIPatched, the receptor of hedgehog, is a lipoprotein receptorProc Natl Acad Sci U S A2008105391291718198278
- BijlsmaMFSpekCAZivkovicDvan de WaterSRezaeeFPeppelenboschMPRepression of smoothened by patched-dependent (pro-)vitamin D3 secretionPLoS Biol200648e23216895439
- WangYDavidowLArvanitesACGlucocorticoid compounds modify smoothened localization and hedgehog pathway activityChem Bio201219897298222921064
- PhilippMFralishGBMeloniARSmoothened signaling in vertebrates is facilitated by a G protein-coupled receptor kinaseMol Biol Cell200819125478548918815277
- OgdenSKFeiDLSchillingNSAhmedYFHwaJRobbinsDJG protein Galphai functions immediately downstream of smoothened in hedgehog signallingNature2008456722496797018987629
- MolnarCHolguinHMayorFJrRuiz-GomezAde CelisJFThe G protein-coupled receptor regulatory kinase GPRK2 participates in hedgehog signaling in DrosophilaProc Natl Acad Sci U S A2007104197963796817483466
- RioboNASaucyBDilizioCManningDRActivation of heterotrimeric G proteins by smoothenedProc Natl Acad Sci U S A200610333126071261216885213
- DouglasAEHeimJAShenFThe alpha subunit of the G protein G13 regulates activity of one or more Gli transcription factors independently of smoothenedJ Biol Chem201128635307143072221757753
- ZhaoYTongCJiangJHedgehog regulates smoothened activity by inducing a conformational switchNature2007450716725225817960137
- CorbitKCAanstadPSinglaVNormanARStainierDYReiterJFVertebrate smoothened functions at the primary ciliumNature200543770611018102116136078
- HuangfuDLiuARakemanASMurciaNSNiswanderLAndersonKVHedgehog signalling in the mouse requires intrafagellar transport proteinsNature20034266962838714603322
- MaySRAshiqueAMKarlenMLoss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of GliDev Biol2005287237838916229832
- HuangfuDAndersonKVCilia and hedgehog responsiveness in the mouseProc Natl Acad Sci U S A200510232113251133016061793
- ZhangQDavenportJRCroyleMJHaycraftCJYoderBKDisruption of IFT results in both exocrine and endocrine abnormalities in the pancreas of Tg737(orpk) mutant miceLab Invest2005851456415580285
- HooverANWynkoopAZengHJiaJNiswanderLALiuAC2cd3 is required for cilia formation and hedgehog signaling in mouseDevelopment2008135244049405819004860
- HillmanRTFengBYNiJNeuropilins are positive regulators of hedgehog signal transductionGenes Dev201125222333234622051878
- SnuderlMBatistaAKirkpatrickNDTargeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastomaCell201315251065107623452854
- CaoYWangLNandyDNeuropilin-1 upholds dedifferentiation and propagation phenotypes of renal cell carcinoma cells by activating Akt and sonic hedgehog axesCancer Res200868218667867218974107
- ParraLMZouYSonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossingNat Neurosci2010131293519946319
- CheungHOZhangXRibeiroAThe kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signalingSci Signal2009276ra2919549984
- Endoh-YamagamiSEvangelistaMWilsonDThe mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during developmentCurr Biol200919151320132619592253
- LawKKMakinoSMoRZhangXPuviindranVHuiCCAntagonistic and cooperative actions of Kif7 and Sufu define graded intracellular Gli activities in hedgehog signalingPLoS One2012711e5019323166838
- LiZJNieuwenhuisENienWKif7 regulates Gli2 through Sufu-dependent and -independent functions during skin development and tumorigenesisDevelopment2012139224152416123034632
- HsuSHZhangXYuCKif7 promotes hedgehog signaling in growth plate chondrocytes by restricting the inhibitory function of SufuDevelopment2011138173791380121795282
- WilsonCWNguyenCTChenMHFused has evolved divergent roles in vertebrate hedgehog signalling and motile ciliogenesisNature200945972439810219305393
- MerchantMEvangelistaMLuohSMLoss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalusMol Cell Biol200525167054706816055717
- ChenMHGaoNKawakamiTChuangPTMice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic developmentMol Cell Biol200525167042705316055716
- EggenschwilerJTEspinozaEAndersonKVRab23 is an essential negative regulator of the mouse sonic hedgehog signalling pathwayNature2001412684319419811449277
- ReiterJFSkarnesWCTectonic, a novel regulator of the hedgehog pathway required for both activation and inhibitionGenes Dev2006201222716357211
- LiCChiSXieJHedgehog signaling in skin cancersCell Signal20112381235124321397013
- HuangSYangLAnYExpression of hedgehog signaling molecules in lung cancerActa Histochem2011113556456920656337
- KinzlerKWRuppertJMBignerSHVogelsteinBThe Gli gene is a member of the Kruppel family of zinc finger proteinsNature198833261623713742832761
- RuppertJMKinzlerKWWongAJThe Gli-Kruppel family of human genesMol Cell Biol198888310431132850480
- BarnfieldPCZhangXThanabalasinghamVYoshidaMHuiCCNegative regulation of Gli1 and Gli2 activator function by suppressor of fused through multiple mechanismsDifferentiation200573839740516316410
- ShengTChiSZhangXXieJRegulation of Gli1 localization by the cAMP/protein kinase A signaling axis through a site near the nuclear localization signalJ Biol Chem2006281191216293631
- KogermanPGrimmTKogermanLMammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1Nat Cell Biol19991531231910559945
- SteccaBMasCClementVMelanomas require hedghog-Gli signaling regulated by interactions between Gli1 and the RAS-MEK/AKT pathwaysProc Natl Acad Sci U S A2007104145895590017392427
- PanYBaiCBJoynerALWangBSonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradationMol Cell Biol20062693365337716611981
- HuntzickerEGEstayISZhenHLoktevaLAJacksonPKOroAEDual degradation signals control Gli protein stability and tumor formationGenes Dev200620327628116421275
- WangBLiYEvidence for the direct involvement of βTrCP in Gli3 protein processingProc Natl Acad Sci U S A20061031333816371461
- Di MarcotullioLFerrettiEGrecoANumb is a suppressor of hedgehog signalling and targets Gli1 for Itch-dependent ubiquitinationNat Cell Biol20068121415142317115028
- JiangJRegulation of Hh/Gli signaling by dual ubiquitin pathwaysCell Cycle20065212457246317102630
- CanettieriGDi MarcotullioLGrecoAHistone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates hedgehog signalling through Gli acetylationNat Cell Biol201012213214220081843
- ConiSAntonucciLD’AmicoDGli2 acetylation at lysine 757 regulates hedgehog-dependent transcriptional output by preventing its promoter occupancyPLoS One201386e6571823762415
- HanLPanYWangBSmall ubiquitin-like modifier (SUMO) modification inhibits Gli2 protein transcriptional activity in vitro and in vivoJ Biol Chem201228724204832048922549777
- ZhangQShiQChenYMultiple Ser/Thr-rich degrons mediate the degradation of Ci/Gli by the Cul3-HIB/SPOP E3 ubiquitin ligaseProc Natl Acad Sci U S A200910650211912119619955409
- ChengSYBishopJMSuppressor of fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complexProc Natl Acad Sci U S A20029985442544711960000
- WangCPanYWangBSuppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressorsDevelopment2010137122001200920463034
- LiZJMackSCMakTHAngersSTaylorMDHuiCCEvasion of p53 and G/M checkpoints are characteristic of Hh-driven basal cell carcinomaOncogene Epub June 10, 2013
- JaganiZMora-BlancoELSansamCGLoss of the tumor suppressor Snf5 leads to aberrant activation of the hedgehog-Gli pathwayNat Med201016121429143321076395
- AtwoodSXLiMLeeATangJYOroAEGli activation by atypical protein kinase C iota/lambda regulates the growth of basal cell carcinomasNature2013494743848448823446420
- YangLXieGFanQXieJActivation of the hedgehog-signaling pathway in human cancer and the clinical implicationsOncogene201029446948119935712
- HahnHWickingCZaphiropoulousPGMutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndromeCell19968568418518681379
- JohnsonRLRothmanALXieJHuman homolog of patched, a candidate gene for the basal cell nevus syndromeScience19962725268166816718658145
- EpsteinEJrGenetic determinants of basal cell carcinoma riskMed Pediatr Oncol200136555555811340611
- GorlinRJNevoid basal-cell carcinoma syndromeMedicine1987662981133547011
- XieJMuroneMLuohSMActivating smoothened mutations in sporadic basal-cell carcinomaNature1998391666290929422511
- LamCWXieJToKFA frequent activated smoothened mutation in sporadic basal cell carcinomasOncogene19991838338369989836
- ReifenbergerJWolterMKnobbeCBSomatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomasBr J Dermatol20051521435115656799
- ReifenbergerJWolterMWeberRGMissense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous systemCancer Res1998589179818039581815
- Couve-PrivatSBouadjarBAvrilMFSarasinADaya-GrosjeanLSignificantly high levels of ultraviolet-specific mutations in the smoothened gene in basal cell carcinomas from DNA repair-deficient xeroderma pigmentosum patientsCancer Res200262247186718912499255
- TostarUMalmCJMeis-KindblomJMKindblomLGToftgardRUndenABDeregulation of the hedgehog signalling pathway: a possible role for the PTCH and SUFU genes in human rhabdomyoma and rhabdomyosarcoma developmentJ Pathol20062081172516294371
- PresseyJGAndersonJRCrossmanDKLynchJCBarrFGHedgehog pathway activity in pediatric embryonal rhabdomyosarcoma and undifferentiated sarcoma: a report from the Children’s Oncology GroupPediatr Blood Cancer201157693093821618411
- ClarkVEErson-OmayEZSerinAGenomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMOScience201333961231077108023348505
- AavikkoMLiSPSaarinenSLoss of SUFU function in familial multiple meningiomaAm J Hum Genet201291352052622958902
- KijimaCMiyashitaTSuzukiMOkaHFujiiKTwo cases of nevoid basal cell carcinoma syndrome associated with meningioma caused by a PTCH1 or SUFU germline mutationFam Cancer201211456557022829011
- FeiDLSanchez-MejiasAWangZHedgehog signaling regulates bladder cancer growth and tumorigenicityCancer Res201272174449445822815529
- Rodriguez-BlancoJSchillingNSTokhuntsRThe hedgehog processing pathway is required for NSCLC growth and survivalOncogene201332182335234522733134
- ShinKLeeJGuoNHedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladderNature2011472734111011421389986
- HahnHWojnowskiLZimmerAMHallJMillerGZimmerARhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndromeNat Med1998456196229585239
- HatleyMETangWGarciaMRA mouse model of rhabdomyosarcoma originating from the adipocyte lineageCancer Cell201222453654623079662
- IgnatiusMSChenEElpekNMIn vivo imaging of tumor-propagating cells, regional tumor heterogeneity, and dynamic cell movements in embryonal rhabdomyosarcomaCancer Cell201221568069322624717
- NitzkiFZibatAFrommholdAUncommitted precursor cells might contribute to increased incidence of embryonal rhabdomyosarcoma in heterozygous patched1-mutant miceOncogene201130434428443621602886
- PelczarPZibatAvan DopWAInactivation of patched1 in mice leads to development of gastrointestinal stromal-like tumors that express PDGFRα but not kitGastroenterology2013144113414423041331
- ParkKSMartelottoLGPeiferMA crucial requirement for hedgehog signaling in small cell lung cancerNat Med201117111504150821983857
- WangDHClemonsNJMiyashitaTAberrant epithelial-mesenchymal hedgehog signaling characterizes Barrett’s metaplasiaGastroenterology201013851810182220138038
- YangLWangLSChenXLHedgehog signaling activation in the development of squamous cell carcinoma and adenocarcinoma of esophagusInt J Biochem Mol Biol201231465722509480
- TianHCallahanCADuPreeKJHedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesisProc Natl Acad Sci U S A2009106114254425919246386
- MaoJLigonKLRakhlinEYA novel somatic mouse model to survey tumorigenic potential applied to the hedgehog pathwayCancer Res20066620101711017817047082
- AszterbaumMEpsteinJOroAUltraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout miceNat Med19995111285129110545995
- GoodrichLVMilenkovicLHigginsKMScottMPAltered neural cell fates and medulloblastoma in mouse patched mutantsScience19972775329110911139262482
- FeldmannGDharaSFendrichVBlockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancersCancer Res20076752187219617332349
- BaileyJMSwansonBJHamadaTSonic hedgehog promotes desmoplasia in pancreatic cancerClin Cancer Res200814195995600418829478
- ChangQFoltzWDChaudaryNHillRPHedleyDWTumor-stroma interaction in orthotopic primary pancreatic cancer xenografts during hedgehog pathway inhibitionInt J Cancer2013133122523423280784
- TangSNFuJNallDRodovaMShankarSSrivastavaRKInhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristicsInt J Cancer20121311304021796625
- FeldmannGFendrichVMcGovernKAn orally bioavailable small-molecule inhibitor of hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancerMol Cancer Ther2008792725273518790753
- GuDLiuHSuGHCombining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasisMol Cancer Ther20131261038104823468532
- BaileyJMMohrAMHollingsworthMASonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancerOncogene200928403513352519633682
- OliveKPJacobetzMADavidsonCJInhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancerScience200932459331457146119460966
- ZhaoCChenAJamiesonCHHedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemiaNature2009458723977677919169242
- BoydALSalciKRShapovalovaZMcIntyreBABhatiaMNonhematopoietic cells represent a more rational target of in vivo hedgehog signaling affecting normal or acute myeloid leukemia progenitorsExp Hematol Epub June 6, 2013
- RomerJTKimuraHMagdalenoSSuppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) miceCancer Cell20046322924015380514
- GuDFanQZhangXXieJA role for transcription factor STAT3 signaling in oncogene smoothened-driven carcinogenesisJ Biol Chem201228745383563836622992748
- XieJAszterbaumMZhangXA role of PDGFRalpha in basal cell carcinoma proliferationProc Natl Acad Sci U S A200198169255925911481486
- JohnsonRWNguyenMPPadaleckiSSTGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical hedgehog signalingCancer Res201171382283121189326
- KeysarSBLePNAndersonRTHedgehog signaling alters reliance on EGF receptor signaling and mediates anti-EGFR therapeutic resistance in head and neck cancerCancer Res201373113381339223576557
- EberlMKlinglerSMangelbergerDHedgehog-EGFR cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cellsEMBO Mol Med20124321823322294553
- FanQHeMShengTRequirement of TGFbeta signaling for SMO-mediated carcinogenesisJ Biol Chem201028547365703657620858897
- WangYDingQYenCJThe crosstalk of mTOR/S6K1 and hedgehog pathwaysCancer Cell201221337438722439934
- ShiSDengYZZhaoJSRACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathwayJ Biol Chem2012287117845785822262830
- HsiehAEllsworthRHsiehDHedgehog/Gli1 regulates IGF dependent malignant behaviors in glioma stem cellsJ Cell Physiol201122641118112720857406
- MainwaringLAKenneyAMDivergent functions for eIF4E and S6 kinase by sonic hedgehog mitogenic signaling in the developing cerebellumOncogene201130151784179721339731
- FernandezLASquatritoMNorthcottPOncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activationOncogene201231151923193721874045
- HellerEHurchlaMAXiangJHedgehog signaling inhibition blocks growth of resistant tumors through effects on tumor microenvironmentCancer Res201272489790722186138
- AbbruzzeseJLNational Institutes of Health (US)Are there new targets for pancreatic cancer therapeutics?2007 Available from: http://videocast.nih.gov/launch.asp?13834Accessed September 10, 2013
- InagumaSKasaiKIkedaHGli1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherinOncogene201130671472320972463
- JoostSAlmadaLLRohnalterVGli1 inhibition promotes epithelial-to-mesenchymal transition in pancreatic cancer cellsCancer Res2012721889922086851
- LiXDengWLobo-RuppertSMRuppertJMGli1 acts through snail and E-cadherin to promote nuclear signaling by beta-cateninOncogene200726314489449817297467
- JavelaudDPierratMJMauvielACrosstalk between TGF-beta and hedgehog signaling in cancerFEBS Lett2012586142016202522609357
- Von HoffDDLoRussoPMRudinCMInhibition of the hedgehog pathway in advanced basal-cell carcinomaN Engl J Med9172009361121164117219726763
- GrahamRALumBLCheetiSPharmacokinetics of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors: the role of alpha-1-acid glycoprotein bindingClin Cancer Res20111782512252021300760
- LoRussoPMRudinCMReddyJCPhase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumorsClin Cancer Res20111782502251121300762
- LorussoPMJimenoADyGPharmacokinetic dose-scheduling study of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumorsClin Cancer Res201117175774578221753154
- MetcalfeCde SauvageFJHedgehog fights back: mechanisms of acquired resistance against smoothened antagonistsCancer Res201171155057506121771911
- TangTTangJYLiDTargeting superficial or nodular basal cell carcinoma with topically formulated small molecule inhibitor of smoothenedClin Cancer Res201117103378338721558397
- SkvaraHKalthoffFMeingassnerJGTopical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitorJ Invest Dermatol201113181735174421430703
- BuonamiciSWilliamsJMorrisseyMInterfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastomaSci Transl Med201025151ra70
- DijkgraafGJAlickeBWeinmannLSmall molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistanceCancer Res201171243544421123452
- KimJAftabBTTangJYItraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonistsCancer Cell2013231233423291299
- KimJTangJYGongRItraconazole, a commonly used anti-fungal that inhibits hedgehog pathway activity and cancer growthCancer Cell201017438839920385363
- TangJYXiaoTZOdaYVitamin D3 inhibits hedgehog signaling and proliferation in murine basal cell carcinomasCancer Prev Res (Phila)20114574475121436386
- ChennaVHuCPramanikDA polymeric nanoparticle encapsulated small-molecule inhibitor of hedgehog signaling (NanoHHI) bypasses secondary mutational resistance to smoothened antagonistsMol Cancer Ther201211116517322027695
- RohnerASpilkerMELamJLEffective targeting of hedgehog signaling in a medulloblastoma model with PF-5274857, a potent and selective smoothened antagonist that penetrates the blood-brain barrierMol Cancer Ther2012111576522084163
- LeeMJHattonBAVillavicencioEHHedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma modelProc Natl Acad Sci U S A2012109207859786422550175
- RudinCMHannCLLaterraJTreatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449N Engl J Med2009361121173117819726761
- FuldaSMolecular targeted therapies for rhabdomyosarcoma: focus on hedgehog and apoptosis signalingKlinische Padiatrie2013225311511923625685
- DierksCBeigiRGuoGRExpansion of Bcr-Abl-positive leukemic stem cells is dependent on hedgehog pathway activationCancer Cell200814323824918772113
- ReadTAFogartyMPMarkantSLIdentification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastomaCancer Cell200915213514719185848
- HofmannIStoverEHCullenDEHedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesisCell Stem Cell20094655956719497284
- GaoJGravesSKochUHedgehog signaling is dispensable for adult hematopoietic stem cell functionCell Stem Cell20094654855819497283
- SigginsSLNguyenNYMcCormackMPThe hedgehog receptor patched1 regulates myeloid and lymphoid progenitors by distinct cell-extrinsic mechanismsBlood20091145995100419483124
- MerchantAJosephGWangQBrennanSMatsuiWGli1 regulates the proliferation and differentiation of HSCs and myeloid progenitorsBlood2010115122391239620107231
- ReyaTMorrisonSJClarkeMFWeissmanILStem cells, cancer, and cancer stem cellsNature2001414685910511111689955
- Sims-MourtadaJIzzoJGApisarnthanaraxSHedgehog: an attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation responseClin Cancer Res200612216565657217085672
- YoshikawaRNakanoYTaoLHedgehog signal activation in oesophageal cancer patients undergoing neoadjuvant chemoradiotherapyBr J Cancer200898101670167418475300
- TakebeNHarrisPJWarrenRQIvySPTargeting cancer stem cells by inhibiting Wnt, notch, and hedgehog pathwaysNat Rev Clin Oncol2011829710621151206
- SongZYueWWeiBSonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancerPLoS One201163e1768721394208
- TanakaHNakamuraMKamedaCThe hedgehog signaling pathway plays an essential role in maintaining the CD44+CD24−/low subpopulation and the side population of breast cancer cellsAnticancer Res20092962147215719528475
- LiuSDontuGMantleIDHedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cellsCancer Res200666126063607116778178
- TakahashiTKawakamiKMishimaSCyclopamine induces eosinophilic differentiation and upregulates CD44 expression in myeloid leukemia cellsLeuk Res201135563864520971508
- LiCHeidtDGDalerbaPIdentification of pancreatic cancer stem cellsCancer Res20076731030103717283135
- VisbalAPLaMarcaHLVillanuevaHAltered differentiation and paracrine stimulation of mammary epithelial cell proliferation by conditionally activated smoothenedDev Biol2011352111612721276786
- BarEEChaudhryALinACyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastomaStem Cells200725102524253317628016
- ClementVSanchezPde TriboletNRadovanovicIRuiz i AltabaAHedgehog-Gli1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicityCurr Biol200717216517217196391
- SuWMengFHuangLZhengMLiuWSunHSonic hedgehog maintains survival and growth of chronic myeloid leukemia progenitor cells through β-catenin signalingExp Hematol201240541842722240607
- Domingo-DomenechJVidalSJRodriguez-BravoVSuppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cellsCancer Cell201222337338822975379
- RamaswamyBLuYTengKYHedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathwayCancer Res201272195048505922875023
- StegADBevisKSKatreAAStem cell pathways contribute to clinical chemoresistance in ovarian cancerClin Cancer Res201218386988122142828
- ChaudaryNPintilieMHedleyDHedgehog pathway signaling in cervical carcinoma and outcome after chemoradiationCancer2012118123105311522028038
- QueirozKCRuela-de-SousaRRFuhlerGMHedgehog signaling maintains chemoresistance in myeloid leukemic cellsOncogene201029486314632220802532
- SinghRRKunkallaKQuCABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphomaOncogene201130494874488621625222
- LinSHGeorgeTJBen-JosefEOpportunities and challenges in the era of molecularly targeted agents and radiation therapyJ Natl Cancer Inst20131051068669323503600